Abstract
Context: Decoctions of Baliospermum montanum Müll. Arg. (Euphorbiaceae) leaves are reported to be useful in the treatment of asthma and other respiratory complications in the Ayurvedic system.
Objective: To evaluate the mast cell stabilization and antihistaminic activities of the chloroform (BMLC) and ethanol (BMLE) extracts of the leaves of Baliospermum montanum.
Materials and methods: The stabilization potential was studied on mouse peritoneal mast cells and the antihistaminic activity was carried out by determining the mortality rate of mice treated with toxicant (compound 48/80) and the effect on elevation of histamine release upon degranulation.
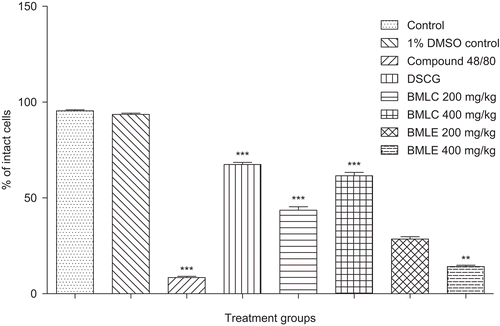
Results: The increased number of intact mast cells (43.640 ± 1.7% and 61.57 ± 1.79% at 200 and 400 mg/ kg, respectively) suggested that the BMLC stabilized the mast cell degranulation and showed decreased elevation of histamine.
Conclusion: BMLC extract was found to be most effective against degranulation and release of histamine from mast cells. Identifying the lead from this plant will be a definite target for treating allergic diseases.
Introduction
Inhibition of allergic mediators upon allergen exposure is considered to be a promising strategy for the treatment of several allergic disorders (CitationChurch & Levi-Schaffer, 1997). Several natural compounds are broadly recognized for their great structural diversity as well as their wide range of therapeutic activities, such as anti-inflammatory, anti-allergic activities (CitationMukherjee & Wahile, 2006).
Baliospermum montanum Müll. Arg. (Euphorbiaceae), commonly known as Danti (Hindi), is an under shrub with herbaceous branches from the roots. A decoction of leaves of the same has been reported to be useful in asthma and to treat other respiratory complications in the Ayurvedic system (CitationPanigarhi & Murti, 1989; CitationMali & Wadekar, 2008). The root has been reported to contain axillarenic acid, baliospermin, and montanin (CitationOgura et al., 1978) possessing a wide range of activities such as anthelmintic, diuretic, purgative, and it is also used for the treatment of bronchitis (CitationKirtikar & Basu, 1999). The effect of the root extract on phagocytosis by human neutrophils was established by CitationWadekar et al. (2008). The anthelmintic potential of alcohol and aqueous extracts of roots of this plant has been reported (CitationMali & Wadekar, 2008). It has also been reported to possess immunomodulatory activity (CitationMali & Wadekar, 2009). The present study was undertaken to evaluate the anti-allergic property of Baliospermum montanum, a well known medicinal plant from Indian systems of medicine including Ayurveda.
Materials and methods
Chemicals and reagents
Compound 48/80 was obtained from Sigma (St. Louis, MO), o-phthalaldehyde (OPT), RPMI-1640 from Himedia Laboratories, Mumbai. Other reagents used were of analytical grade and were obtained from local suppliers at Kolkata, India.
Preparation of plant extract
Leaves of Baliospermum montanum were collected from the Malda district of West Bengal, India in May 2008. A sample was identified and authenticated by S. Rajan, Field Botanist, Ootacamund, Tamil Nadu. A voucher specimen (SNPS-1039) was preserved at the School of Natural Product Studies. The chloroform (BMLC) extract was prepared from 1 kg Baliospermum montanum leaves (BML) by soxhlation, it yielded 2.8% w/w and the same marc was used to obtain ethanol (BMLE) extract (1.9% w/w). The extracts were dried under rotary vacuum evaporator to obtain solvent free extract. Both the extracts were subjected to qualitative phytochemical tests (CitationMukherjee, 2002).
Animals
The original stock of Swiss albino mice (20–25 g) were acclimatized in standard conditions (n = 6/group; n = 10/group for mortality testing). Animal experiments were approved by the Institutional Animal Ethical Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), constituted under the directives of the Ministry of Social Justice and Empowerment, Government of India. They were maintained on a standard pellet diet with free access to water and housed in a temperature-controlled room at the Indian Institute of Chemical Biology, Kolkata.
Acute toxicity study
The acute oral toxicity study (CitationLitchfield & Wilcoxon, 1949) was carried out as per the guidelines set by the Organization for Economic Co-operation and Development (OECD) received from the CPCSEA. BMLC and BMLE extracts at a dose range of 200–2000 mg/kg were administered orally to different groups of mice and mortality was observed up to 72 h.
Mast cell stabilization activity
The mast cell stabilization study was performed based on the method of CitationGupta et al. (1995) with little modification. Briefly, BMLC and BMLE were administered at various doses (200 and 400 mg/kg, p.o.) for 4 days and the standard drug disodium cromoglycate (DSCG) (50 mg/kg) was administered intraperitoneally due to its poor absorption through the oral route). On day 5, 5 mL cold phosphate buffer saline (containing NaCl 137 mM, NaHCO3 12 mM, NaH2PO4 0.3 mM, KCl 12.7 mM, MgCl2 1 mM, CaCl2 1.8 mM, dextrose 5.6 mM) was injected (i.p.) to all groups of mice. The peritoneal fluid-containing mast cells were collected immediately after gentle abdominal massage for 10 sec; the fluid was collected in RPMI-1640 media (pH 7.2–7.4). The cell viability was checked by exclusion test with trypan blue (0.4%). The viable cells were washed several times with RPMI-1640 media by centrifugation at low speed (500–600 rpm), discarding the supernatant and re-suspending the mast cell pellets in the medium. Mast cells obtained from the treated and control groups were incubated with compound 48/80 (1 μg/mL), a mast cell degranulator, at 37°C for 10 min in a water bath. Then the cells were stained with toluidine blue (1%) and protection from degranulation was observed under high power microscope and calculated accordingly.
Compound 48/80-induced systemic anaphylaxis
Compound 48/80-induced systemic anaphylactic reaction was examined based on an earlier reported method (CitationYi et al., 2001). One group of mice (n = 10/group) were administered with an injection of 0.008 g/kg, i.p. of the toxicant. BMLC and BMLE (200 and 400 mg/mL) were dissolved in carboxymethyl cellulose and administered orally for 4 days prior to injection of compound 48/80. DSCG was used as standard. Mortality was monitored for 1 h after induction of anaphylactic shock and blood was collected from the heart of each mouse. Mortality (%) within 1 h following compound 48/80 injection was represented as the number of dead mice × 100/total number of experimental mice.
Histamine estimation
The blood tissues were homogenized and centrifuged at 400 g for 10 min and the content of histamine was measured in plasma by o-phthalaldehyde spectrofluorimetric method (CitationShore et al., 1959). From alkalinized perchloric acid tissue extracts, histamine was extracted with n-butanol:water; and then histamine returned to the aqueous solution. This solution was condensed with o-phthalaldehyde, which yielded a product having strong and stable fluorescence. The fluorescent intensity was measured at 438 nm (excitation at 353 nm) in a spectrofluorimeter (Perkin-Elmer LS-50B, USA).
Statistical analysis
Each datum represents the mean and standard error of the mean (SEM) of the different experiments under the same conditions. The one-way ANOVA test was used to make a statistical comparison between the groups. Bonferroni’s multiple comparison test was carried out to compare between toxicant and test groups. Results with p <0.001 were considered statistically significant.
Results
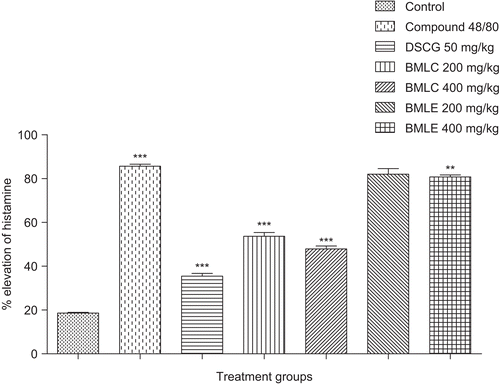
The presence of alkaloids (BMLC, BMLE), triterpenoids (BMLC, BMLE), diterpenoids (BMLC) and glycosides (BMLC) were observed in BML through qualitative phytochemical studies. No mortality was observed in the acute toxicity test with mice and there were no behavioral changes observed up to 2000 mg/kg of BMLC and BMLE. 67.46 ± 1.06% protection of mast cells from degranulation was enabled by the standard DSCG, whereas BMLC extracts showed significant mast cell stabilization activity (), i.e., 61.57 ± 1.79% of intact mast cells at 400 mg/kg which was comparable to that of standard drug. The lower rate of mortality of mice from the anaphylactic shock was 60% at 400 mg/kg (BMLC), whereas the BMLE had a lesser effect on the anaphylactic shock (). BMLC at a dose of 400 mg/kg inhibited 20% of mortality (p < 0.01). The elevation of histamine in plasma from different groups of mice was determined by spectrofluorimetric method (). BMLC exerted inhibition of mast cell-dependent anaphylactic reaction in mice and reduced the elevation of histamine to 53.62 ± 1.16% at 47.86 ± 0.98% at 200 and 400 mg/kg, respectively. BMLC was shown to inhibit the skin reactions induced by histamine in mice, which indicated that the extract directly reduced the allergic mediators, and pre-treatment with BMLC extract inhibited the release of histamine significantly (p<0.001).
Table 1. Effect of BML extracts on anaphylactic shock.
Discussion
The proposed medicinal properties of BML may be attributed to the presence of alkaloids, triterpenoids, diterpenoids, and glycosides. From the results of mast cell degranulation study, it can be postulated that the protection of mast cells in non-sensitized mice indicates the possibility of inhibition of the phenomenon of sensitization. Various agents used in the treatment of asthma or allergic reactions, like DSCG and dexamethasone, inhibit phosphodiesterase and thereby increase the intracellular cyclic AMP levels (CitationRoy & Warren, 1974). An increased level in intracellular cyclic AMP is known to have mast cell stabilizing property (CitationSpataro & Bosmann, 1976). It is likely that BMLC has shown membrane stabilizing effect by inhibition of release of allergic mediators.
It is especially noteworthy that the extent and potency of inhibition shown by BMLC against compound 48/80-induced mast cell degranulation and anaphylaxis was almost similar to that of DSCG. The results of the study demonstrated that the extract of B. montanum showed marked mast cell stabilization and antihistaminic activities. This remarkable activity is probably due to the inhibition of histamine release from the mast cells activation and degranulation by both IgE dependent and IgE independent stimuli.
Conclusion
Out of both the ethanol and chloroform extracts tested, the chloroform extract of B. montanum (BMLC) showed most potential effect against degranulation and release of histamine from mast cells remarkably. The precise molecular mechanism underlying this effect and estimation of the bioactive phytoconstituent for this activity is underway in our laboratory.
Acknowledgements
The authors are grateful to the Department of Science and Technology, Government of India and the Japan Society for Promotion of Science (F. No. DST/INT/JAP/P-62/08), through the India–Japan Cooperative Science programme.
Declaration of interest
Thanks are due to the Indian Council of Medical Research, Government of India, New Delhi, for providing a Senior Research Fellowship to P. Venkatesh (F. No. 45/29/2007/BMS/TRM).
References
- Church MK, Levi-Schaffer FJ (1997): The human mast cell. J Allergy Clin Immunol 99: 155–160.
- Gupta PP, Srimal RC, Srivastava M, Singh KL, Tandon JS (1995): Anti-allergic activity of arbortristosides from Nyctanthus arbortristis. Int J Pharmacog 33: 70–72.
- Kirtikar KR, Basu BD (1999): Indian Medicinal Plants, Vol 3, second edition. Dehradun, India: International Book Distributors.
- Litchfield JT, Wilcoxon FA (1949): Simplified method of evaluating dose effect experiments. J Pharmacol Exp Ther 96:99–133.
- Mali RG, Wadekar RR (2008): In vitro anthelmintic activity of Baliospermum montanum Muell. Arg. roots. Ind J Pharm Sci 70: 131–133.
- Mali RG, Wadekar RR (2009): Baliospermum montanum (Danti): Ethnobotany, phytochemistry and pharmacology – A review. Int J Green Pharm 2: 194–199.
- Mukherjee PK (2002). Quality Control of Herbal Drugs. New Delhi, India: Business Horizons, pp. 90.
- Mukherjee PK, Wahile A (2006): Integrated approaches towards drug development from ayurveda and other Indian system of medicines. J Ethnopharmacol 103: 25–35.
- Ogura M, Koike K, Cordell GA, Farnsworth NR (1978): Potential anticancer agents VIII. Constituents of Baliospermum montanum (Euphorbiaceae). Planta Med 33: 128–143.
- Panigarhi G, Murti SK (1989). Flora of Bilaspur District of Madhya Pradesh, Botanical Survey of India, Vol 1, pp. 46–71.
- Roy AC, Warren BY (1974): Inhibition of cyclic AMP phosphodiesterase by disodium cromoglycate. Biochem Pharmacol 23: 917–920.
- Shore PA, Burkhalter A, Cohn VH Jr (1959): A method for the fluorometric assay of histamine in tissues. J Pharmacol Exp Ther 127: 182–186.
- Spataro AC, Bosmann HB (1976): Mechanism of action of disodium cromoglycate on mast cell calcium influx after a histamine releasing stimulus. Biochem Pharmocol 25: 505–510.
- Wadekar RR, Agrawal SV, Tewari KM, Shinde RD, Mate S, Patil K (2008): Effect of Baliospermum montanum root extract on phagocytosis by human neutrophils. Int J Green Pharm 2: 46–49.
- Yi JM, Kim MS, Seo SW, Lee KN, Yook CS, Kim HM (2001): Acanthopanax senticosus root inhibits mast cell-dependent anaphylaxis. Clin Chim Acta 312: 163–168.