Abstract
Mycobacterium tuberculosis is responsible for over 8 million cases of tuberculosis (TB) annually. Natural products may play important roles in the chemotherapy of TB. The antimycobacterial activity and the innate immune response of methanol (METH) and dichloromethane (DCM) extracts of Indigofera suffruticosa Miller (Fabaceae) were evaluated. We observed that the minimum inhibitory concentrations (MICs) for METH and DCM extracts were 125 and 1000 µg/mL, respectively. However, they were able to induce the innate immune response through the production of high levels of NO and TNF-α (p < 0.001) by peritoneal exudate cells (PECs). These results suggest that I. suffruticosa extracts may have an important immunological role in the control of TB once macrophage activity is induced by them.
Introduction
Mycobacterium tuberculosis is a serious threat to humankind, with over 8 million cases of tuberculosis (TB) annually, killing almost 3 million of people per year around the world (CitationWHO, 2008). Moreover, side effects from first-line anti-TB drugs can cause significant morbidity, and compromise treatment regimens for TB (CitationYee et al., 2003). Most healthy individuals are able to control TB infection with a vigorous immune response, halting the progression of the disease, but not necessarily eradicating the microorganism (CitationMcKinney, 2000). The bacterium resides within macrophages, allowing them to resist the antimicrobial effector mechanisms of the host (CitationRaupach & Kaufmann 2001). Macrophages constitute one of the main phagocytic cells of the immunological system, and they are the first cells involved in an immunological response. Part of their effectiveness is due to the production of nitric oxide (NO), hydrogen peroxide (H2O2), and other reactive nitrogen intermediates (RNIs), as well as phagocytosis of alien particles (CitationKeil et al., 1999; CitationCarlos et al., 2004; CitationAllavena et al., 2008). Hydrogen peroxide generated by macrophages in a reaction catalyzed by an NADPH (reduced nicotinamide adenine dinucleotide phosphate) oxidase was the first identified effector molecule that mediated mycobactericidal effects of mononuclear phagocytes (CitationWalker & Lowrie, 1981; CitationLopes et al., 2005). NO, formed by the action of the inducible form of nitric oxide synthase (iNOS), reacts with oxygen radical-forming RNIs. NO and related RNIs have been reported to possess antimycobacterial activity (CitationKwon, 1997; CitationYang et al., 2009). Tumor necrosis factor-α (TNF-α), a cytokine that plays multiple roles in immune and pathologic responses in tuberculosis, is also required for acute infection control (CitationFlynn et al., 1995; CitationPalladino et al., 2003; CitationBabbar & Casero, 2006).
Indigofera suffruticosa Miller (Fabaceae) is found in tropical and subtropical areas and is well adapted to growth in semi-arid regions and soils of low fertility (CitationPaiva et al., 1987). A chemical investigation of extracts of leaves of I. suffruticosa in Natural Products Alert (CitationNAPRALERT, 2003) and Chemical Abstracts databases has revealed the presence of alkaloids, flavonoids, steroids, proteins, carbohydrates, and indigo. Some recent reports have demonstrated the in vitro bioassay activity of plant-derived terpenoids against M. tuberculosis (CitationCantrell et al., 2001; CitationHastings, 1990; CitationHiguchi et al., 2008a). The literature also reports the antimycobacterial activity of many classes of natural products such as alkanes, phenolics, acetogenic quinines, flavonoids, triterpenes, flavonones, and chalcones (CitationCopp, 2003; CitationHiguchi et al., 2008b; CitationLeite et al., 2006; CitationPavan et al., 2009).
At present, there is no new drug generation able to eliminate the bacillus. Thus, we investigated the antimycobacterial and immunological activity of methanol (METH) and dichloromethane (DCM) extracts of I. suffruticosa.
Materials and methods
Plant material and samples
Aerial parts of I. suffruticosa were collected in Rubião Junior, Botucatu City, São Paulo State, Brazil, and identified by Prof. Dr. Jorge Yoshio Tamashiro. Immunological assays were performed as soon as the plant was collected. A voucher specimen (HUEC 129598) was deposited at the Herbarium of the Universidade Estadual de Campinas (Unicamp), Campinas, SP, Brazil. The aerial parts of I. suffruticosa (1.1 kg) were dried (40°C), powdered, and extracted exhaustively at room temperature with dichloromethane and methanol, successively. Solvents were evaporated at 40°C under reduced pressure to afford the DCM (15.2 g) and METH (30.0 g) extracts.
Each extract was first solubilized in dimethyl sulfoxide (DMSO) and then diluted in an appropriate culture medium, RPMI-1640 for immunological assays and Middlebrook 7H9 for the determination of antimycobacterial activity (62.5–4000 μg/mL).
Peritoneal macrophages
Peritoneal macrophages, thioglycollate-elicited peritoneal exudate cells (PECs), were harvested from Swiss mice using 5.0 mL of sterile phosphate-buffered saline (PBS, pH 7.4). The cells were washed twice by centrifugation at 200 g for 5 min at 4°C and re-suspended in RPMI-1640 medium (Sigma). The adherent cells were obtained by incubation for 1 h at 37°C in an atmosphere of air/CO2 (95:5, v/v) (Forma Scientific), and incubated with lipopolysaccharide (LPS) or RPMI-1640 medium. This protocol was in agreement with the regulations of the Research Ethics Committee (# 01/2005).
MTT assay for cell viability
PECs (5 × 106 cells/mL) was re-suspended in RPMI-1640 medium. The suspension (100 μL) and the extracts (100 μL) were added to each well of a 96-well tissue culture plate and they were incubated for 24 h. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) colorimetric assay was performed as described by CitationMosmann (1983). Cells only and culture medium (RPMI-1640) were used as a control that corresponded to 100% of macrophage viability.
Measurement of H2O2 production
Hydrogen peroxide production of adherent PECs (2 × 106 cells/mL) was measured using the horseradish peroxidase-dependent phenol red oxidation microassay (CitationPick & Mizel, 1981). Phorbol myristate acetate (PMA; Sigma, St. Louis, MO) was used as a positive control.
Measurement of NO production
NO production of adherent PECs (5 × 106 cells/mL) was measured using Griess reagent (CitationGreen et al., 1982). Escherichia coli O111B lipopolysaccharide (LPS 1 µg/mL) solution was used as a positive control.
Measurement of TNF-α production
Determination of TNF-α in the supernatant was based in its property to destroy the L929 tumoral cell line (mouse tumor fibroblast) (CitationCarlos et al., 1994). LPS (1 µg/mL) was used as a positive control.
Determination of antimycobacterial activity by MABA
The minimum inhibitory concentration (MIC) of METH and DCM extracts was determined against M. tuberculosis H37Rv (American Type Culture Collection 27294) in Middlebrook 7H9 medium using the microplate Alamar blue assay (MABA) (CitationCollins & Franzblau, 1997). For the standard test, the MIC value of isoniazid (Sigma) was determined each time. The acceptable MIC of isoniazid ranged from 0.015 to 0.05 µg/mL.
Statistical analysis
The results are expressed as mean ± SD of at least four experiments. One-way analysis of variance (ANOVA) with Dunnett’s post-test was performed using GraphPad InStat software (San Diego, CA, USA) with the level of significance set at p < 0.05.
Results and discussion
TB multiple drug resistance has become a major threat worldwide, and thus calls for an urgent search for new and effective treatments for this deadly disease. Naturally occurring compounds obtained as extracts from plants indicate that inhibitory activity against M. tuberculosis is widespread in nature (CitationOkunade et al., 2004).
The cytotoxic effect of the extracts was evaluated by the determination of MTT (a tetrazolium salt: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide) (CitationMosmann, 1983). The half maximal inhibitory concentration (IC50) was found to be 200 µg/mL ().
Table 1. Effect of methanol and dichloromethane extracts of Indigofera suffruticosa on the viability of peritoneal macrophages.
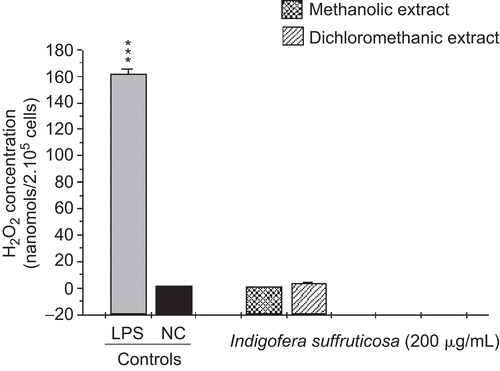
This study evaluated the antimycobacterial activity of extracts of I. suffruticosa and their action in the innate immune system. The antimycobacterial activities of METH and DCM are presented in . CitationGu et al. (2004) considered plant extracts with an MIC value ≤ 128 µg/mL to be active. Thus, we considered the result of MIC 125 µg/mL found for the METH crude extract to be promising. The extracts presented a high production of nitric oxide with statistically significant values compared to the negative control (p < 0.001). The amount of NO produced by the METH extract (105.99 µmol/5 × 105 cells) was larger than the production of the DCM extract (58.9 µmol/5 × 105 cells) (). The results regarding TNF-α confirmed a significant production of this cytokine, at levels near that of the positive control (252.7 and 234.6 units/mL, METH and DCM extracts, respectively), confirming a correlation between the synthesis of TNF-α and NO () (CitationBogdan et al., 1991; CitationCarli et al., 2009). I. suffruticosa did not produce significant amounts of H2O2 when compared with the negative control (p > 0.05): METH, 0.59 nmol/2 × 105cells and DCM, 3.3 nmol/2 × 105cells) (). This fact can be justified by the presence of tannins, such as gallic acid, in extracts of I. suffruticosa. This class of substances has been shown to have antioxidant potential, being responsible for the scavenging of free radicals such as hydrogen peroxide (CitationAkira et al., 2002). Thus, this screening suggests that both extracts of I. suffruticosa promoted the activation of macrophages. The significant production of studied mediators (NO and TNF-α) by activated macrophages in the presence of I. suffruticosa is very important, since macrophages produce several effector molecules that can enhance or restore the ability of the innate immune system to fight against TB infection.
Table 2. Minimal inhibitory concentration (MIC) against M. tuber-culosis of I. suffruticosa methanol and dichloromethane extracts.
Figure 1. Induction of nitric oxide (NO) production by methanolic and dichloromethanic extracts of I. suffruticosa from peritoneal macrophages. Cells incubated with lipopolysaccharide (1 µg/mL) were used as a positive control (C+) and cells in culture medium (RPMI-1640) were used as a negative control (C–). Data are reported as mean ± SD for at least four independent experiments carried out in triplicate. One-way ANOVA with Dunnett’s post-test was performed; ***p < 0.001 vs. C–.
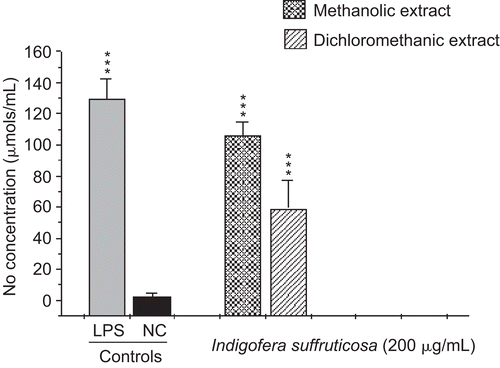
Figure 2. Induction of tumor necrosis factor-α (TNF-α) production by methanolic and dichloromethanic extracts of I. suffruticosa from peritoneal macrophages. Cells incubated with lipopolysaccharide (1 µg/mL) were used as a positive control (C+) and cells in culture medium (RPMI-1640) were used as a negative control (C–). Data are reported as mean ± SD for at least four independent experiments carried out in triplicate. One-way ANOVA with Dunnett’s post-test was performed; ***p < 0.001 vs. C–.
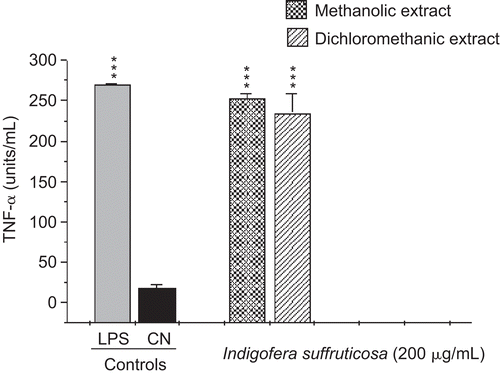
Figure 3. Induction of hydrogen peroxide (H2O2) production by methanolic and dichloromethanic extracts of I. suffruticosa from peritoneal macrophages. Cells incubated with phorbol myristate acetate (0.2 mM) were used as a positive control (C+) and cells in potassium phosphate buffer as a negative control (C–). Data are reported as mean ± SD for at least four independent experiments carried out in triplicate.
Nitric oxide (NO), formed by the action of the inducible form of nitric oxide synthase (iNOS), reacts with oxygen radical-forming RNIs. NO and related RNIs have been reported to possess antimycobacterial activity (CitationKwon, 1997). Phagocytes kill intracellular organisms during an initial oxidative phase dependent on NADPH oxidase, followed by a prolonged nitrosative phase, during which bacterial growth is inhibited by iNOS (CitationNathan & Shiloh, 2000). There are several potential mechanisms that can explain how NO may affect the microbial life-cycle. NO and other RNIs can modify bacterial DNA, protein, and lipids at both the microbial surface and intracellularly. They can alter cytokine production and induce or prevent apoptosis of host cells by controlling caspase activity (CitationRaupach & Kaufmann, 2001).
M. tuberculosis strongly induces the release of several cytokines during infection. TNF-α is a cytokine that plays multiple roles in immune and pathologic responses in tuberculosis, and is also required for acute infection control (CitationFlynn et al., 1995). It plays a major role in the recruitment of inflammatory cells to the site of infection and in the formation and maintenance of granulomas (CitationGaemperli et al., 2006). This cytokine is necessary for optimal co-ordination of both the differentiation of specific T cells to secrete the appropriate T helper 1 cytokines and the development of granulomas in which activated macrophages restrict mycobacterial growth (CitationEhlers, 2003). TNF-α is required for control of latent TB, and it is also a key element for activating macrophages to produce iNOS and thus in maintaining the pathway for generating NO and preventing reactivation of the disease (CitationAdams et al., 1995).
We suggest that the extracts may be an important bactericidal source against M. tuberculosis of natural origin and do not present the toxic effects provoked by the drugs currently used in the treatment of tuberculosis. Moreover, possible association with traditional drugs can be suggested, considering that most standard drugs do not present the same simultaneous microbiological and immunological effect of the extracts tested here. The results described herein highlight the importance of conducting an in-depth study of the species of the Brazilian biome, and show the great potential of its biodiversity in the treatment of infectious diseases.
Declaration of interest
The authors thank FAPESP for its financial support.
References
- Adams LB, Mason CM, Kolls JK, Scollard D, Krahenbuhl JL, Nelson S (1995): Exacerbation of acute and chronic murine tuberculosis by administration of a tumor necrosis factor receptor-expressing adenovirus. J Infect Dis 171: 400–405.
- Allavena P, Sica A, Solinas G, Porta C, Mantovani A (2008): The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol 66: 1–9.
- Akira N, Yuto U, Kunihiko T, Keisuke M (2002): Effect of tannin compounds on uranium-hydrogen peroxide system. Nippon Kagakkai Koen Yokoshu 81:634.
- Babbar NRA, Casero J (2006): Tumor necrosis factor-alpha increases reactive oxygen species by inducing spermine oxidase in human lung epithelial cells: a potential mechanism for inflammation-induced carcinogenesis. Cancer Res 66: 11125–11130.
- Bogdan C, Vodovotz Y, Nathan C (1991): Macrophage deactivation by interleukin 10. J Exp Med 174: 1549–1555.
- Cantrell CL, Franzblau SG, Fischer NH (2001): Antimycobacterial plant terpenoids. Planta Med 67: 1–10.
- Carli CBA, Matos DC, Lopes FCM, Maia DCG, Dias MB, Sannomiya M, Rodrigues CM, Andreo MA, Vilegas W, Colombo LL, Carlos IZ (2009): Isolated flavonoids against mammary tumour cells LM2. Z Naturforsch 64: 32–36.
- Carlos IZ, Monnazzi LGS, Falcão DP, De Medeiros BMM (2004): TNF, H2O2 and NO response of peritoneal macrophages to Yersinia terocolitica O:3 derivatives. Microb Infect 6: 207–212.
- Carlos IZ, Sgarbi DBG, Angluster J, Alviano CS, Silva CL (1994): Disturbances in the production of interleukin-1-necrosis and tumor necrosis factor in disseminated murine sporotrichosis. Mycopathologia 127: 189–194.
- Collins LS, Franzblau SG (1997): Microplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob Agents Chemother 41: 1004–1009.
- Copp BR (2003): Antimycobacterial natural products. Nat Prod Rep 20: 535–557.
- Ehlers S (2003): Role of tumour necrosis factor (TNF) in host defence against tuberculosis: implications for immunotherapies targeting TNF. Ann Rheum Dis 62(Suppl. 2): ii37–ii42.
- Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, Schreiber R, Mak TW, Bloom BR (1995): Tumor necrosis factor-α is required in the protective immune response against M. tuberculosis in mice. Immunity 2: 561–572.
- Gaemperli A, Hauser T, Speck RF (2006): Risk of infection during treatment with tumor necrosis factor-alpha inhibitors. Z Rheumatol 65: 24–31.
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982): Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem 126: 131–138.
- Gu JQ, Wang Y, Franzblau SG, Montenegro G, Yang D, Timmermann BN (2004). Antitubercular constituents of Valeriana laxiflora. Planta Med 70: 509–514.
- Hastings RB (1990): Medicinal legumes of Mexico: Fabaceae, Papilionoideae, Part One. Econ Bot 44: 336–348.
- Higuchi CT, Pavan FR, Sannomiya M, Vilegas W, Leite SRA, Sacramento LVS, Sato DN, Leite CQF (2008a): Triterpenes and antitubercular activity of Byrsonima crassa. Quim Nova 31: 1719–1721.
- Higuchi CT, Sannomiya M, Pavan FR, Leite SRA, Sato DN, Franzblau SG, Sacramento LVS, Vilegas W, Leite CQF (2008b): Byrsonima fagifolia Niedenzu a polar compound with anti-tubercular activity. Evid Based Complement Alternat Med 2008 Dec 17. [Epub ahead of print]
- Keil DE, Luebke RW, Pruett BS (1999): Differences in the effects of dexamethasone on macrophage nitrite production: dependence on exposure regimem (in vivo or in vitro) and activation stimuli. Int J Immunopharmacol 17: 157–166.
- Kwon OJ (1997): The role of nitric oxide in the immune response of tuberculosis. Korean Med Sci 12: 481–487.
- Leite SP, Vieira JRC, de Medeiros PL, Leite RMP, Lima VLM, Xavier HS, Lima ED (2006): Antimicrobial activity of Indigofera suffruticosa. eCAM 3: 261–265.
- Lopes FCM, Benzatti FP, Jordão Junior CM, Moreira RRD, Carlos IZ (2005): Effect of the essential oil of Achillea millefolium L. in the production of hydrogen peroxide and tumor necrosis factor-α in murine macrophages. Braz J Pharm Sci 41: 401–405.
- McKinney JD (2000): In vivo veritas: the search for TB drug targets goes live. Nat Med 6: 1330–1333.
- Mosmann T (1983): Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65: 55–63.
- NAPRALERT (2003): Natural Products Alert, Illinois University, Chicago. Available at: http://www.uic.edu/phamacy/depts/PCRPS/NAPRALERT.htm. Accessed 15 July 2009.
- Nathan C, Shiloh MU (2000): Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc Natl Acad Sci USA 97: 8841–8848.
- Okunade AL, Elvin-Lewis MP, Lewis WH (2004): Natural antimycobacterial metabolites: current status. Phytochemistry 65: 1017–1032.
- Paiva MAS, Barbosa ACD, Alves HLJB (1987): Indigofera suffruticosa Mill (Leguminosae) com potencial forrageiro em uma região de Caatinga no Semi-árido de Pernambuco (Alagoinha). In: Proceedings of the XXXVIII Congresso Nacional de Botânica. São Paulo, Brasil: Sociedade Nacional de Botânica, p. 422.
- Palladino MA, Bahjat FR, Theodorakis EA, Moldawer LL (2003): Anti-TNF-α therapies: the next generation. Nat Rev Drug Discov 2: 736–746.
- Pavan FR, Leite CQF, Coelho RG, Coutinho ID, Honda NK, Cardoso CAL, Vilegas W, Leite SRA, Sato DN (2009): Evaluation of anti-Mycobacterium tuberculosis activity of Campomanesia adamantium (MYRTACEAE). Quim Nova 32: 1222–1227.
- Pick E, Mizel DJ (1981): Superoxide anion and hydrogen peroxide production by chemically elicited peritoneal macrophages induction by multiple nonphagocytic stimuli. J Immunol Methods 46: 211–226.
- Raupach B, Kaufmann SHE (2001): Immune responses to intracellular bacteria. Curr Opin Immunol 13: 417–428.
- Walker L, Lowrie DB (1981): Killing of Mycobacterium microti by immunologically activated macrophages. Nature 293: 69–70.
- WHO (2008): Available at: http://www.who.int/tb/publications/global_report/2008/chapter_1/en/index3.html. Accessed 3 December 2008.
- Yang C, Yuk J, Jo E (2009): The role of nitric oxide in mycobacterial infections. Immune Netw 9: 46–52.
- Yee D, Valiquette C, Pelletier M, Parisien I, Rocher I, Menzies D (2003): Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am J Respir Crit Care Med 167: 1472–1477.