Abstract
Context: Ethnobotanical surveys conducted on Pelargonium reniforme Curtis (Geraniaceae) have shown that the aqueous root extracts are used to treat alcohol-induced liver damage.
Objective: We evaluated the antioxidant properties of the extract and its effects on alcohol-induced hepatotoxicity using Wistar rats.
Materials and methods: Alcohol-induced hepatotoxicity studies were carried out by observing the effect of the aqueous root extract on some liver marker enzymes, bilirubin, and total protein after liver damage. The levels of some phenolic compounds were determined by standard methods. Also, the reducing power of the plant extract and its ability to scavenge 1,1-diphenyl-2-picrylhydrazyl (DPPH*) and 2,2′-azinobis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS*+) radicals were determined to evaluate its antioxidant activity.
Results and discussion: The results obtained show that the plant extract possessed significant antioxidant activity. It had a significant level of phenolic compounds, scavenged DPPH* and ABTS*+ radicals effectively, and demonstrated good reducing power. This may indicate that the plant contained compounds which can remove toxic metabolites following alcohol abuse. Serum analysis of animals treated with only ethanol showed a significant increase in the levels of liver marker enzymes and total and unconjugated bilirubin, while a significant decrease was observed in the levels of conjugated bilirubin and total proteins. Administration of the plant extract restored the levels of these markers to normal levels, and this indicates the ability of the plant extract to restore normal functioning of a damaged liver.
Conclusion: The study shows that P. reniforme is a potential source of antioxidants and compounds which are useful in treating alcoholic liver damage.
Introduction
Alcohol toxicity is one of the world’s major health problems; many people are affected due to several fatal diseases caused by alcohol (CitationSingha et al., 2007). The liver is one of the organs known to be severely damaged due to chronic alcohol intake (CitationKundu et al., 2008). It has been observed that almost all ingested alcohol is metabolized in the liver and excessive alcohol use can lead to acute and chronic liver disease. Also, most of the consumed alcohol is eventually broken down by the liver, and the products generated and accumulated during alcohol metabolism (e.g., acetaldehyde) are more toxic than alcohol itself (CitationKurose et al., 1996). Alcohol abuse can elicit disturbances in the delicate balance between the pro- and antioxidant systems of the organism, therefore leading to oxidative stress. Increased generation of oxygen- and ethanol-derived free radicals has been observed at the microsomal level (particularly at the ethanol-inducible cytochrome P450 isoform), the cytosolic xanthine, and aldehyde oxidase, as well as through the mitochondrial respiratory chain (CitationNordmann et al., 1992). Polyunsaturated fatty acids are probably the most susceptible target to free radical attack that occurs due to alcohol abuse. The reaction of free radicals with the membrane lipid components leads to lipid peroxidation. This process can eventually cause increased membrane permeability and cell death (CitationRakonczay et al., 2003). To counteract these oxidants, cells have several enzymatic antioxidants and nonenzymatic antioxidants, but their levels are altered in alcoholics (CitationSaravanan & Nalini, 2007). This results in covalent modification of cellular macromolecules, morphological changes leading to tissue damage, and aberrant biochemistry of the liver (CitationLieber, 1991; CitationMcCuskey, 1991).
Although important progress has been made in understanding the pathogenesis of alcoholic liver disease, treatment strategies such as lifestyle changes and pharmacological and nutrition therapy have been employed, but these therapies for this disease are not very effective (CitationSaravanan et al., 2006), and liver transplantation is expensive and often beyond the reach of the common man (CitationFaremi et al., 2008). A phytotherapeutic approach to modern drug development can provide many invaluable drugs from traditional medicinal plants (CitationSaravanan et al., 2006).
Pelargonium reniforme Curtis (Geraniaceae) is a shrublet of up to 1 m height with kidney-shaped leaves and pink flowers. It is indigenous to the Eastern Cape Province in South Africa and it occurs mainly in coastal regions. It is widely used by traditional healers in areas of southern Africa for the treatment of diarrhea, dysentery, fever, respiratory tract infections, liver complaints, and wounds (CitationWatt & Breyer-Brandwijk, 1962). Information obtained from traditional healers and rural dwellers during ethnobotanical surveys carried out in Nkonkobe Municipality, Eastern Cape Province of South Africa showed that the aqueous root extracts are commonly used in the treatment of alcohol-induced liver disorders. Infusions and decoctions of the tubers are commonly taken, while a traditional method of using the roots is to boil the tuber in milk. Also, the roots may be directly chewed or powdered and mixed with food (CitationLatté & Kolodziej, 2004). Although the root of this plant is used in the treatment of alcohol-induced liver disorders among several ethnic groups in areas of southern Africa, there is a paucity of scientific evidence regarding its usage in liver disorders. Hence, the present study was undertaken to evaluate the in vitro antioxidant activity as well as the protective and curative effects of P. reniforme root extracts against alcohol-induced damage of rat liver.
Materials and methods
Collection and identification of plant material
The plant samples were collected in December 2008 from a natural population of P. reniforme from Grahamstown in the Eastern Cape Province of South Africa. The plant was identified by Professor D. S. Grierson of the Department of Botany, University of Fort Hare, and a voucher specimen (GER 3928) was deposited at the Giffen Herbarium of the University.
Animals
Adult rats of Wistar strain (158.6 ± 33.18 g) were used for the study. They were obtained from the animal house of the Agricultural and Rural Development Research Institute, University of Fort Hare. They were kept in rat cages and fed on commercial rat pellets (EPOL Feeds, South Africa Ltd.) and allowed free access to fresh water. The project was approved by the Ethics Committee at the University of Fort Hare.
Chemicals
The assay kits for albumin, bilirubin, total protein, alkaline phosphatase, gamma glutamyl transferase, and alanine and aspartate aminotransferases were obtained from Roche Diagnostic GmbH, Mannhein, Germany. Ethanol was purchased from E. Merck, Darmstadt, Germany. 1,1-Diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azinobis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS), potassium ferricyanide, butylated hydroxytoluene (BHT), ascorbic acid, catechin, tannic acid, quercetin, and FeCl3 were purchased from Sigma Chemical Co. (St. Louis, MO, USA), vanillin from BDH Chemicals Ltd. (Poole, England), and Folin–Ciocalteu phenol reagent and sodium carbonate were from Merck Chemical supplies (Damstadt, Germany). All other reagents used were of analytical grade and were supplied by Merck Chemicals (Pty) Ltd., Bellville, South Africa.
Preparation of aqueous extract
The roots of P. reniforme were air-dried at room temperature for 7 days. The dried material was then comminuted into coarse powder using the Waring commercial laboratory blender. The powder (100 g) was extracted in 1000 mL of distilled water for 48 h on an orbital shaker (Stuart Scientific Orbital Shaker, UK). The extract was filtered using a Buchner funnel and Whatman no. 1 filter paper. The resulting filtrate was freeze-dried (Savant Refrigerated Vapor Trap, RV T41404, USA) to give a yield of 6.15 g. This was reconstituted separately in distilled water to give the required doses used in this study.
In vitro assays
Determination of total phenolics
The total phenolic content of the extract was determined by the modified Folin–Ciocalteu method (CitationWolfe et al., 2003). The extract (1 mg/mL) was mixed with 5 mL Folin–Ciocalteu reagent (previously diluted with water 1:10 v/v) and 4 mL (75 g/L) of sodium carbonate. The mixture was vortexed for 15 s and allowed to stand for 30 min at 40°C for color development. Absorbance was then measured at 765 nm using a Hewlett-Packard UV-VIS (ultraviolet-visible) spectrophotometer. Samples of extract were evaluated at a final concentration of 1 mg/mL. Total phenolic content was expressed as mg/g tannic acid equivalent using the following equation based on the calibration curve: y = 0.1216x, R2 = 0.9365, where x is the absorbance and y is the tannic acid equivalent (mg/g).
Determination of total flavonols
Total flavonol was estimated using the method of CitationKumaran and Karunakaran (2007). To 2 mL of sample (standard), 2 mL of 2% AlCl3 ethanol and 3 mL (50 g/L) sodium acetate solutions were added. The absorbance at 440 nm was read after 2.5 h at 20°C. Extract samples were evaluated at a final concentration of 1 mg/mL. Total flavonoid content was calculated as quercetin (mg/g) using the following equation based on the calibration curve: y = 0.0255x, R2 = 0.9812, where x is the absorbance and y is the quercetin equivalent (mg/g).
Determination of total proanthocyanidins
The procedure reported by CitationSun et al. (1998) was used to determine the total proanthocyanidin content. A volume of 0.5 mL of 0.1 mg/mL extract solution was mixed with 3 mL of 4% vanillin–methanol solution and 1.5 mL hydrochloric acid; the mixture was allowed to stand for 15 min. The absorbance was measured at 500 nm. Extract was evaluated at a final concentration of 0.1 mg/mL. Total proanthocyanidin content was expressed as catechin equivalents (mg/g) using the following equation based on the calibration curve: y = 0.5825x, R2 = 0.9277, where x is the absorbance and y is the catechin equivalent (mg/g).
Determination of total flavonoids
Total flavonoid contents were determined using the method of CitationOrdonez et al. (2006). A volume of 0.5 mL of 2% AlCl3 ethanol solution was added to 0.5 mL of sample solution. After 1 h at room temperature, the absorbance was measured at 420 nm. A yellow color indicated the presence of flavonoids. Extract samples were evaluated at a final concentration of 1 mg/mL. Total flavonoid content was calculated as quercetin (mg/g) using the following equation based on the calibration curve: y = 0.025x, R2 = 0.9812, where x is the absorbance and y is the quercetin equivalent (mg/g).
ABTS radical scavenging assay
The method of CitationRe et al. (1999) was adopted for the ABTS radical scavenging assay. The stock solution, which was allowed to stand in the dark for 16 h at room temperature, contained equal volumes of 7 mM ABTS salt and 2.4 mM potassium persulfate. The resultant ABTS*+ solution was diluted with methanol until an absorbance of about 0.70 ± 0.01 at 734 nm was reached. Varying concentrations of the plant extracts (1 mL) were reacted with 1 mL of the ABTS*+ solution and the absorbance taken at 734 nm between 3 and 7 min using the spectrophotometer. The ABTS*+ scavenging capacity of the extract was compared with that of BHT and rutin and the percentage inhibition calculated as:
where Abscontrol is the absorbance of ABTS radical + methanol, and Abssample is the absorbance of ABTS radical + sample extract/standard.
DPPH radical scavenging assay
The effect of extracts on the DPPH radical was estimated using the method of CitationLiyana-Pathirana and Shahidi (2005). A solution of DPPH (0.135 mM) in methanol was prepared and 1 mL of this solution was mixed with 1 mL of varying concentrations of the methanol extract. The reaction mixture was vortexed thoroughly and left in the dark at room temperature for 30 min. The absorbance of the mixture was measured at 517 nm using rutin and BHT as references. The ability to scavenge DPPH radical was calculated as:
where Abscontrol is the absorbance of DPPH radical + methanol, and Abssample is the absorbance of DPPH radical + sample extract/standard.
Determination of ferric reducing power
The ferric reducing potential of the extract was assayed as described by CitationDuh et al. (1999). The different concentrations of the extract and the standards, rutin and BHT (0.025–0.5 mg/mL; 1 mL), were mixed with phosphate buffer (2.5 mL, 0.2 M, pH 6.6) and potassium ferricyanide [K3Fe(CN)6] (2.5 mL, 1% w/v). The mixture was incubated at 50°C for 20 min. Trichloroacetic acid (TCA; 2.5 mL) (10% w/v) was added to the mixture, which was then centrifuged for 10 min at 1000 rpm. The upper layer of the solution (2.5 mL) was mixed with 2.5 mL distilled water and 0.5 mL of 0.1% w/v FeCl3. The absorbance was measured at 700 nm in a spectrophotometer. Higher absorbance of the reaction mixture indicated greater reducing power. BHT and vitamin C were used as standards.
Alcohol-induced hepatotoxicity studies
The animals were randomly divided into 10 groups, each comprising six, and were orally administered as follows:
Group 1 (alcohol control) received 0.5 mL of 20% ethanol (5 g/kg body weight) (CitationFaremi et al., 2008).
Group 2 (normal control) received 0.5 mL of distilled water.
Groups 3, 4, and 5 received aqueous extract of P. reniforme at 50, 100, and 200 mg/kg, respectively, 2 h before administration of 20% ethanol.
Groups 6, 7, and 8 received aqueous extract of P. reniforme at 50, 100, and 200 mg/kg, respectively, 2 h after administration of 20% ethanol.
Group 9 (positive control) received silymarin, the known hepatoprotective compound (Sigma Chemical Co.) at 25 mg/kg, 2 h before administration of 20% ethanol.
Group 10 (positive control) received silymarin (25 mg/kg) 2 h after administration of 20% ethanol.
The administration was done repeatedly on a daily basis for 3 weeks using a metal oropharyngeal cannula. All rats from each group were sacrificed 24 h after their respective 21 daily doses. Blood samples were collected for evaluating the biochemical parameters. The study was carried out following approval from the Ethical Committee on Animal Use and Care of the University of Fort Hare, South Africa.
Biochemical estimations
The activities of serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were assayed by the method of CitationReitman and Frankel (1957). Serum bilirubin level was estimated by the method of CitationMalloy and Evelyn (1937). Serum total protein, albumin, and globulin levels were estimated by the Biuret method (CitationReinhold, 1953). Gamma glutamyl transferase (GGT) and alkaline phosphatase (ALP) activities were determined by the method of CitationRosalki and Rau (1972).
Statistical analysis
The data for the various biochemical parameters were analyzed by one-way analysis of variance (ANOVA) followed by Student’s t-test using SAS. Values of p < 0.05 were considered statistically significant. The experimental results for the in vitro assays are expressed as mean ± standard deviation of three replicates.
Results
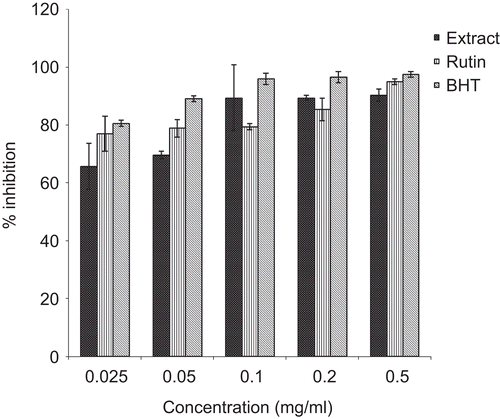
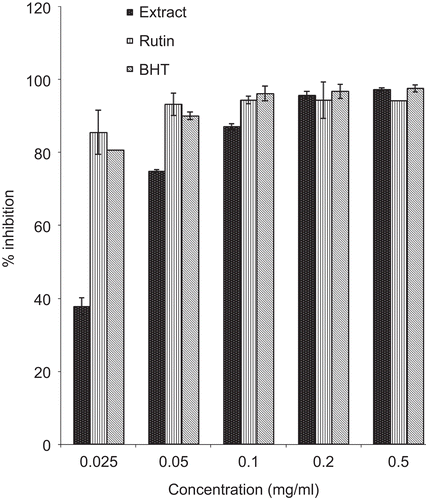
The aqueous extract of P. reniforme root was characterized by the presence of phenolic compounds. The levels of these compounds are shown in . The plant extract had a high level of total phenols (300.9 ± 0.1 mg tannic acid/g extract) and a low level of total flavonols (12.13 ± 0.01 mg quercetin/g extract). The plant extract also exhibited significant inhibition of DPPH* and ABTS*+ radicals in a dose-dependent manner ( and ).
Table 1. Polyphenolic contents of the aqueous root extract of P. reniforme.
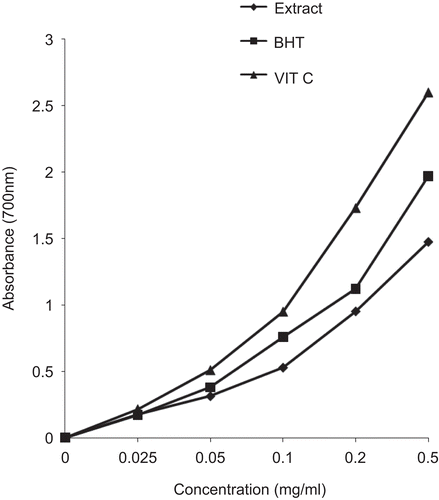
Significant DPPH* and ABTS*+ radical scavenging activity was evident at all the tested concentrations of the extract and compared well with the standards – butylated hydroxytoluene (BHT) and rutin. As illustrated in , Fe3+ was transformed to Fe2+ in the presence of the plant extract and the reference compounds, BHT and vitamin C, to measure the reductive capability. The results indicated that the reductive capability of the plant extract was dose-dependent. At 0.5 mg/mL, the absorbance was 1.48, while those of BHT and vitamin C were 1.97 and 2.6, respectively.
shows the effect of pre-treatment and post-treatment of P. reniforme extract on alcohol-induced liver damage based on the activities of some liver marker enzymes (ALT, AST, ALP, and GGT), and levels of total, conjugated, and unconjugated bilirubin. Animals in group 1 (alcohol only) developed hepatic damage when compared with animals in group 2 (distilled water only). This was evidenced by a significant elevation in the levels of the hepatic enzyme markers studied. Pre-treatment of the animals with three different doses of the plant extract before alcohol administration (groups 3, 4, and 5) ensured protection of the liver, as shown by the significant decrease in the levels of all the hepatic enzyme markers studied. The effects of post-treatment of P. reniforme extract after alcohol administration (groups 6, 7, and 8) indicated that the plant could enhance recovery from tissue damage, as shown by the significant decrease in levels of the liver marker enzymes under consideration. The levels of total and conjugated bilirubin are also good indices for hepatotoxicity. The levels of total and unconjugated bilirubin increased significantly in group 1 in comparison with group 2, which is evidence of hepatic damage, while they were reduced significantly in both the pre- and post-treated groups. Also, the levels of conjugated bilirubin were reduced significantly in group 1, while a significant increase was observed in the treated groups. The effects of pre-treatment and post-treatment with silymarin, a known hepatoprotective compound (groups 9 and 10), on all parameters are also shown in . The activities of the liver marker enzymes and the levels of total and unconjugated bilirubin were reduced significantly, while a significant increase was observed in the level of conjugated bilirubin, in all groups treated with silymarin, and this compares well with the results obtained for the plant extracts.
Table 2. Effect of aqueous extract of P. reniforme roots on hepatic markers in the serum of control and ethanol-administered rats.
The levels of serum total protein decreased significantly in ethanol alone-fed rats compared with those in control animals (). There was an improvement in the levels of proteins in rats pre-treated and post-treated with the plant extract (groups 3–8). However, no significant changes were observed in the levels of albumin and globulin, though their levels increased slightly in the normal control and treated groups in comparison with the alcohol control group. The results obtained from the treated animals also compare well with those obtained from animals treated with silymarin (groups 9 and 10), as a significant increase in the levels of serum total proteins was also observed.
Table 3. Effect of aqueous extract of P. reniforme roots on the serum protein components of control and ethanol-administered rats.
Discussion
There is increasing evidence that oxidative stress plays a vital role in the pathogenesis of alcohol liver disease (CitationLindros, 1995; CitationZima et al., 2001). Alcohol-induced hepatic tissue damage is mediated by acetaldehyde and reactive oxygen species (CitationZima et al., 2001). The removal and neutralization of these noxious toxic metabolites are considered to be vital initial steps in the prevention of alcohol-related liver diseases (CitationOzaras et al., 2003).
In the present study, we found that the aqueous root extract of P. reniforme had significant antioxidant activity. The extract was able to scavenge DPPH* and ABTS*+ free radicals in a concentration-dependent manner. It was also observed to contain a significant level of phenolic compounds, which are very important because of their free radical scavenging ability due to their hydroxyl groups (CitationHatano et al., 1989). Phenolics are the major plant compounds with antioxidant activity. This activity is believed to be mainly due to their redox properties, which play an important role in adsorbing and neutralizing free radicals, quenching singlet and triplet oxygen, or decomposing peroxides (CitationZheng & Wang, 2001). Flavonoids were included among the several phenolic compounds present in the aqueous root extracts of the plant. The hydrogen-donating substituents (hydroxyl groups) attached to the aromatic ring structures of flavonoids enable them to undergo a redox reaction, which, in turn, helps them scavenge free radicals (CitationBrand-Williams et al., 1995). In addition, the plant showed very good reducing capacity, which may also serve as a significant indicator of its potential antioxidant activity (CitationHazra et al., 2008). The observed antioxidant property of P. reniforme may enable it to mop up noxious toxic metabolites released when alcohol is abused, and this may explain the observed protection of the liver cells from damage and improvement in the functional status of the cells after damage.
Excess alcohol consumption has been linked with altered liver metabolism and liver damage, with leakage of cytoplasmic liver enzymes into the blood (CitationJames, 1993). AST and ALT are considered among the most sensitive markers of hepatocellular injury. ALP, which is secreted from the lysosomes, is also a marker enzyme for assessing liver damage (CitationSingha et al., 2007). When the integrity of the lysosomal membrane changes and/or the membrane of the lysosome is ruptured by deleterious influences, this acid hydrolase enters the bloodstream, producing a transient increase in the activity of lysosomal enzymes in the serum. The GGT index has been reported to be high in alcoholic liver disease, and measurement of GGT has been claimed to be an extremely sensitive test and marker of ethanol-induced hepatic damage (CitationSandhir & Gill, 1999). Increased levels of these enzymes (AST, ALT, ALP, and GGT) in the serum were observed in alcohol-administered rats, which indicates increased permeability, damage, and necrosis of hepatocytes (CitationGoldberg & Watts, 1965). Pretreatment with the extract of P. reniforme significantly decreased levels of serum enzyme markers, thus suggesting that the extract possessed compounds that protected the hepatocytes from alcohol-induced liver injury and subsequent leakage of the enzymes into the circulation. Decreased levels of the enzyme markers in the post-treated group compared to control were an indication that the extract also possessed a curative effect.
Serum bilirubin is one of the most sensitive tests employed in the diagnosis of hepatic diseases. It provides useful information on how well the liver is functioning (CitationSaravanan et al., 2006). Bilirubin, a chemical breakdown product of hemoglobin, is conjugated with glucuronic acid in hepatocytes to increase its water solubility. Unconjugated hyperbilirubinemia was observed in alcohol-fed rats, which may be a result of mass inhibition of the conjugation reaction and release of unconjugated bilirubin from damaged and dead hepatocytes. The decrease in serum bilirubin after treatment with P. reniforme indicated the effectiveness of the drug in the maintenance of normal functional status of the liver.
Hypoalbuminemia is most frequent in the presence of advanced chronic liver diseases. Hence, a decline in total protein content can be deemed a useful index of the severity of cellular dysfunction in chronic liver diseases. The lowered level of total proteins recorded in alcohol-treated rats revealed the severity of hepatopathy. Stabilization of serum protein levels in the pre- and post-treatment groups administered with P. reniforme is further a clear indication of the improvement of the functional status of the liver cells.
Conclusion
On the basis of the results obtained in the present study, it is concluded that the aqueous extract of the roots of Pelargonium reniforme, which contains significant amounts of phenolic compounds, exhibits high antioxidant and free radical scavenging activities. It also has very good reducing power. These in vitro assays indicate that this plant extract is a significant source of natural antioxidants, which might be helpful in removing and neutralizing noxious toxic metabolites released when alcohol is abused. Also, the results show that the plant extract has protective and curative effects on alcohol-induced liver damage, as it restored the liver marker enzymes, serum bilirubin, and protein to normal levels.
The potency of P. reniforme root extract compares well with silymarin with respect to the hepatic markers observed. Multiple mechanisms may interplay in its protective and curative effects on the liver. Hence, it merits further development for exploiting it as a therapeutic agent.
Declaration of interest
The authors wish to thank the Govan Mbeki Research and Development Centre of the University of Fort Hare and the National Research Foundation of South Africa for financial assistance.
References
- Brand-Williams W, Cuvelier ME, Berset C (1995): Use of a free-radical method to evaluate antioxidant activity. LWT 28: 25–30.
- Duh PD, Tu YY, Yen GC (1999): Antioxidant activity of water extract of Harng Jyur (Chrysanthemum morifolium Ramat). LWT 32: 269–277.
- Faremi TY, Suru SM, Fafunso MA, Obioha UE (2008): Hepatoprotective potentials of Phyllanthus amarus against ethanol-induced oxidative stress in rats. Food Chem Toxicol 46: 2658–2664.
- Goldberg DM, Watts C (1965): Serum enzyme changes as evidence of liver reaction to oral alcohol. Gastroenterology 49: 256–261.
- Hatano T, Edamatsu R, Hiramatsu M, Mori A, Fujita Y, Yasuhara T, Yoshida T, Okuda T (1989): Effects of tannins and related polyphenols on superoxide anion radical, and on 1,1-diphenyl-2-picrylhydrazyl radical. Chem Pharm Bull 37: 2016–2021.
- Hazra B, Biswas S, Mandal N (2008): Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complement Altern Med 8: 63.
- James WPT (1993): Alcohol: its metabolism and effects. In:Garrow JS, James WPT, eds., Human Nutrition and Dietetics. London, Churchill Livingstone, pp. 103–118.
- Kumaran A, Karunakaran RJ (2007): In vitro antioxidant activities of methanol extracts of Phyllanthus species from India. LWT 40: 344–352.
- Kundu R, Dasgupta S, Biswas A, Bhattacharya A, Pal BC, Bandyopadhyay D, Bhattacharya S, Bhattacharya S (2008): Cajanus cajan Linn. (Leguminosae) prevents alcohol-induced rat liver damage and augments cytoprotective function. J Ethnopharmacol 118: 440–447.
- Kurose I, Higuchi H, Kato S, Miura S, Ishii H (1996): Ethanol-induced oxidative stress in the liver. Alcohol Clin Exp Res 20: 77A–85A.
- Latté KP, Kolodziej H (2004): Antioxidant properties of phenolic compounds from Pelargonium reniforme. J Agric Food Chem 52: 4899–4902.
- Lieber CS (1991): Hepatic, metabolic and toxic effects of ethanol: 1991 update. Clin Exp Res 15: 573–592.
- Lindros KO (1995): Alcoholic liver disease: Pathobiological aspects. J Hepatol 23: 7–15.
- Liyana-Pathirana CM, Shahidi F (2005): Antioxidant activity of commercial soft and hard wheat (Triticum aestivum L) as affected by gastric pH conditions. J Agric Food Chem 53: 2433–2440.
- Malloy HT, Evelyn KA (1937): The determination of bilirubin with the photoelectric colorimeter. J Biol Chem 119: 481–490.
- McCuskey RS (1991): In vivo microscopy of the effects of ethanol on the liver. In:Watson RR, ed., Liver Pathology and Alcohol. Totowa, NJ, Humana Press, pp. 563–570.
- Nordmann R, Ribiere C, Rouach H (1992): Implication of free radical mechanisms in ethanol-induced cellular injury. Free Radic Biol Med 12: 219–240.
- Ordonez AAL, Gomez JD, Vattuone MA, Isla MI (2006): Antioxidant activities of Sechium edule (Jacq.) Swart extracts. Food Chem 97: 452–458.
- Ozaras R, Tahan V, Aydin S, Uzun H, Kaya S, Senturk H (2003): N-Acetylcysteine attenuates alcohol-induced oxidative stress in animals. World J Gastroenterol 9: 791–794.
- Rakonczay Z, Boros I, Jarmay K, Hegyi P, Lonovics J, Takacs T (2003): Ethanol administration generates oxidative stress in the pancreas and liver, but fails to induce heat-shock proteins in rats. J Gastroenterol Hepatol 18: 858–867.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999): Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26: 1231–1237.
- Reinhold JG (1953): Manual determination of serum total protein, albumin and globulin fractions by Biuret method. In: Reiner M, ed., Standard Methods in Clinical Chemistry. New York, Academic Press, p. 88.
- Reitman S, Frankel S (1957): A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28: 56–63.
- Rosalki SB, Rau D (1972): Serum gamma-glutamyl transpeptidase activity in alcoholism. Clin Chim Acta 39: 41–47.
- Sandhir R, Gill KD (1999): Hepatoprotective effects of Liv-52 on ethanol induced liver damage in rats. Indian J Exp Biol 37: 762–766.
- Saravanan N, Nalini N (2007): Antioxidant effect of Hemidesmus indicus on ethanol-induced hepatotoxicity in rats. J Med Food 10: 675–682.
- Saravanan R, Viswanathan P, Pugalendi KV (2006): Protective effect of ursolic acid on ethanol-mediated experimental liver damage in rats. Life Sci 78: 713–718.
- Singha PK, Roy S, Dey S (2007): Protective activity of andrographolide and arabinogalactan proteins from Andrographis paniculata Nees against ethanol-induced toxicity in mice. J Ethnopharmacol 111: 13–21.
- Sun JS, Tsuang YW, Chen IJ, Huang WC, Hang YS, Lu FJ (1998): An ultra-weak chemiluminescence study on oxidative stress in rabbits following acute thermal injury. Burns 24: 225–231.
- Watt C, Breyer-Brandwijk MG (1962): The Medicinal and Poisonous Plants of Southern and Eastern Africa. Edinburgh, Livingstone, pp. 449–455.
- Wolfe K, Wu X, Liu RH (2003): Antioxidant activity of apple peels. J Agric Food Chem 51: 609–614.
- Zheng W, Wang SY (2001): Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem 49: 5165–5170.
- Zima T, Fialova L, Mestek O, Janebova M, Crkovska J, Malbohan I, Stipek S, Mikulikova L, Popov P (2001): Oxidative stress, metabolism of ethanol and alcohol-related diseases. J Biomed Sci 8: 59–70.