Abstract
Context: The pharmaceutical alkaloid huperzine A (HupA), currently used in herbal supplements and medicines worldwide, is predominantly sourced from the Chinese lycopod Huperzia serrata (Thunb. ex Murray) Trev. (Lycopodiaceae), which on average contains only 0.08 mg HupA g−1 dry weight, and is experiencing a rapid decline in China due to over-harvesting.
Objective: To find a high-yielding, natural source of HupA and/or the related huperzine B (HupB) that could potentially be used as the starting material in a commercial propagation program.
Materials and methods: We surveyed 17 Huperzia species (15 indigenous to Australia and southeast Asia) for their foliar HupA and HupB concentrations. We also studied intra-specific variation for the huperzines in four species that were available in sufficient numbers, and determined tissue-specific accumulation in larger specimens.
Results: HupA was detected in 11 Australasian and southeast Asian species, with eight also containing HupB, albeit at much lower concentrations. A H. elmeri (Herter) Holub plant from the Philippines had one of the highest HupA concentrations recorded (1.01 mg g−1 dry wt) and it also had the highest HupB content of all plants surveyed (0.34 mg g−1 dry wt). Intra-specific HupA and HupB concentrations were extremely variable, and at the intra-plant level, reproductive strobili were found to accumulate the highest HupA concentrations.
Discussion and conclusion: Select Huperzia species from Australia and southeast Asia have potential as the starting material for establishing commercial HupA plantations, but the high intra-specific variability observed suggests that detailed screening is needed to isolate high huperzine-yielding individuals.
Introduction
The alkaloids huperzine A (HupA) and huperzine B (HupB) belong to the lycopodine group of Lycopodium alkaloids and both are highly selective and potent inhibitors of acetylcholine esterase (AChE). Recent medical research has focused on HupA, the more potent AChE inhibitor, as a potential therapeutic in the treatment of diseases such as Alzheimer’s disease, which result from reduced function of cholinergic neurons (CitationDesilets et al., 2009). HupA and HupB were originally isolated from leaves of the Chinese club-moss Huperzia serrata (Thunb. ex Murray) Trevis. (Lycopodiaceae), which has a record of use as the traditional Chinese medicine “Qian Ceng Ta” since the Tang dynasty (ad 618–907; CitationMa et al., 2007). Although it is possible to chemically synthesise HupA (CitationKozikowski et al., 1990), the resulting racemic mixture has been found to be much less efficient at AChE inhibition when compared to natural HupA derived from plant extracts (CitationMa et al., 2007). Hence, commercial HupA is still predominantly extracted from H. serrata foliage and this has led to over-collection and a dramatic decline in natural H. serrata populations throughout China (CitationMa et al., 2006).
The inherently low levels of HupA in H. serrata foliage (mean of 0.08 mg g−1 dry weight; CitationMa et al., 2005) have prompted several investigations of related species to identify higher HupA yielders. Thus far, HupA has only been isolated from species of Huperzia Bernhardi (accepted in this study as including Phlegmariurus Holub) (CitationBorloz et al., 2006; CitationGoodger et al., 2008; CitationMa et al., 2005, Citation2007; CitationOrtega et al., 2004a; CitationSzypuła et al., 2005). An extensive investigation into the HupA contents of 40 Huperzia indigenous to China found H. carinata (Desv. ex Poir.) Trevis [= Phlegmariurus carinatus (Devs. ex Poir.) Ching] to be the highest HupA yielding species, with a maximum foliar concentration of 0.56 mg g−1 dry wt (CitationMa et al., 2005). In Europe, HupA has been detected in Swiss (CitationBorloz et al., 2006) and Polish (CitationSzypuła et al., 2005) H. selago (L.) Bernhardi ex Schrank & C.F.P. Martius, with one individual from the latter study exhibiting the highest known HupA concentration of 1.27 mg g−1 dry wt. Finally, in the only study of Australasian Huperzia, CitationGoodger et al. (2008) reported that most of the species had higher levels of HupA than the current main commercial source (H. serrata), with one individual of H. carinata containing over 1 mg g−1 dry wt.
The aim of this research was to identify high HupA yielding individuals or species that may potentially be used to initiate commercial propagation and harvesting programs. The study by CitationGoodger et al. (2008) screened for HupA and HupB in 11 of the approximately 35 Huperzia species that occur throughout the Australasian region. Here, we aimed to extend that screen to include both more Australasian species, and a wider geographic range in the southeast Asia region. We also aimed to characterize the intra-specific variability of HupA and HupB in selected species, and to determine whether there is a level of tissue-specific accumulation of HupA and HupB. These results may have implications for the choice of explant material in alternative propagation systems such as HupA-producing Huperzia cell cultures.
Materials and methods
Plant material
A total of 17 Huperzia species from 14 localities were screened for HupA and HupB. Fifteen species were from the Australasian and southeast Asian region. Two species from outside this region were included because their huperzine contents were previously unknown: H. aqualupiana (Spring.) Rothm. from Brazil and H. taxifolia (Sw.) Trevis. from Mexico. All plant samples were sourced in 2007 and 2008 mainly from natural populations, but also from commercial nursery plants of known provenance. Voucher specimens of each species have been deposited in the University of Melbourne Herbarium, James Cook University Herbarium, Cairns Herbarium or Brisbane Herbarium, Australia (see for voucher specimen numbers). Due to the scarcity of Huperzia plants in natural populations and the difficulty in sampling rainforest epiphytes, limited amounts of material from each plant was collected. Most samples consisted entirely of leaf (lycophylls) and stem tissues, but in some cases, reproductive stobili (sporophyll and sporangia) were also collected. All plant material was frozen in liquid nitrogen, freeze-dried and ground into fine powder using an A10 analytical mill (IKA, Staufen, Germany).
Table 1. Seventeen Huperzia species‡ were screened for foliar huperzine A (HupA) and huperzine B (HupB) concentrations. Samples were predominantly from Australasia and southeast Asia (15 species), but included one species from Brazil and Mexico. One standard error of the mean (SE) is presented for species with multiple plant samples.
Extraction and isolation
Huperzines were extracted from each freeze-dried Huperzia sample (~0.5 g) as described in CitationGoodger et al. (2008), and the alkaloid extraction efficiency of this protocol was evaluated to be 98% (mean ± 1 SE = 97.6 ± 1.46 µg HupA recovered) by the addition of 100 µg of a HupA standard (purity >95%; Chromadex, Santa Ana, CA, USA) to each of three Huperzia samples pre-determined to contain no native HupA. After extraction and solvent removal, plant extracts were re-suspended in MeOH (0.5 mL) and passed through a syringe filter (0.45 µm; Millipore, Billerica, MA, USA) prior to high performance liquid chromatography (HPLC) analysis. All resulting HupA and HupB concentrations are the mean values of duplicate extractions performed on each sample.
High performance liquid chromatography analysis
To quantify HupA and HupB in each plant sample, extracts were fractionated using reverse phase HPLC as described in CitationGoodger et al. (2008). An external HupA standard series (0.05-1 mg mL−1) was also prepared and used to quantify HupA concentrations. The standard curve was linear in the range from 0 to 1 mg HupA mL−1. The limit of detection and quantification was set at 0.001 mg HupA mL−1. HupB concentrations in plant extracts were also calculated from the HupA standard series by accounting for the minor difference in molar extinction coefficient between HupA and HupB.
Mass spectrometry analysis
The consistency of retention times for HupA and HupB and their characteristic dual UV optima around 313 and 231 nm were sufficient to confidently identify the compounds in the HPLC traces. Nevertheless, to confirm the identity of HupA and HupB, representative extracts of Huperzia species together with an authentic sample of HupA were subjected to liquid chromatography mass spectrometry (LCMS) analysis. The LCMS system comprised a Finnigan Surveyor pump equipped with a photodiode array detector connected to an electro-spray ionization (ESI) source and a linear quadrupole ion trap (LTQ) coupled to a Fourier transform ion cyclotron Resonance (FT-ICR) MS (ThermoFinnigan, Bremen, Germany). The instrument was calibrated using a solution of caffeine and Ultramark 1621 (Lancaster Synthesis, Ward Hill, MA, USA). High resolution mass measurements were made in positive mode after fractionation on a Luna C18 (2) column (150 × 4.6 mm, 5 μm; Phenomenex, Torrance, CA) using the same HPLC conditions as described in CitationGoodger et al. (2008). The following MS source conditions were used: 5.27 kV source voltage, 37.48 µA source current, −63.79°C vaporiser temperature, 7.99 sheath gas flow rate, 33.21 V capillary voltage, 250°C capillary temperature and 79.82 V tube lens voltage and a scanning range m/z 100-1100. Chromatograms and mass spectra were evaluated using Xcalibur software (ThermoElectron, Manchester, UK).
Huperzine A (standard; Chromadex)
UV (MeOH) λmax 232, 313 nm; ESI MS (pos. ion mode) m/z 243.14912 [M+H]+ (calcd for C15H19O1N2 243.14974); MS-MS (m/z [email protected]) = 226.12262 (100), 243.14923 (19.2), 208.11208 (17.0), 211.09917 (10.4).
Huperzine A (Huperzia extracts)
UV (MeOH) λmax 232, 313 nm; ESI MS (pos. ion mode) m/z 243.14905 [M+H]+ (calcd for C15H19O1N2 243.14974); MS-MS (m/z [email protected]) = 243.14905 (100), 226.12270 (27.6), 208.11224 (4.6), 211.09933 (3.6).
Huperzine B (Huperzia extracts)
UV (MeOH) λmax 231, 311 nm; ESI MS (pos. ion mode) m/z 257.16556 [M+H]+ (calcd for C16H21O1N2 257.16539); MS-MS (m/z [email protected]) = 215.08321 (100), 257.16492 (47.7), 240.06483 (47.7), 239.15433 (39.4).
Results
A total of 107 varieties from 17 Huperzia species (15 Australasian and southeast Asian, 1 Brazilian and 1 Mexican) were screened for foliar HupA and HupB, which were found to vary widely between species (). Of the 15 Australasian and southeast Asian Huperzia examined, HupA was detected in 11 species, with concentrations ranging from 0.004 to 1.012 mg g−1 dry wt, while HupB was detected in eight species (0.008 to 0.339 mg g−1 dry wt). The highest concentration of both HupA and HupB was observed in a variety of H. elmeri originating from the Philippines. However, the concentrations of HupA and HupB among the three sampled varieties of H. elmeri were highly variable. In addition, HupA (0.204 mg g−1 dry wt) and HupB (0.074 mg g−1 dry wt) were detected in the Brazilian species H. aqualupiana.
Four Huperzia species (H. carinata, H. phlegmaria, H. Phlegmariodies, and H. squarrosa) were available in sufficient numbers (n ≥ 12) to allow for intra-specific comparison. Wide variations in HupA and HupB concentrations were observed within the four species (). Huperzia carinata had the highest mean (± SE) foliar concentrations of 0.15 ± 0.026 mg HupA g−1 dry wt and 0.021 ± 0.006 mg HupB g−1 dry wt among the four species (). Nevertheless, the amount of HupA and HupB in H. carinata ranged from undetectable levels to 0.649 mg g−1 dry wt and 0.144 mg g−1 dry wt, respectively (). The highest HupA and HupB concentrations were recorded in plants from Indonesia and Australia, respectively.
Figure 1. Foliar huperzine A concentrations in individual plants of (A) Huperzia carinata, n = 32; (B) H. phlegmaria, n = 28; (C) H. squarrosa, n = 12; and (D) H. phlegmariodies, n = 17. Plants are ordered according to increasing HupA concentrations to illustrate the range of concentrations within each species. Countries in the legend represent original source localities of the samples.
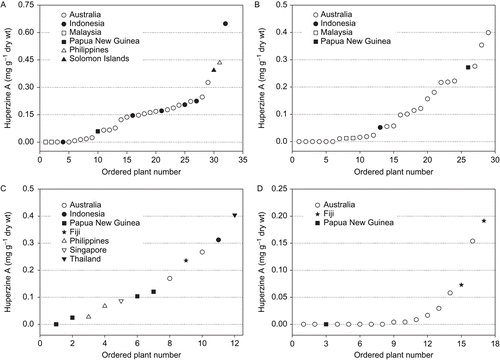
Of the other three species (), H. phlegmarioidies (Gaudich.) Rothm. had both the lowest mean HupA concentration and the lowest range (). Its mean HupB concentration and range (mean = 0.013 ± 0.08 mg g−1 dry wt; range = 0 to 0.128 mg g−1 dry wt) was, however, higher than that of H. phlegmaria (L.) Rothm (mean = 0.015 ± 0.004 mg g−1 dry wt; range = 0 to 0.084 mg g−1 dry wt) and H. squarrosa (C. Foster) Trevisan, which had the lowest of all the species (mean = 0.009 ± 0.006 mg g−1 dry wt, range = 0 to 0.067 mg g−1 dry wt). Interestingly, the individual with the highest HupA and HupB concentrations came from a different region for each species: Australia for H. phlegmaria, Fiji for H. phlegmarioidies, and Thailand (HupA) and Indonesia (HupB) for H. squarrosa.
HupA and HupB concentrations varied widely among tissue types (). In general, tassels had the highest HupA and HupB concentrations, followed by leaves and stems. The highest concentration of HupA (1.30 mg g−1 dry wt) and HupB (0.46 mg g−1 dry wt) was recorded from the tassels of H. elmeri. In a more detailed examination of a single H. squarrosa (HupA: 0.169 mg g−1 dry wt) sample, the sporophylls (spore-bearing leaves) were found to contain the highest concentrations of HupA (0.586 mg g−1 dry wt), followed by the sporangia (0.180 mg g−1 dry wt), the leaves (0.142 mg g−1 dry wt) and the stem (0.079 mg g−1 dry wt).
Table 2. Concentrations of HupA and HupB within different plant tissue types across five Huperzia species.
Discussion
This screen for HupA and HupB in Australasian and southeast Asian Huperzia has yielded promising results. Of the 15 Australasian Huperzia species examined, HupA was detected in 11 species and HupB was also detected in eight of these species. Both HupA and HupB concentrations varied widely between and within species. Therefore, more individuals of the four species (plus the Mexican species) for which no HupA was detected need to be screened in order to definitively conclude that these species do not have the capacity to produce HupA.
An individual of H. elmeri originating from the Philippines yielded the highest HupA (1.012 mg g−1 dry wt) and HupB (0.339 mg g−1 dry wt) concentrations. These levels are substantially greater than those of H. serrata, which on average only contains only 0.082 mg g−1 dry wt HupA (CitationMa et al., 2005). Within the samples that contained both alkaloids, HupB always occurred at a much lower concentration. Although the biosynthetic pathway of HupA has not been established, it has been suggested that HupB is a precursor to HupA, which might explain why HupB appears to consistently occur at much lower concentrations than HupA (CitationMa & Gang, 2004, Citation2008; CitationGoodger et al., 2008).
Interestingly, HupA and Hup B were also detected in the H. aqualupiana sample from Brazil. This is the first time HupA and HupB have been reported in a South American Huperzia species. While a previous study had reported AChE inhibitory effect from an alkaloid extract of H. saururus (Lam.) Trevis. collected from Argentina, HupA or HupB were not detected within the alkaloid extracts (CitationOrtega et al., 2004b). South America has been reported as another region where numerous Huperzia species exist (CitationWikström et al. 1999), and further work on HupA from plants in this region is warranted.
In the intra-plant study, the tassels or the reproductive parts were observed to contain the highest HupA content, followed by the leaves and the stem. The sporophylls were found to contain three times as much HupA when compared to the whole plant. Similar tissue-specific accumulation of HupA has also been reported in H. serrata, with leaves (lycophylls) yielding the most HupA, followed by stem tissue (CitationMa et al., 2005). The tissue-specific accumulation of HupA suggests that explant choice may be important for the potential establishment of axenic cultures of Huperzia cells for HupA production.
Conclusion
This study has provided a greater understanding of Australasian and southeast Asian Huperzia as potential sources of huperzine alkaloids. HupA was found in 11 species, with eight also containing HupB, albeit at much lower levels. The Huperzia species in these regions do indeed have potential as the starting material for establishing commercial huperzine plantations, with a H. elmeri plant from the Philippines having one of the highest HupA concentrations recorded for a plant and it also had the highest HupB content of all plants surveyed. Nevertheless, the high intra-specific variability observed for HupA and HupB concentrations in the examined Huperzia species suggests that extensive screening is needed to isolate more high-yielding individuals.
Acknowledgments
A.R.F. acknowledges the support of Michelle Waycott. We thank Harry and Rita Kupke, Owen and Coral Rawlins, Bruce Gray and Holly Field for access to Huperzia plants.
Declaration of interest
This research was supported by funds from the Holsworth Wildlife Research Fund (Victorian Community Foundation, ANZ Charitable Trusts). The authors report no conflicts of interest.
References
- Borloz A, Marston A, Hostettmann K (2006): The determination of huperzine A in European Lycopodiaceae species by HPLC-UV-MS. Phytochem Anal 17: 332–336.
- Desilets AR, Gickas JJ, Dunican KC (2009): Role of huperzine A in the treatment of Alzheimer’s disease. Ann Pharmacother 43: 514–518.
- Goodger JQD, Whincup AL, Field AR, Holtum JAM, Woodrow IE (2008): Variation in huperzine A and B in Australasian Huperzia species Biochem Sys Ecol 36: 612–618.
- Kozikowski AP, Yamada F, Tang XC, Hanin I (1990): Synthesis and biological evaluation of ± Z-huperzine A. Tetrahedron Lett 31: 6159–6162.
- Ma X, Gang DR (2004): The Lycopodium alkaloids. Nat Prod Rep 21:752–772.
- Ma X, Gang DR (2008): In vitro production of huperzine A, a promising drug candidate for Alzheimer’s disease. Phytochemistry 69: 2022–2028.
- Ma X, Tan C, Zhu D, Gang DR (2005): Is there a better source of huperzine A than Huperzia serrata? Huperzine A content of Huperziaceae species in China. J Agric Food Chem 53: 1393–1398.
- Ma X, Tan C, Zhu D, Gang DR (2006): A survey of potential huperzine A natural resources in China: The Huperziaceae. J Ethnopharmacol 104: 54–67.
- Ma X, Tan C, Zhu D, Gang DR, Xiao P (2007): Huperzine A from Huperzia species – An ethnopharmacolgical review. J Ethnopharmacol 113: 15–34.
- Øllgaard B (1987): A revised classification of the Lycopodiaceae s. Lat Opera Botanica 92: 153–178.
- Ortega MG, Agnese AM, Cabrera JL (2004a): Anticholinesterase activity in an alkaloid extract of Huperzia saururus. Phytomedicine 11: 539–543.
- Ortega MG, Agnese AM, Cabrera JL (2004b): Sauroine – A novel Lycopodium alkaloid extract of Huperzia saururus. Tetrahedron Lett 45: 7003–7005.
- Szypuła W, Pietrosiuk A, Suchocki P, Olszowska O, Furmanowa M, Kazimierska O (2005): Somatic embryogenesis and in vitro culture of Huperzia selago shoots as a potential source of huperzine A. Plant Science 168: 1443–1452.
- Wikström N, Kenrick P, Chase M (1999): Epiphytism and terrestrialization in tropical Huperzia (Lycopodiaceae). Plant Sys Evol 218: 221–243.