Abstract
Context: The Thai Lanna region has its own folklores and wisdoms in various fields such as traditional medicines. The galls of Terminalia chebula Retz. (Combretaceae) frequently appear in many Thai Lanna medicinal plant recipes for promoting longevity.
Objectives: To investigate the in vitro anti-aging activities of the extracts from 15 plants including T. chebula gall selected from the Thai medicinal plant recipes that have been traditionally used for longevity.
Materials and methods: The plant extracts were prepared by four extraction methods including hot (HW) and cold (CW) aqueous processes and hot (HM) and cold (CM) methanol processes. These extracts were tested for antioxidative and tyrosinase inhibition activity as well as the proliferative and MMP-2 inhibition activity on early aging human skin fibroblasts in order to evaluate their in vitro anti-aging activity.
Results: At 0.1 mg/mL, the CW extract of T. chebula gall exhibited the highest DPPH radical scavenging activity with scavenging of 84.64% ± 2.22%, whereas ascorbic acid, α-tocopherol and butylated hydroxyl toluene gave 96.50% ± 0.1%, 35.74% ± 0.2% and 27.43% ± 0.1%, respectively. The CW extract of T. chebula gall indicated the highest stimulation index (SI) on normal human fibroblast proliferation of 1.441 which was more active than ascorbic acid (SI 1.21). This extract has also demonstrated MMP-2 inhibition on fibroblasts determined by zymography 1.37 times more potent than ascorbic acid.
Discussion and conclusion: This study has confirmed the traditional use of T. chebula gall in many Thai medicinal plant recipes for longevity which will be beneficial for further development of anti-aging products.
Introduction
The global market values of anti-aging products which help the body fight off the damage caused by aging are increasing continuously. The largest group of compounds in anti-aging products is antioxidants. Aging is a very complex biological process including the damage from free radicals and the dark spots of the skin from melanin overproduction. Free radicals are highly reactive molecules with unpaired electrons which can cause damage to cell membranes, lipids, proteins, and DNA. Damage to DNA eventually results in collagen breakdown. Dark spots of the aged skin are from melanin overproduction which may be caused by chronic sun exposure, melasma, or other hyperpigmentation diseases (CitationBriganti et al., 2003). Tyrosinase, a copper-containing monooxygenase, is a key enzyme that catalyzes melanin synthesis in melanocytes (CitationSturm et al., 2001). Applications of tyrosinase inhibitors may be the least invasive procedure for maintaining skin whiteness (CitationKadekaro et al., 2003). Fibroblasts which produce collagens, glycosaminoglycans, reticular and elastic fibers, and glycoproteins are found in the extracellular matrix. Collagen, the major structural component of the skin in the dermis has been suggested to be the cause of the clinical changes observed in naturally aged and photo-aged skin (CitationMillis et al., 1989; CitationChung et al., 2001). Collagen synthesis has been studied by serial cultures of human dermal fibroblasts (CitationMillis et al., 1989). The effects on skin collagen synthesis for anti-aging evaluation can be determined from the proliferation activity of the human fibroblasts by the sulforhodamine B (SRB) assay. Gelatinase A (MMP-2) digests native collagen types I, II and III in a similar manner to the collagenases (CitationAimes & Quigley, 1995; CitationPatterson et al., 2001). MMP-2 induction was mediated by phenomena accelerated in aged human skin. Increased expression of MMP-2 is involved with collagen degradation in aged human skin (CitationSteinbrenner et al., 2003), leading to wrinkle formation and aged appearance. Thus, MMP-2 inhibitors which delay collagen degradation can be used to evaluate anti-aging activity.
Traditional herbs provide interesting and largely unexplored sources for the development of potential new cosmetic and pharmaceutical products. The Lanna region covered many provinces and cities in China, Myanmar and Thailand. It was an independent country about 700 year ago. In Thailand, this area called “Thai Lanna” which included seven provinces of Chiang Mai, Chiang Rai, Lamphun, Lampang, Phayao, Phrae and Nan. The Thai Lanna region has its own folklore and wisdoms in various fields such as politics, agriculture and traditional medicines. The Thai Lanna medicinal plant recipes are still currently used by the people in the northern part of Thailand. The Thai Lanna medicinal plant recipes were recorded as Lanna scripts in palm leaves, mulberry paper or Streblus asper Lour. (Moraceae) paper. The Natural Products Research and Development Center (NPRDC) at Chiang Mai University in Thailand has developed a database containing the Thai Lanna medicinal plant recipes collected from many institutes, temples and folklore doctors (CitationManosroi et al., 2006). Twenty-one Thai Lanna medicinal textbooks with over 19,161 recipes, 2,556 diseases, and 4,672 medicinal plants were collected. Many Thai Lanna medicinal plant recipes have implied the anti-aging application of being slow down the aging process and promoting vitality and well being. Terminalia chebula Retz. (Combretaceae) is one of the Thai Lanna medicinal plants which appear in many of these recipes. It is widely grown in tropical regions. The dried galls of T. chebula are frequently sold at fresh markets in Southeast Asia. Its medical applications include astringent, purgative, supplements for anti-aging and imparting of longevity as well as boosting of the immune system (CitationPharmacopoeia Commission of PRC, 1997; CitationZhu, 1998; CitationLemmens & Bunyapraphatsara, 2003). Moreover, T. chebula fruits have been shown to have antioxidant (CitationChen et al., 2003), antimicrobial (CitationBurapadaja & Bunchoo, 1995), and anticancer activities (CitationLee et al., 1995; CitationSaleem et al., 2002).
In this present study, 15 plants including T. chebula gall were selected from the Thai Lanna medicinal plant recipes database and their extracts were prepared by the aqueous and methanol extraction using hot and cold processes. These extracts were tested for antioxidative and tyrosinase inhibition activity as well as the proliferative and MMP-2 inhibition activity on early aging human skin fibroblasts in order to evaluate their in vitro anti-aging activity.
Materials and methods
Materials
L-(+)-Ascorbic acid (vitamin C), α-tocopherol, butylated hydroxytoluene (BHT), 2,2-diphenyl-1-picryhydrazyl radical (DPPH), EDTA, sulforodamine B, dimethyl sulfoxide (DMSO), kojic acid, ferrozine and ferric chloride (FeCl2) were purchased from Sigma (St. Louis, MO). Tyrosinase from mushroom (4187 U/mg) and L-tyrosine were purchased from Fluka (Buchs, Switzerland). Alpha-modified Eagle’s culture medium, antibiotics penicillin and streptomycin, fetal bovine serum and tripsin were purchased from Hyclone (Logan, Utah). All other chemicals and reagents were analytical grade.
Plant selection
Fifteen medicinal plants including T. chebula gall were selected from the Thai Lanna Medicinal Plant Textbook Database version “Manosroi 2” developed by NPRDC, Science and Technology Research Institute (STRI), Chiang Mai University in Thailand. The frequent traditional use and scientific evidence for anti-aging and longevity indicated in the recipes were used to select the plants. The selected plants were collected from Chiang Mai Province in Thailand during January to February in 2008 (). The specimen was authenticated by Suda Saowakhon, a botanist at Faculty of Pharmacy, Chiang Mai University, Thailand and deposited at NPRDC, STRI; Chiang Mai University in Thailand.
Table 1. Comparison of percentage yields of the 60 extracts from the 15 selected Thai Lanna plants including T. chebula gall prepared by aqueous and methanol cold and hot processes.
Preparation of the extracts
The plants were washed, cut into pieces, dried at 40° ± 2°C in a hot air oven, ground to powder and kept in an airtight plastic bag at 4° ± 2°C until use. For the extraction process, the dried plant powder (100 g) was extracted using four different conditions. For hot methanol (HM), the powder was extracted by continuous Soxhlet extraction for 1 h in 400 mL methanol until exhausted (65° ± 2°C). For hot water (HW), the powder was heated for 1 h with 400 mL distilled water at 100° ± 2°C and then cooled to room temperature (27° ± 2°C). For cold methanol (CM) and cold water (CW), 100 g of the powder was macerated in 400 mL of methanol or distilled water and sonicated in a bath sonicator for 1 h at room temperature (27° ± 2°C). The mixtures were filtered through Whatman No. 1 filter paper and the plant residues were re-extracted twice under the same conditions. The filtrates were pooled and concentrated under vacuum by a rotary evaporator (R-124 Buchi, Flawil, Switzerland), and lyophilized. The dried extracts were stored at 4° ± 2°C prior to use. Sixty extracts were obtained and the percentage yields were calculated on a dry weight basis.
Phytochemical test of the extracts
The extract (20 mg), dissolved in 20 mL of 80% methanol, was used for detecting the presence of alkaloids, flavonoids, glycosides, saponins, tannins and xanthones according to the methods previously described. For alkaloid, 2 mL of the extract solution mixed with 1 mL of 1% HCl was boiled over a water bath and 6 drops of Dragendorff’s reagent were added. Cream-ish or brownish-red or orange precipitate indicated the presence of alkaloids (CitationBrimer et al., 1989). Quinine sulfate (Sigma) was used as a positive control. For anthraquinone (Borntrager’s test) determination, 0.1 g of the powder extract was boiled with 4 mL of alcoholic KOH for 2–3 minutes and diluted with 4 mL of water and filtered. The filtrate was acidified with dilute HCl, filtered, cooled and shaken with benzene. The benzene layer was separated and put into a clean test tube and shaken with 2 mL of the dilute ammonia solution. Extracts consisting of anthraquinones gave an orange-red to deep orange-red color in the aqueous layer (CitationAllen, 1974; CitationHarbone, 1976). Anthraquinone from Fluka was used as a positive control. For the presence of flavonoids (Shinoda test), 2 mL of the extract solution mixed with 1 mL of concentrated HCl and magnesium ribbon gave the pink tomato-red color (CitationAllen, 1974). Luteolin from Sigma was used as a positive control. For the qualitative assay of glycoside (Fehling’s test for reducing sugars), 2 mL of the extract solution mixed with 1 mL of Fehling’s solution was heated in a water bath for 10 min. The brick-red precipitate indicated the presence of reducing sugar contained in glycosides (CitationHarbone, 1976; CitationOnwukaeme et al., 2007). Glucose, fructose and galactose from Sigma were used as positive controls. For saponin (Frothing test), 0.5 mL of the extract solution was mixed with 5 mL of distilled water. The frothing persistence indicated the presence of saponins (CitationAllen, 1974; CitationHarbone, 1976). Saponaria officinalis extract from NPRDC, STRI, Chiang Mai University was used as a positive control. For tannins, 2 mL of the extract solution were mixed with 2 mL of 15% FeCl3 solution. The blue-black precipitate indicated the presence of tannins (CitationVanMiddlesworth & Cannell, 1998; CitationOnwukaeme et al., 2007). Tannic acid from Fluka was used as a positive control. For xanthones, 2 mL of the extract solution was mixed with 1 mL 5% KOH reagent. The formation of yellow precipitate indicated the presence of xanthones (CitationAllen, 1974; CitationHarbone, 1976). Garcinia mangostana Linn. extract from NPRDC, STRI, Chiang Mai University was used as a positive control.
Antioxidative assays
DPPH radical scavenging activity
The DPPH radical scavenging activity of all extracts was determined by a modified method previously described (CitationTachibana et al., 2001). Briefly, 50 µL of the five serial concentration extracts, 0.001-10 mg/mL dissolved in methanol and 20%v/v DMSO (1:1), and 50 µL of ethanol solution of DPPH were put into each well of a 96-well microplate (Nalge Nunc International, NY). The reaction mixture was allowed to stand for 30 min at 27° ± 2°C, and the absorbance was measured at 515 nm by a well reader (Bio-Rad, model 680 microplate reader, Philadelphia, PA 19102-1737, USA) against a blank (methanol mixed with 20% v/v DMSO, 1:1). Ascorbic acid, BHT and α-tocopherol (0.001-10 mg/mL) were used as positive controls. The experiments were done in triplicate. The IC50 value which was the concentration of the sample that scavenged 50% of the DPPH radical was determined. The percentages of DPPH radical scavenging activity were calculated:
The histogram of the percentages of DPPH radical scavenging activity of the extract at 0.1 mg/mL was presented.
Chelating assay
The Fe2+ chelating ability of the extract was measured by the ferrous iron-ferrozine complex method (CitationDecker & Welch, 1990). Briefly, the reaction mixture containing 2 mM FeCl2 (10 µL) and 5 mM ferrozine (10 µL) and 100 µL of the five serial concentration extracts (0.001-10 mg/mL dissolved in methanol and 20% v/v DMSO 1:1 solution) were mixed in a 96-well plate and incubated for 10 min at 27° ± 2°C. The absorbance was recorded by a well reader at 570 nm. The absorbance of the control was determined by replacing the extract with methanol. EDTA (0.001-10 mg/mL) was used as a positive control. The experiments were done in triplicate. The IC50 value which was the concentration of the sample that chelated 50% of the ferrous iron was determined. The ability of the sample to chelate ferrous ion was calculated:
The histogram of the percentages of chelating effect of the extract at 0.1 mg/mL was presented.
Tyrosinase inhibition assay
The extracts were assayed by the modified tyrosinase inhibition method previously described (CitationShimizu et al., 1998). Briefly, 120 µL of 1.66 mM of tyrosine solution in 0.1 M phosphate buffer (pH 6.8), 60 µL of five serial concentrations of the extracts (0.001-10 mg/mL dissolved in methanol and 20% v/v DMSO 1:1) and 60 µL phosphate buffer were mixed in a 96-well plate and incubated at 37° ± 2°C for 60 min. Then, 60 µL of tyrosinase enzyme solution (0.6 mg/mL) in phosphate buffer were added. The enzyme activity at 37° ± 2°C was measured by a well reader at 450 nm. Ascorbic acid and kojic acid (0.001–10 mg/mL) were used as positive controls. The experiments were done in triplicate. The IC50 value which was the concentration of the sample that inhibited 50% of the enzyme activity was determined. The inhibition percentage of tyrosinase was calculated:
The histogram of the percentages of the inhibition effect of the extract at 0.1 mg/mL was presented.
Cell culture
The normal human skin fibroblasts were provided by Natthanej Luplertlop at the Department of Tropical Hygiene, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand. Cells were cultured under standard conditions in the complete culture medium containing α-modified Eagle’s culture medium (MEM-Alpha) supplemented with 10% (v/v) fetal bovine serum (FBS), penicillin (100 U/ml) and streptomycin (100 mg/ mL). Cells were incubated in a temperature-controlled, humidified incubator (Shel Lab, model 2123TC, Cornelius, OR 97113, USA) with 5% CO2 at 37°C. Cells were used at the 15th passage.
Cell proliferation activity by the SRB assay
The extracts from the three plants showing the highest activity of DPPH scavenging, chelating and tyrosinase inhibition activities were selected to test for cell proliferation activity of the 15th passage normal human skin fibroblasts by SRB assay according to the method of CitationPapazisis et al. (1997). Ascorbic acid (0.001–10 mg/mL) was used as a positive control. The cells were plated at a density of 1 × 105 cells/well in 96-well plates and left for cell attachment on the plate overnight in 5% CO2 at 37°C. Cells were then exposed to five serial concentrations of the extracts (0.001–10 mg/mL) for 24 h. After incubation, the adherent cells were fixed in situ, washed and dyed with SRB. The bound dye was solubilized and the absorbance was measured at 540 nm by a well reader. The assays were done in three independent and separate experiments. The percentage of cell growth was calculated using the follow equation:
Stimulation index (SI) which was the ratio between the percentages of %G treated with the extracts at 0.1 mg/mL and the control (no treatment) was presented.
Gelatinolytic activity on MMP-2 inhibition of the plant extracts (Zymography)
The CM, CW, HM and HW extract of the three plants which showed the highest activity of human fibroblast proliferation activity were selected to assay for gelatinolytic activity of MMP-2 inhibition in comparing with ascorbic acid. A monolayer of 5 × 105 cells at the 15th passage normal human skin fibroblasts was maintained in the culture medium without FBS for 24 h, treated with the extracts and ascorbic acid at concentration 0.1 mg/mL and incubated for 48 h. The culture supernatants were collected. To assess the gelatinolytic activities of MMP-2 in culture media, SDS-PAGE zymography using gelatin as a substrate was performed. Briefly, 20 µL of cell culture supernatant were suspended in loading buffer,0.125M Tris (pH 6.8), 4% SDS and 0.04% bromophenol blue, and, without prior denaturation, run on 10% SDS-polyacrylamide gel containing 1 mg/mL gelatin. After electrophoresis, gels were washed to remove SDS and incubated for 20 min in renaturing buffer (50 mM Tris, 5 mM CaCl2, 0.02% NaN3, 2.5% Triton X-100). The gels were then incubated for 24 h at 37°C in developing buffer 50 mM Tris (pH 7.5), 5 mM CaCl2, 0.02% NaN3 and 1% Triton X-100. Gels were subsequently stained with 0.5% Coomassie brilliant blue G-250 and de-stained in 30% methanol and 10% acetic acid (v/v) to detect gelatinolytic activity (CitationKim et al., 2007). The gel was documented by a gel documentation system (Bio-Rad Laboratories, UK) and analyzed by Quantity 1-D analysis software. The area multiplied by intensity (mm2) of the bands on the gel was determined as the relative MMP-2 content (CitationCarmeliet et al., 1997; CitationArican & Ceylan, 1999). The percentages of MMP-2 inhibition in comparing to the control (the untreated systems) were calculated by using the following equation:
The assays were done in three independent separate experiments. The potency of MMP-2 inhibition of the samples was compared with the positive control (ascorbic acid).
Statistical analysis
All assays were performed in triplicate in three independent and separate experiments. The data were presented as means ± standard deviations (SD) from three independent analyses, separate experiments.
Results and discussion
Percentage yields of the plant extracts prepared by different processes
The percentage yields of the 60 extracts prepared by four different extraction processes (CM, HM, CW and HW) of the 15 selected Thai Lanna medicinal plants were presented in . Aqueous extracts of most plants gave higher percentage yield than the methanol extracts. The plants may contain more water-soluble than water-insoluble constituents. The highest percentage yields were from T. chebula gall by CM, CW and HW at 59.02%, 49.85% and 60% respectively. For HM, P. parkinsonii flower gave the highest percentage yield at 29.25% while T. chebula gall gave 23.28%. HW of all plants gave higher yields than CW, while CM showed higher yields than HM. Most plants may contain heat labile water-insoluble components. Thus, the hot process appeared to be the superior process for water-soluble components, whereas the cold process was advantageous for water-insoluble substances.
Phytochemical tests of the extracts
shows the phytochemical constituents of the 60 extracts. Alkaloid was a basic secondary metabolite in all extracts, while glycosides and tannin were in most extracts. Few extracts contained anthraquinones. Interestingly, the extracts of C. fistula fruit prepared by all processes (CM, HM, CW and HW) contained all phytochemical compounds including alkaloids, anthraquinones, flavonoids, glycosides, saponins, tannins and xanthones. All extracts by all extraction processes contained thesame phytochemicals, except for anthraquinones in M. fragrans, P. indica and P. zeylanica which were found in HM and CM, but not found in HW and CW. Saponins in P. nigrum and tannins in A. gramineus were found in CW and HW; CM and HM, but not found in CM and HM; CW and HW, respectively. For all T. chebula gall extracts, they contained alkaloids, flavonoids, saponins, tannins and xanthones but no anthraquinones and glycosides. Phytochemicals in the extracts appeared to depend on types of plants more than the extraction conditions. Generally, solvents and temperatures are important parameters to obtain high yields. Temperature has a positive effect on the extraction yields and rates by enhancing the solubility of the compounds. However, some heat labile phytochemicals may be degraded by high temperature as CW gave higher yield than HW of most plants.
Table 2. Qualitative determination of constituents by phytochemical tests in 60 extracts from the 15 selected Thai Lanna plants including T. chebula gall prepared by various extraction processes.
DPPH radical scavenging activity
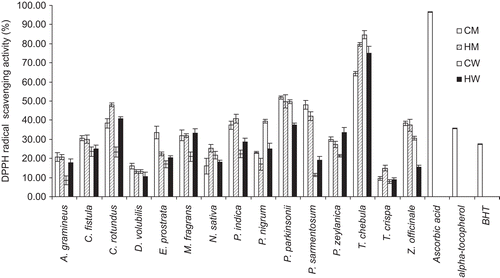
shows the IC50 values of the DPPH radical scavenging assay of the 60 extracts. demonstrated the percentages of DPPH radical scavenging activity of the 60 extracts at 0.1 mg/mL. The standard antioxidants (ascorbic acid, α-tocopherol and BHT at 0.1 mg/ml) gave the scavenging activity of 96.5% ± 0.1%, 35.74% ± 0.2% and 27.43% ± 0.1% with IC50 values of 0.014 ± 0.001, 0.038 ± 0.002 and 0.049 ± 0.002 mg/mL, respectively. The methanol extract of most plants exhibited higher activity than the aqueous extracts. Alcoholic solvents have been commonly employed to extract phenolic compounds from plants, because of obtaining high yield although they were not highly selective for phenols (CitationSpigno & De Faveri, 2007). The phenolic compounds which have been reported to scavenge DPPH include flavonoids, anthraquinones, anthocyanidins, xanthones, and tannins. They also scavenged superoxide and hydroxyl radical by single electron transfer (CitationHo et al., 1999; CitationChoi et al., 2002). Extracts from T. chebula gall prepared by all processes exhibited higher DPPH radical scavenging activity than those of other plants. The highest scavenging activity of T.chebula gall extract at 84.64% ± 2.22% was found (at 0.1 mg/mL) by CW process with the IC50 value of 0.016 ± 0.001 mg/mL, which were 2.37 and 3.09 times more potent than α-tocopherol (IC50 value of 0.038 ± 0.002 mg/mL) and BHT (IC50 value of 0.049 ± 0.002 mg/mL), respectively. The phytochemicals found in this extract including alkaloids, flavonoids, saponins, tannins and xanthones () may be synergistic and responsible for this activity. T. chebula and its galls have been used traditionally in combination with other Thai Lanna medicinal plants in many recipes for promoting longevity. Thus, this result has supported the folklore wisdom application of T. chebula gall in Thai Lanna medicines.
Table 3. IC50 values of the 60 extracts from the 15 selected Thai Lanna plants including T. chebula gall determined by the DPPH radical scavenging, chelating and tyrosinase inhibition assays.
Figure 1. Comparison of the percentages of DPPH radical scavenging activity of the 60 extracts at 0.1 mg/mL from the 15 selected Thai Lanna plants including T. chebula gall and the standard antioxidants (ascorbic acid, α-tocopherol and BHT at 0.1 mg/mL). CM, cold methanol process; HM, hot methanol process; CW, cold aqueous process; HW, hot aqueous process.
Chelating activity
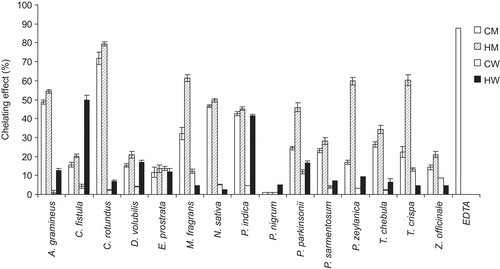
shows the IC50 values of chelating activity assay of the 60 extracts. presents the percentages of the chelating effect of 60 extracts at 0.1 mg/mL. None of the extracts indicated better chelating activity than EDTA at 0.1 mg/mL which gave 87.74% ± 0.1% and the IC50 value of 0.079 ± 0.001 mg/mL. The CM, HM, CW and HW extracts of T. chebula gall gave the IC50 values of 0.282 ± 0.002, 0.217 ± 0.002, 2.287 ± 0.275 and 1.151 ± 0.006, respectively. The highest chelating activity of 79.49% ± 1.1% was found in HM of C. rotundus root with the IC50 value of 0.094 ± 0.001 mg/mL. In fact, C. rotundus has been reported to contain many phenolic compounds, such as gallic acid, p-coumaric acid and epicatechin (CitationProestos et al., 2005) which are potent antioxidants by ferric reducing antioxidant power and Trolox equivalent antioxidant capacity assays along with metal chelating properties (CitationAmin & Razieh, 2007). From our phytochemical test, C. rotundus extract by HM contained alkaloids, glycosides and tannins ().
Figure 2. Comparison of the percentages of the chelating effect (%) by the ferrous iron-ferrozine complex method of the 60 extracts at 0.1 mg/mL from the 15 selected Thai Lanna plants including T. chebula gall and the standard chelating agent (EDTA at 0.1 mg/mL). CM, cold methanol process; HM, hot methanol process; CW, cold aqueous process; HW, hot aqueous process.
Tyrosinase inhibition activity
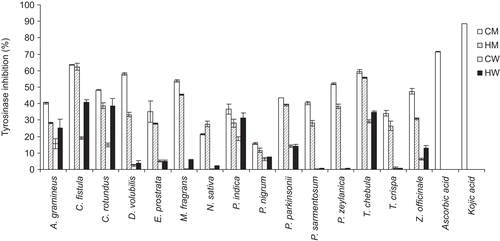
shows the IC50 values of the tyrosinase inhibition activity of 60 extracts. demonstrates the percentages of tyrosinase inhibition of 60 extracts at 0.1 mg/ mL. The CM, HM, CW and HW extracts of T. chebula gall gave the IC50 values of 0.082 ± 0.002, 0.088 ± 0.001, 0.393 ± 0.008 and 0.151 ± 0.006 respectively. The CM extract of C. fistula fruit gave the highest tyrosinase inhibition activity of 63.55% ± 0.16% with the IC50 value of 0.076 ± 0.001 mg/mL, but lower than ascorbic acid and kojic acid (at 0.1 mg/mL) which gave 71.53% ± 0.2% and 88.63% ± 0.1% with the IC50 values of 0.046 ± 0.001 and 0.03 ± 0.001 mg/mL, respectively. From , the CM extract of C. fistula contained anthraquinones, flavonoids, glycosides, saponins and tannins. The methanol extract of C. fistula has been evaluated in in vitro models in protecting free radical-induced lipid peroxidation in model membranes (CitationSunil & Muller, 1998). Flavonoids in this plant have also been reported to inhibit tyrosinase due to their ability to chelate copper in the active site (CitationMaity et al., 1998; CitationCuellar et al., 2001). The fruit tissue of this plant was found to be a rich source of potassium, calcium, iron and manganese (CitationBarthakur et al., 1995) which may be competitive inhibitors of the tyrosinase enzyme. Proanthocyanidins including flavan-3-ol (epiafzelechin and epicatechin) units with an abnormal 2S-configuration together with the common flavan-3-ols and proanthocyanidins like catechin, epicatechin, procyanidin B-2 and epiafzelechin which are strong tyrosinase inhibitors have also been found in the pods of C. fistula (CitationKashiwada et al., 1990).
Figure 3. Comparison of the percentages of tyrosinase inhibition of the 60 extracts at 0.1 mg/mL from the 15 selected Thai Lanna plants including T. chebula gall and the standard whitening agents (ascorbic acid and kojic acid at 0.1 mg/mL). CM, cold methanol process; HM, hot methanol process; CW, cold aqueous process; HW, hot aqueous process.
Proliferation of normal human skin fibroblasts by the SRB assay
From the DPPH radical scavenging, chelating and tyrosinase inhibition assays, three plants including T. chebula gall, C. rotundus root and C. fistula fruit were selected to investigate for human skin fibroblasts proliferative assay. The stimulation index (SI) of the extracts at 0.1 mg/mL on normal human skin fibroblasts (15th passage) is shown in . Extracts of all three plants by HW and CW showed higher SI than by HM and CM. The methanol extracts appeared to be more toxic to the cells than the aqueous extracts. The CW extract of T. chebula gall gave the highest cell stimulative effect with the SI value of 1.441 ± 0.084 which was more potent than ascorbic acid (at 0.1 mg/mL) that gave the SI value of 1.21 ± 0.033. The higher cell stimulative effect on fibroblasts of the aqueous extracts in comparing to the methanol extracts might be due to the presence of more polar compounds in the aqueous extracts (CW and HW) which are usually more bioactive and less toxic to cells. This result can anticipate the effect of the extract on collagen synthesis from the stimulation of human fibroblast proliferation.
Table 4. Comparison of the stimulation index (SI) of the 12 extracts at 0.1 mg/mL of the three selected Thai Lanna plants including T. chebula gall on normal human skin fibroblasts (15th passage).
Gelatinolytic activity on MMP-2 inhibition of the plant extracts (Zymography)
Although many studies have reported on the biological activities of T.chebula (CitationLee et al., 1995; CitationBurapadaja & Bunchoo, 1995; CitationSaleem et al., 2002; CitationChen et al., 2003), there was no report on the effects of the extract of this plant on MMP-2 expression in human dermal fibroblasts. shows the comparison of the inhibition of MMP-2 between T. chebula gall extracts (CW, CM, HW and HM) and ascorbic acid by zymography. All extracts of T. chebula gall as well as ascorbic acid inhibited the MMP-2 expression. The CW extract of T. chebula gall at 0.1 mg/mL showed the inhibition of MMP-2 at 89.94% of the control which was about 1.37 times more potent than ascorbic acid that gave 65.79% of the control. This has suggested that the CW extract of T. chebula gall was a potent inhibitor of MMP-2 expression on early aging human skin fibroblasts (15th passage). This effect may be not only from the cell proliferation stimulation, but also from the phytochemicals existing in the CW extract of this plant including alkaloids, flavonoids, tannins and xanthones. This result has also supported the traditional use for longevity of T. chebula gall since the indication of this plant in many Thai Lanna recipes was by cold aqueous extraction (maceration).
Figure 4. Comparison of the gelatinolytic activity on MMP-2 inhibition between T. chebula gall extracts (CW, CM, HW and HM) at 0.1 mg/mL and ascorbic acid at 0.1 mg/mL. (A) zymograms of three independent separate experiments, (B) MMP-2 contents [area × intensity; (mm2)] (left) and the percentages of MMP-2 inhibition (right). CM, cold methanol process; HM, hot methanol process; CW, cold aqueous process; HW, hot aqueous process).
![Figure 4. Comparison of the gelatinolytic activity on MMP-2 inhibition between T. chebula gall extracts (CW, CM, HW and HM) at 0.1 mg/mL and ascorbic acid at 0.1 mg/mL. (A) zymograms of three independent separate experiments, (B) MMP-2 contents [area × intensity; (mm2)] (left) and the percentages of MMP-2 inhibition (right). CM, cold methanol process; HM, hot methanol process; CW, cold aqueous process; HW, hot aqueous process).](/cms/asset/67467c55-e003-4c25-9783-fa50c53643f6/iphb_a_459137_f0004_b.gif)
Conclusion
This present study has demonstrated the in vitro anti-aging activities of T. chebula gall in comparison to 14 Thai Lanna medicinal plants with the indication for longevity selected from the database of Thai Lanna medicinal plant recipes. The biological activities which are related to anti-aging including antioxidative, tyrosinase inhibition and proliferative stimulation as well as the MMP-2 expression inhibition on human skin fibroblasts were used to evaluate the 60 extracts prepared by aqueous and methanol hot and cold processes. For all 15 plants including T. chebula gall, an aqueous hot extraction process (HW) gave higher yields than the aqueous cold process (CW), whereas the methanol cold process (CM) gave better yield than the methanol hot process (HM). The methanol extract either by the hot or cold process of most plants exhibited higher DPPH radical scavenging, chelating and tyrosinase inhibition activities than the aqueous extracts. The three Thai Lanna medicinal plants, including T. chebula gall, C. rotundus root and C. fistula fruit which gave the highest DPPH radical scavenging, chelating and tyrosinase inhibition activity respectively, were selected to test for proliferation stimulation and MMP-2 expression inhibition on normal human skin fibroblasts (15th passage). The aqueous extracts exhibited stronger proliferation stimulation activity on normal human fibroblasts than the methanol extracts. For the CW extracts, T. chebula gall showed more stimulative effect than ascorbic acid, while C. fistula fruit and C. rotundus root gave almost the same effect as ascorbic acid. Moreover, the inhibition of MMP-2 expression determined by zymography of T.chebula gall CW extract was 1.37 times more potent than ascorbic acid. This present study has not only demonstrated the potent in vitro anti-aging effects of the cold aqueous extract of T.chebula gall, but also supported the traditional indication for longevity of this plant in the Thai Lanna medicinal plant recipes as well.
Acknowledgments
The authors would like to thank Dr. Natthanej Luplertlop at Department of Tropical Hygiene, Faculty of Tropical Medicine, Mahidol University in Thailand for providing the human skin fibroblasts.
Declaration of interest
This work was supported by the Thailand Research Fund (TRF) under the RGJ-PhD program and Natural Products Research and Development Center (NPRDC), Science and Technology Research Institute (STRI), Faculty of Pharmacy, Chiang Mai University, Chiang Mai 50200, Thailand. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aimes RT, Quigley JP (1995): Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J Biol Chem 270: 5872–5876.
- Allen ST (1974): Chemical Analysis of Ecological Material. New York, Blackwell Scientific, p. 313.
- Amin A, Razieh Y (2007): Cyperus rotundus suppresses AGE formation and protein oxidation in a model of fructose-mediated protein glycoxidation. Int J Biol Macromol 41: 572–578.
- Arican M, Ceylan C (1999): Metalloproteinases in canine experimental traumatic keratoconjunctivitis. J Vet Med 46: 527–532.
- Barthakur NN, Arnold NP, Alli I (1995): The Indian Labernum (Cassia fistula L.) fruit: An analysis of its chemical constituents. Plant Food Hum Nutr 47: 55–62.
- Briganti S, Camera E, Picardo M (2003): Chemical and instrumental approaches to treat hyperpigmentation. Pigm Cell Res 16: 101–110.
- Brimer L, Lorentzen B, Smitt V (1989): Evelser I Farmakognosi K-25/9 [Pharmacognosy]. Copenhagen, Royal Danish School of Pharmacy.
- Burapadaja S, Bunchoo A (1995): Antimicrobial activity of tannins from Terminalia citrine. Planta Med 61: 365–366.
- Carmeliet P, Moons L, Herbert JM, Crawley J, Lupu F, Lijnen R, Collen D (1997): Urokinase but not tissue plaminogen activator mediates arterial neointima formation in mice. Circ Res 81: 829–839.
- Chen HY, Lin TC, Yu KH, Yang CM, Lin CC (2003): Antioxidant and free radical scavenging activities of Terminalia chebula. Biol Pharm Bull 26: 1331–1335.
- Choi CW, Kim SC, Hwang SS, Choi BK, Ahn HJ, Lee MY, Park SH, Kim SK (2002): Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison. Plant Sci 163: 1161–1168.
- Chung JH, Seo JY, Choi HR, Lee MK, Youn CS, Rhie G, Cho KH, Kim KH, Park KC, Eun HC (2001): Modulation of skin collagen metabolism in aged and photoaged human skin in vivo. J Invest Dermatol 117: 1218–1224.
- Cuellar MJ, Giner RM, Recio MC, Mánez S, Rios JL (2001): Topical anti-inflammatory activity of some Asian medicinal plants used in dermatological disorders. Fitoterapia 72: 221–229.
- Decker EA, Welch B (1990): Role of ferritin as a lipid oxidation catalyst in muscle food. J Agric Food Chem 38: 674–677.
- Harbone JR (1976): Phytochemical Methods. A Guide to Modern Techniques of Plant Analysis. London, Charpan & Hall, p. 78.
- Ho KY, Huang JS, Tsai CC, Lin TC, Hsu YF, Lin CC (1999): Antioxidant activity of tannin components from Vaccinium vitisidaea L. J Pharm Pharmacol 51: 1075–1078.
- Kadekaro AL, Kanto H, Kavanagh R, Abdel-Malek ZA (2003): Significance of the melanocortin 1 receptor in regulating human melanocyte pigmentation, proliferation and survival. Ann N Y Acad Sci 994: 359–365.
- Kashiwada Y, Toshika K, Chen R, Nonaka G, Nishioka I (1990): Tannins and related compounds. XCIII. Occurrence of enantiomeric proanthocyanidins in the Leguminosae plants, Cassia fistula L.; Cassia javanica L. Chem Pharm Bull 38: 888–893.
- Kim S, Kim Y, Kim JE, Cho KH, Chung JH (2007): Berberine inhibits TPA-induced MMP-9 and IL-6 expression in normal human keratinocytes. Phytomedicine 15: 340–347.
- Maity TK, Mandal SC, Mukherjee PK, Saha K, Das J, Pal M, Saha BP (1998): Studies on antiinflammatory effect of Cassia tora leaf extract (fam. Leguminosae). Phytother Res 12: 221–223.
- Lee SH, Ryu SY, Choi SU, Lee CO, No Z, Kim SK, Ahn JW (1995): Hydrolysable tannins and related compound having cytotoxic activity from the fruits of Terminalia chebula. Arch Pharm Res 18: 118–120.
- Lemmens RHMJ, Bunyapraphatsara N (2003): Plant resources of South-East Asia No.12 (3): Medicinal and poisonous plants. Leiden, Backhuys, p. 3.
- Manosroi J, Manosroi A, Rungruangsri U (2006): Translation of Lanna medicinal plant recipes for research and development of modern pharmaceuticals and the understanding of the Lanna Thai cultures/histories. CMU Journal 5: 437–441.
- Millis AT, Sottile J, Hoyle M, Mann DM, Diemer V (1989): Collagenase production by early and late passage cultures of human fibroblasts. Exp Gerontol 24: 559–575.
- Onwukaeme DN, Ikuegbvweha TB, Asonye CC (2007): Evaluation of phytochemical constituents, antibacterial activities and effect of exudates of Pycanthus angolensis Weld Warb (Myristicaceae) on corneal ulcers in rabbits. Trop J Pharm Res 6: 725–730.
- Papazisis KT, Geromichalos GD, Dimitriadis KA, Korsaris AH (1997): Optimization of the sulforhodamine B colorimetric assay. J Immunol Methods 208: 151–158.
- Patterson ML, Atkinson SJ, Knuper V, Murphy G (2001): Specific collagenolysis by gelatinase A, MMP-2, is determined by the hemopexin domain and not the fibronectin-like domain. FEBS Lett 503: 158–162.
- Pharmacopoeia Commission of PRC (1997): Pharmacopoeia of the People’s Republic of China (I). Beijing, Chemical Industry Press, p. 61.
- Proestos C, Chorianopoulos N, Nychas GJE, Komaitis M (2005): RP-HPLC analysis of the phenolic compounds of plant extracts. Investigation of their antioxidant capacity and antimicrobial activity. J Agric Food Chem 53: 1190–1195.
- Saleem A, Husheem M, Harkonen P, Pihlaja K (2002): Inhibition of cancer cell growth by crude extract and phenolics of Terminalia chebula Retz. fruit. J Ethnopharmacol 81: 327–336.
- Shimizu K, Kondo R, Sakai K, Lee S, Sato H. (1998). The inhibitory components from Artocarpus incisus on melanin biosynthesis. Planta Med 64: 408–412.
- Spigno D, De Faveri DM (2007): Antioxidants from grape stalks and marc: Influence of extraction procedure on yield, purity and antioxidant power of the extracts. J Food Eng 78: 793–801.
- Steinbrenner H, Ramos MC, Stuhlmann D, Sies H, Brenneisen P (2003): UVA-mediated downregulation of MMP-2 and MMP-9 in human epidermal keratinocytes. Biochem Bioph Res Co 308: 486–491.
- Sturm RA, Teasdale RD, Box NF (2001): Human pigmentation genes: identification, structure and consequences of polymorphic variation. Gene 277: 49–62.
- Sunil KC, Müller K (1998): Inhibition of leukotriene biosynthesis and lipid peroxidation in biological models by the extract of Cassia fistula. Phytother Res 12: 526–528.
- Tachibana Y, Kikuzaki H, Hj-Lajis N, Nakatani N (2001): Antioxidant activity of carbazoles from Murraya koenigii leaves. J Agric Food Chem 49: 5589–5594.
- VanMiddlesworth F, Cannell RJP (1998): Dereplication and partial identification of natural products, in: Cannell RJP, ed., Methods in Biotechnology: Natural Product Isolation. Totowa, New Jersey, Humana Press, p. 291.
- Zhu PY (1998): Chinese Materia Medica. Amsterdam, Harwood Academic, pp. 663–666.