Abstract
Context: Medicinal plants are nature′s gift to human beings to make disease free healthy life, and play a vital role to preserve our health. They are believed to be much safer and proven elixir in the treatment of various ailments. The genus Artemisia (Astraceae) consists of about 500 species, occurring throughout the world. The present review comprises the ethnopharmacological, phytochemical and therapeutic potential of various species of Artemisia.
Objective: The aim of this this review is to bring together most of the available scientific research conducted on the genus Artemisia, which is currently scattered across various publications. Through this review the authors hope to attract the attention of natural product researchers throughout the world to focus on the unexplored potential of Artemisia species.
Methods: This review has been compiled using references from major databases such as Chemical Abstracts, Medicinal and Aromatic Plants Abstracts, ScienceDirect, SciFinder, PubMed, King′s American Dispensatory, Henriette′s Herbal Homepage, Dr. Duke′s Phytochemical and Ethnobotanical Databases.
Results: An exhaustive survey of literature revealed that the different species of Artemisia have a vast range of biological activities including antimalarial, cytotoxic, antihepatotoxic, antibacterial, antifungal and antioxidant activity. Some very important drug leads have been discovered from this genus, notably artemisinin, the well known antimalarial drug isolated from the Chinese herb Artemisia annua. Terpenoids, flavonoids, coumarins, caffeoylquinic acids, sterols and acetylenes constitute major classes of phytoconstituents of the genus.
Conclusion: Various species of Artemisia seems to hold great potential for in-depth investigation for various biological activities, especially their effects on the central nervous and cardiovascular systems.
Introduction
A survey by the World Health Organization reported that about 80% of the world′s populations rely on non-conventional medicines, especially herbal sources, in their primary healthcare (CitationChan, 2003). Medicinal herbs are the local heritage with global importance. The world is endowed with a rich wealth of medicinal herbs. Owing to the global trend towards improved “quality of life”, there is considerable evidence of an increase in demand for medicinal plants (CitationKotnis et al., 2004). Use of plants for treating various ailments of both humans and animal is a practice as old as human life itself. India is richly endowed with a wide variety of plants having medicinal value. These plants are widely used by all sections of society whether directly as folk remedies or indirectly as pharmaceutical preparations of modern medicine (Bhagwati, Citation2003). In the current scenario, focus on plant research has increased all over the world and a large body of evidence has collected to show the immense potential of medicinal plants used in various traditional systems. Medicinal plants are a major source of biodynamic compounds of therapeutic value, but the different variety of plants with different therapeutic properties is quite astonishing (CitationHarsha et al., 2002).
The present review emphasizes the botanical, ethnopharmacological, phytochemical, and pharmacological reports and clinical studies on the various species of Artemisia. Through this review, the authors hope to attract the attention of natural product researchers throughout the world to focus on the unexplored potential of the Artemisia species. This genus needs to be investigated systematically so that potential species can be exploited as therapeutic agents.
The genus Artemisia
The genus Artemisia is one of the largest and most widely distributed genera of the family Astraceae (Compositae). It is a heterogenous genus, consisting over 500 diverse species distributed mainly in the temperate zones of Europe, Asia and North America. These species are perennial, biennial and annual herbs or small shrubs (CitationWatson et al., 2002; CitationMehrdad et al., 2007).
General morphology
General morphological features of the genus Artemisia is described as leaves alternate, capitula small, usually racemouse, paniculate or capitate, inflorescence, rarely solitary; involucral bracts in few rows, receptacle flat to hemispherical, without scales and sometimes hirsute; florets all tubular, achenes obovoid, pappus absent or sometimes a small scarious ring (CitationHeywood & Humphries, 1997; CitationMucciarelli & Maffel, 2002; CitationPolyakov & Shishkin, 1995).
Ethnopharmacology
Traditionally, Artemisia absinthium (L.) has been used as an antispasmodic, febrifuge, stomachic, cardiac stimulant, anthelmintic, for the restoration of declining mental function and inflammation of the liver, and to improve memory (CitationWake et al., 2000; CitationGuarrera, 2005). Artemisia afra (Jacq.) has been used in the treatment of a variety of ailments such as coughs, colds, headaches, dyspepsia, colic, malaria, diabetes, bladder and kidney disorders, and also used as a purgative (CitationThring & Weitz, 2006). Artemisia annua (L.) listed in the Chinese pharmacopoeia has been used as a remedy for various fevers including malaria (CitationMueller et al., 2000). Artemisia asiatica (Nakai) have been used in traditional oriental medicine for the treatment of cancer, inflammation, infections and ulcerogenic diseases (CitationLim et al., 2008). Artemisia douglasiana (Bess.) has been used to treat premenstrual syndrome and dysmenorrhea (CitationGarcia & Adams, 2005). Artemisia dracunculus (L.) has been used as antidiabetic and anticoagulant (CitationSwanston-Flatt et al., 1991; CitationShahriyary & Yazdanparast, 2007). Artemisia judaica (L.), an Egyptian medicinal plant has been used in the treatment of gastrointestinal disorders (CitationLiu et al., 2004). In the western USA, Artemisia tripartite (Rydberg) (three-tip sagebrush) has been used in the treatment of colds, sore throats, tonsillitis, headaches and wounds by Native Americans (CitationMoerman, 1998). Artemisia verlotorum (Lamotte) has been used in folk medicine of some countries of Tuscany, Italy, as a remedy for hypertension (CitationCalderone et al., 1999). Artemisia vestita (Wall. ex Bess.) is a common traditional Tibetan medicinal plant which has been used widely in China for treating various inflammatory diseases (CitationYe et al., 2008). Artemisia vulgaris (L.) has been used as an analgesic, antiinflammatory, antispasmodic and in liver diseases (CitationGilani et al., 2005; CitationTemraz & El-Tantawy, 2008).
Phytochemistry
An exhaustive literature survey on phytochemical reports of the genus Artemisia reveals that the Artemisia species comprise mainly terpenoids, flavonoids, coumarins, caffeoylquinic acids, sterols and acetylenes. Amongst various species of Artemisia, A. absinthium, A. afra, A. annua, A. maritima and A. scoparia (Waldst et Kit) are especially rich in terpenoids. summarizes the phytoconstituents of various species of Artemisia, and represents the chemical structures of the most commonly occurring major volatile compounds.
Figure 1. Chemical structures of the commonly occurring major volatile components of Artemisia species.
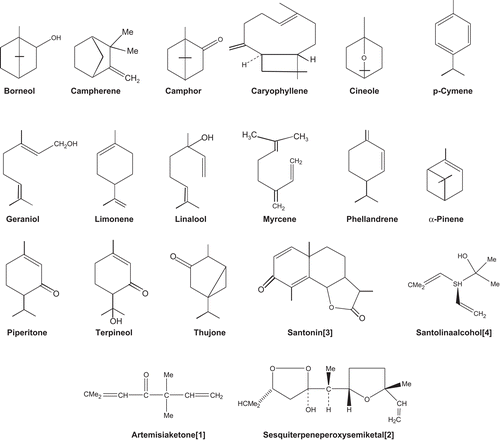
Table 1. Phytoconstituents of various species of Artemisia.
Pharmacological and clinical reports
The essential oils distilled from the aerial parts of A. absinthium inhibited in vitro growth of Candida albicans and Saccharomyces cerevisiae (CitationJuteau et al., 2003). Free-radical scavenging activity of A. absinthium extracts has been reported (CitationJasna et al., 2004). The antioxidative activity was tested by measuring their ability to scavenge stable 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical and reactive hydroxyl radical during the Fenton reaction trapped by 5,5-dimethyl-1-pyrroline-N-oxide, using electron spin resonance spectroscopy. It has been reported that crude aqueous extracts and crude ethanol extracts of the aerial parts of A. absinthium exhibit anthelmintic activity in comparison to albendazole against the gastrointestinal nematodes of sheep (CitationTariq et al., 2009). Recently, we have shown that A. absinthium exhibited neuroprotective effects against focal ischemia and reperfusion-induced cerebral injury in rats (CitationAdams & Garcia, 2006).
CitationRemberg et al. (2004) reported that a nasal spray formulation containing an extract characterised by a mixture of essential oils and flavonols from the A. abrotanum genotype “Tycho”, appear to be clinically useful and suitable for the prophylactic and therapeutic management of patients with allergic rhinitis and adjuvant symptoms. Flavonols isolated from the methanol extract of A. abrotanum exhibited spasmolytic activity against carbacholine-induced contraction of guinea-pig trachea (EC50 value 20-30 µm) (CitationBergendorff & Sterner, 1995). Moreover, A. abrotanum extract exhibited in vitro antimicrobial activity against Malassezia spp., Candida albicans and Staphylococcus aureus.
A. afra has been reported to have a broad spectrum of inhibitory activity against microorganisms (CitationMuyima et al., 2002). All other pharmacological activities of this species have been reported by CitationLiu et al. (2009).
Artemisinin, an endoperoxide sesquiterpene lactone isolated from the Chinese medicinal plant A. annua, has provided a new class of highly effective antimalarials. Artemisinin-based combination therapies are now generally considered as the best current treatment for uncomplicated Plasmodium falciparum malaria (CitationHe et al., 2009). In addition, the essential oil has shown antioxidant activity equivalent to 18% of the reference compound (α-tocopherol). Dihydro-epideoxyarteannuin B and deoxyartemisinin isolated from the sequiterpene lactone-enriched fraction obtained from the crude ethanol extract of A. annua exhibited antiulcerogenic activity against ethanol and indomethacin-induced ulcer models in rats (CitationFoglio et al., 2002). It has been reported that oral administration of artemisinin (35 or 75 mg/kg) isolated from A. annua can adversely affect post-implantation development and pregnancy in the rat (CitationBoareto et al., 2008).
Essential oil isolated from Artemisia arborescens (L.) has been shown antiviral activity against HSV-1 and HSV-2 (CitationSaddi et al., 2007).
DA-9601, a standardized extract of A. asiatica has been shown to exhibit chemopreventive effects against azoxymethane-initiated and dextran sulfate sodium-promoted mouse colon carcinogenesis (CitationRyu et al., 1998). The ethanol extract of A. asiatica has been reported to inhibit inflammatory activation of mouse microglial cells as determined by the production of nitric oxide and the expression of inducible nitric oxide synthase and inflammatory cytokine. The extract also protected nerve growth factor-differentiated PC12 cells against microglial cytotoxicity (CitationLim et al., 2008). The monoterpene alcohol fraction isolated from A. asiatica exhibited antibacterial and antifungal activity (CitationKalemba et al., 2002).
Extract of Artemisia campestris (L.) has been reported to scavenge radicals formed by carbon tetrachloride treatment resulting in protection against carbon tetrachloride-induced liver toxicity (CitationAniya et al., 2000).
CitationHong and Lee (2009) reported that the ethyl acetate fraction of Artemisia capillaris (Thunb.) (ACE) exhibit excellent protective effect by strengthening the antioxidant defense system, reducing the generation of reactive oxygen species (ROS) and damaging oxidative substances in the liver of high-fat diet induced obese mice. Moreover, ACE also exhibited significant ROS scavenging and protective effect against DPPH radical, superoxide, hydroxyl and nitric oxide radical (CitationHong et al., 2007). Chloroform extract of A. capillaris has been reported to have anticarcinogenic activity versus DMBA-induced mouse epidermal carcinogenesis (CitationKim et al., 2008). Flavonoids and a coumarin (6,7-dimethylesculetin) isolated from the buds of A. capillaris, showed significant antihepatotoxic activity by means of carbon tetrachloride-induced liver lesions in vivo and in vitro (CitationKiso et al., 1984). Tablets prepared from A. capillaris have potential antiviral activity against hepatitis B virus replication in vitro (CitationJin et al., 2005). Capillin, a component of the essential oil isolated from A. capillaris exhibited antimicrobial activity against Trichophyton mentagrophytes, Pyricularia oryzae, Candida albicans, Bacillus subtilis, Escherichia coli and Cochliobolus miyabeanus (CitationTanaka, 1961). CitationWu et al. (2001) reported that flavonoids, artemisidin A, coumarins, artemicapins A, B, C and D, and 70 other known compounds isolated and characterized from the aerial part of A. capillaris exhibited antiplatelet aggregation activity, and significant activity against HIV replication in H9 lymphocytic cells. It has been shown that water extract of A. capillaris greatly increased the volume of bile secreted and its dry weight without any significant change in the IR spectrum (CitationMashimo et al., 1963). A. capillaris extract has been shown to protect beta cells on cytokine-induced beta-cell damage, by suppressing NF-kappaB activation (CitationKim et al., 2007). A water extract of A. capillaris exhibited protective effects against oxidative stress induced by 2,2′-azobis (2-amidinopropane) dihydrochloride in Sprague-Dawley male rats (CitationHan et al., 2006). It has been reported that β-pinene, β-caryophyllene and capillene isolated from A. capillaris exhibited antimicrobial activity against 15 different genera of oral bacteria (CitationCha et al., 2005).
A. douglasiana was, and still is, used to treat premenstrual syndrome and dysmenorrhea (CitationGarcia & Adams, 2005). The tea prepared from A. douglasiana has been used to relieve premenstrual syndrome. Dysmenorrhea is treated by chewing A. douglasiana seeds (CitationJames & Cecilia, 2006).
Ethanol extract of A. dracunculus has shown antihyperglycemic activity in diabetic and non-diabetic animals (CitationRibnicky et al., 2006). CitationBenli et al. (2007) reported that methanol, chloroform and acetone extracts of A. dracunculus exhibit antimicrobial activity against nine bacteria and four yeasts strains by the disc diffusion method. Methanol extract and chloroform fraction of A. dracunculus at a concentration of 200 mug/mL, inhibited platelet adhesion to laminin coated wells by 50% and 60%, respectively (CitationShahriyary & Yazdanparast, 2007).
Two flavones, 4′,6,7-trihydroxy-3′,5′-dimethoxy-flavone and 5′,5-dihydroxy-3′,4′,8-trimethoxyflavone isolated from A. giraldii showed antibiotic activity against Staphylococcus aureus, Sarcina lutea, Escherichia coli, Pseudomonas aeruginosa, Aspergillus flavus and Trichoderma viride (CitationZheng et al., 1996). Santolinylol, a monoterpene isolated from Artemisia giraldii (Pamp.) has been reported to have antifungal activity (CitationTan et al., 1999).
The ethanol-soluble part of a hot-water extract from Artemisia iwayomogi (Kitam.) has been reported to inhibit liver fibrosis induced by carbon tetrachloride in rats (CitationPark et al., 2000).
Piperitone and trans-ethyl cinnamate isolated from A. judaica showed pronounced insecticidal and antifeedant activity against the third instar larvae of Spodoptera littoralis (Boisd), and antifungal activity against four plant pathogenic fungi (CitationAbdelgaleil-Samir et al., 2008).
Aqueous methanol extract of Artemisia maritima (L.) at a dose of 500 mg/kg has been reported to exhibit hepatoprotective activity against acetaminophen and carbon tetrachloride-induced hepatic damage (CitationJanbaz & Gilani, 1995). Cineole, a volatile oil isolated from the seeds of A. maritima shown to have more toxic effect as compared to that of santonin solution (0.0175%) (CitationJanot & Mouton, 1930).
Essential oil isolated from flowering tops and leaves of Artemisia monospermal (Delile) has shown insecticidal activity against Musca domestica vicina and Drosophila melanogaster (CitationFahmy et al., 1968). Moreover, air-dried powdered drug of A. monospermal exhibited antispasmodic activity in the treatment of colic or in conditions associated with arterial hypertension (CitationSharaf et al., 1959).
The chloroform, diethyl ether, ethanol, hexane, methanol and petroleum ether extracts of Artemisia nilagirica (C.B. Clarke) leaf exhibited antibacterial activity against clinical and phytopathogenic bacteria. Methanol and hexane extracts showed high inhibition against clinical and phytopathogens, respectively (CitationAbdul & Waheeta, 2010).
The monoterpenoid-rich essential oil isolated from A. scoparia (25-200 μg/mL) has shown a strong antioxidant and radical scavenging activity against hydroxyl ion and hydrogen peroxide (CitationSingh et al., 2008). The essential oil isolated from A. scoparia exhibited strong insecticidal activity against stored-product insects (CitationNegahban et al., 2006). A. scoparia has been reported to have anticholesterolemic, antipyretic, antiseptic, antibacterial, diuretic, purgative and vasodilator activity. Moreover, it has been used in the treatment of hepatitis, jaundice, and gall bladder inflammation (CitationYeung, 1985).
Artemisetin and chrysosplenetin isolated from A. sieversiana exhibited marked antitumor activity against melanoma B16, but only weakly retarded growth of Pliss lymphosarcoma (CitationChemesova et al., 1987). Moreover, essential oil isolated from A. sieversiana exhibited marked anti-inflammatory properties, apparently due to the azulenes in essential oil (CitationSaratikov et al., 1986).
Biologically active polysaccharide fractions isolated from A. tripartita have been reported to exhibit macrophage-activating activity, enhancing production of intracellular ROS and release of nitric oxide, interleukin 6, interleukin 10, tumor necrosis factor alpha, and monocyte chemotactic protein 1. In addition, all fractions exhibited scavenging activity for ROS generated enzymatically or produced extracellularly by human neutrophils (CitationXie et al., 2008). Moreover, A. tripartite has been shown to have anti-fungal property (CitationTan et al., 1998).
Aqueous dried extract of A. verlotorum showed marked, but transient, hypotensive activity on the blood pressure of anaesthetized rats and on in vitro rat′s isolated aortae. This effect was mediated by a strong vasodilator action, closely linked to the release of endothelial nitric oxide and to the nitric oxide-guanosine 3′,5′-cyclic monophosphate pathway, caused by muscarinic receptor agonism (CitationCalderone et al., 1999). Methanol and aqueous extracts of A. verlotorum exhibited in vitro antimycotic activity against Saprolegnia ferax (CitationMacchioni et al., 1999).
The ethanol extract of the A. vestita exhibited significant inhibitory activity against the picryl chloride-induced contact hypersensitivity in mice. Cirsilineol, apigenin and 6-methoxytricin found to be responsible for the immunosuppressive activity of A. vestita (CitationYe et al., 2008). In addition, the potential of these three components suggested new effective remedies for the treatment of T cell-mediated inflammatory diseases.
The hydroalcohol extract of A. vulgaris at doses of 500 and 1000 mg/kg significantly inhibited abdominal contortions by 48 and 59%, respectively. Rutin, a flavonoid glycoside, and caffeic acid derivatives were identified in this hydroalcohol extract (CitationPires et al., 2009). Recently, antioxidant properties of this plant have shown correlation with oxidative stress defense and different human diseases. The aqueous extract exhibited scavenging potential with IC50 value of 11.4 µg/mL for DPPH, the values were found to be close to those of standard rutin (10 µg/mL). On the other hand, A. vulgaris extract exhibited nitric oxide scavenging activity with IC50 value of 125 mg/mL (CitationTemraz & El-Tantawy, 2008). Both aqueous leaf extract, and essential oil obtained by steam distillation of the leaves of A. vulgaris have been shown to have insecticidal and larvicidal effects, whereas, the dry powder of A. vulgaris leaves was not found to be a contact poison for flies (Chopra et al., Citation1940; CitationFerrolino-Calumpang & Padolina, 1985). Aqueous leaf extract of A. vulgaris has been shown to have a protective effect on tissue damage brought about by ischemia-reperfusion injury in the rat mesentery (CitationTigno et al., 2000). The crude extract of the aerial parts of A. vulgaris exhibited hepatoprotective effects against d-galactosamine and lipopolysaccharide-induced hepatitis in mice (CitationGilani et al., 2005).
Discussion and conclusion
Herbal medicines are used worldwide in the traditional treatment of various ailments and diseases. Some of these have undergone in vitro screening but the efficacy of such herbal preparations has seldom been rigorously proven in controlled clinical trials. Conventional drugs provide effective therapeutic property for certain types of diseases, but for antibiotics there is an increasing issue of drug resistance, and consequently, a further need to discover new bioactive natural products. Although natural products are not necessarily safer than the synthetic analogues, still many patients undergoing treatment choose herbal medicines. Hence, health care professionals should be aware of the available pharmacological evidence of several herbal preparations.
The present review emphasizes the botanical, phytochemical, ethnopharmacological, pharmacological reports, clinical study and toxicological information on the various species of Artemisia. An exhaustive survey of literature revealed that sporadic information is available on more than 30 species. These species have been investigated for their phytoconstituents and pharmacological activities. Terpenoids, flavonoids, coumarins, caffeoylquinic acids and sterols constitute major classes of phytoconstituents of the genus. All species are rich in terpenoids but A. absinthium, A. afra, A. annua, A. maritima, A. scoparia and A. vulgaris possess high percentage of terpenoids. While A. capillaris, A. annua, A. dracunculus and A. scoparia are rich in flavonoids and coumarins.
A. absinthium, A. afra and A. nilagirica have been reported to have a broad spectrum of inhibitory activity against a variety of microorganisms due to presence of essential oil. Nasal spray formulation containing an extract characterised by a mixture of essential oils and flavonols from A. abrotanum is found to be clinically useful and suitable for the prophylactic and therapeutic management of patients with allergic rhinitis and adjuvant symptoms (CitationRemberg et al., 2004).
A Chinese medicinal plant, A. annua, has provided a new class of highly effective antimalarials due to presence of an endoperoxide sesquiterpene lactone, artemisinin. Artemisinin-based combination therapies are now considered as the best current treatment for uncomplicated Plasmodium falciparum malaria (CitationHe et al., 2009). DA-9601, a standardized extract of A. asiatica exhibits hepatoprotective and chemopreventive effect; A. capillaries, A. scoparia and A. vulgaris exhibits significant ROS scavenging and protective effect against DPPH radical, superoxide and hydroxyl radicals (CitationAniya et al., 2000; CitationHammoda et al., 2008; CitationTemraz & El-Tantawy, 2008) due to presence of flavonoids and volatile oil. Moreover, tablets of A. capillaris have potential antiviral activity (CitationJin et al., 2005).
Despite a long tradition of use of some species of Artemisia for treatment of various ailments, little pharmacological work so far has been carried out to validate their traditional uses. Some species of Artemisia seem to hold great potential for in-depth investigation for various biological activities, especially their effects on the central nervous system (CNS) and cardiovascular system. Presently, the authors are involved in evaluating the CNS effects of traditionally used medicinal plants with a view to isolating bioactive constituents following the bioactivity directed fractionation protocols.
Declaration of interest
Support from the L.L.R. Educational Trust, Solan, Himachal Pradesh, India, which runs the L.R. Institute of Pharmacy, is gratefully acknowledged.
References
- Abdelgaleil-Samir AM, Abbassy Moustafa A, Belal Abdel-Salam H, Abdel Rasoul MA. (2008). Bioactivity of two major constituents isolated from the essential oil of Artemisia judaica. Bioresource Technol, 99, 5947–5950.
- Abdul RA, Waheeta H. (2010). Antibacterial activity of Artemisia nilagirica leaf extracts against clinical and phytopathogenic bacteria. BMC CAM, 10, 1–20.
- Adams JD, Garcia C. (2006). Women′s health among the Chumash. eCAM, 1–7.
- Ali MS, Jahangir M, Saleem M. (2003). Structural distinction between sabandins A and B from Artemisia scoparia Waldst. Nat Prod Res, 17, 1–4.
- Aniya Y, Shimabukuro M, Shimoji M, Kohatsu C, Kunii D, Takayama F, Egashira T. (2000). Antioxidant and hepatoprotective actions of the medicinal herb Artemisia campestris from the Okinawa Islands. Biol Pharm Bull, 23, 309–312.
- Bayrak A, Dogan A, Akgul A. (1986). Research on essential oil components from tarragon (Artemisia dracunculus). Turk Tarimve Ormancilik Dergisi, 10, 314–318.
- Benli M, Kaya I, Yigit N. (2007). Screening antimicrobial activity of various extracts of Artemisia dracunculus. Cell Biochem Funct, 25, 681–686.
- Bergendorff O, Sterner O. (1995). Spasmolytic flavonols from Artemisia abrotanum. Planta Med, 61, 370–371.
- Bhagwati U. (2003.) Utilization of medicinal plants by the rural women of Kulu, Himachal Pradesh. Indian J Tradi Knowl, 2, 366–370.
- Bhakuni DS, Goel AK, Jain S, Mehrotra BN, Srimal RC. (1990). Screening of Indian plants for biological activity, part XIV. Indian J Exp Biol, 28, 619–637.
- Boareto AC, Muller JC, Bufalo AC, Botelho GGK, de Araujo SL, Foglio MA, de Morais RN, Dalsenter PR. (2008). Toxicity of artemisinin (Artemisia annua L.) in two different periods of pregnancy in Wistar rats. Reprod Toxicol, 25, 239–246.
- Borsutzki LH. (1955). Chemistry of Artemisia maritima. Archiv der Pharmazie und Berichte der Deutschen Pharmazeutischen Gesellschaft, 288, 336–340.
- Calderone V, Martinotti E, Baragatti B, Breschi MC, Morelli I. (1999). Vascular effects of aqueous crude extracts of Artemisia verlotorum, in vivo and in vitro pharmacological studies in rats. Phytother Res, 13, 645–648.
- Carnat A, Heitz A, Fraisse A, Carnat AP, Lamaison JL. (2000). Major dicaffeoylquinic acids from Artemisia vulgaris. Fitoterapia, 71, 587–589.
- Cavaleiro JAS. (1986). Iso-sakuranetin, a flavonone from Artemisia campestris sub-sp. maritima. Fitoterapia, 57, 278–279.
- Cha JD, Jeong MR, Jeong SI, Moon SE, Kim JY, Kil BS, Song YH. (2005). Chemical composition and antimicrobial activity of the essential oils of Artemisia scoparia and Artemisia capillaries. Planta Med, 71, 186–190.
- Chan K. (2003). Some aspects of toxic contaminants in herbal medicines. J Chemosphere, 52, 1361–1371.
- Chandrasekharan I, Khan HA, Ghanim A. (1981). Flavonoids from Artemisia scoparia. Planta Med, 43, 310–311.
- Chemesova II, Belenovskaya LM, Stukov AN. (1987). Antitumor activity of flavonoids from some species of Artemisia L. Rastitel′nye Resursy, 23, 100–103.
- Chopra RN, Roy DN, Ghosh SM. 1940. Blumea densiflora and Artemisia vulgaris: Their insecticidal and larvicidal properties. J Malaria Inst India, 3, 495, 1066.
- Covello M. (1941). Composition of the essential oil of Artemisia verlotorum Lamotte. Napoli, 15, 61–71.
- Daise LL, Daniela SA, Celuta SA, Paul PK. (2008). Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry, 69, 1732–1738.
- Fahmy IR, Ahmed ZF, Maher A, Moneim FA. (1968). The insecticidal properties of the essential oil of Artemisia monospermal. Bulletin de l′Institut du Desert d′Egypte, 8, 49–59.
- Ferrolino-Calumpang SM, Padolina WG. (1985). Insecticidal activity screening and isolation of the major crystalline fraction of Artemisia vulgaris L. Philippine Agriculturist, 68, 249–261.
- Foglio MA, Dias PC, Antonio MA, Possenti A, Rodrigues RAF, da Silva EF, Rehder VL, de Carvalho JE. (2002). Antiulcerogenic activity of some sesquiterpene lactones isolated from Artemisia annua. Planta Med, 68, 515–518.
- Garcia C, Adams J. (2005). Healing with Medicinal Plants of the West – Cultural and Scientific Basis for their Use. La Crescenta, CA: Abedus Press.
- Gilani AH, Yaeesh S, Jamal Q, Ghayur MN. (2005). Hepatoprotective activity of aqueous-methanol extract of Artemisia vulgaris. Phytother Res, 19, 170–172.
- Goryaev MI, Shabanov IM. (1953). Essential oil of Artemisia mogoltavica. Ser Khim, 7, 75–78.
- Govindaraj S, Kumari BDR, Cioni PL, Flamini G. (2008). Mass propagation and essential oil analysis of Artemisia vulgaris. J Biosci Bioeng, 105, 176–183.
- Govorko D, Logendra S, Wang Y, Komarnytsky S, Cefalu WT, Raskin I. (2007). Polyphenolic compounds from Artemisia dracunculus L. inhibit PEPCK gene expression and gluconeogenesis in an H4IIE hepatoma cell line. Am J Physiol, 293, E1503–1510.
- Greger H, Hofer O. (1980). New unsymmetrical substituted tetrahydrofurofuran lignans from Artemisia absinthium. Assignment of the relative stereochemistry by lanthanide induced chemical shift. Tetrahedron, 36, 3551–3558.
- Guarrera PM. (2005). Traditional phytotherapy in central Italy (Marche, Abruzzo, and Latium). Fitoterapia, 76, 1–25.
- Guven KC. (1963). Turkish Artemisia species. II. Artemisia campestris. Folia Pharm, 5, 586–591.
- Hammoda HM, Ela MA, El-Lakany AM, El-Hanbali O, Zaki CS, Ghazy NM. (2008). New constituents of Artemisia monosperma. Del Die Pharmazie, 63, 611–614.
- Han J, Ye M, Qiao X, Xu M, Wang BR, Guo DA. (2008). Characterization of phenolic compounds in the Chinese herbal drug Artemisia annua by liquid chromatography coupled to electrospray ionization mass spectrometry. J Pharmaceut Biomed, 47, 516–525.
- Han KH, Jeon YJ, Athukorala Y, Choi KD, Kim CJ, Cho JK, Sekikawa M, Fukushima M, Lee CH. (2006). A water extract of Artemisia capillaris prevents 2,2′-azobis(2-amidinopropane) dihydrochloride-induced liver damage in rats. J Med Food, 9, 342–347.
- Han X, Ma X, Zhang T, Zhang Y, Liu Q, Ito Y. (2007). Isolation of high-purity casticin from Artemisia annua by high-speed counter-current chromatography. J Chromatogr, 1151, 180–182.
- Harsha VH, Hebbar SS, Hegde GR, Shripatti V. (2002). Ethnomedical knowledge of plants used by Kunabi tribe of Karnataka in India. Fitoterapia, 73, 281–287.
- Heo HJ, Yang HC, Cho HY, Hong B, Lim ST, Park HJ, Kim KH, Kim HK, Shin DH. (2000). Inhibitory effect of Artemisia asiatica alkaloids on acetylcholinesterase activity from rat PC12 cells. Mol Cell, 10, 253–262.
- Heywood VH, Humphries CJ. (1997). Anthemideae systematic review. In: Heywood VH, ed. The Biology and Chemistry of the Compositae. London: Academic Press, 868.
- Hofer O, Szabo G, Greger H. (1986). 2-Hydroxy-4-methoxy-trans-cinnamic acid as a precursor of herniarin in Artemisia dracunculus. Monatshefte Fuer Chemie, 117, 1219–1222.
- Hong JH, Lee IS. (2009). Effects of Artemisia capillaris ethyl acetate fraction on oxidative stress and antioxidant enzyme in high-fat diet induced obese mice. Chemico-Biol Interact, 179, 88–93.
- Hong JH, Lee JW, Park JH, Lee IS. (2007). Antioxidative and cytoprotective effects of Artemisia capillaris fractions. Biofactors, 31, 43–53.
- Irwin MA, Geissman TA. (1969). Sesquiterpene lactones. Constituents of Artemisia nova and A. tripartita subspecies rupicola. Phytochemistry, 8, 305–311.
- Ishibashi K, Katsuhara J, Hashimoto K, Kobayashi M. (1965). Terpenes and terpenoids in the neutral fraction of the essential oil of Artemisia maritima. Kogyo Kagaku Zasshi, 68, 1224–1228.
- Jaitak V, Singh B, Kaul VK. (2008). Variability of volatile constituents in Artemisia maritima in western Himalaya. Nat Prod Res, 22, 565–568.
- Jakupovic J, Tan RX, Bohlmann F, Jia ZJ, Huneck S. (1991). Acetylenes and other constituents from Artemisia dracunculus. Planta Med, 57, 450–453.
- Janbaz KH, Gilani AH. (1995). Evaluation of the protective potential of Artemisia maritima extract on acetaminophen- and CCl4-induced liver damage. Pakistan J Ethnopharmacol, 47, 43–47.
- Janot MM, Mouton R. (1930). The toxicity of the seeds from Artemisia maritima L. compared with that of santonin. Pharmacologiques, 37, 593–599.
- Jasna M, Canadanovic B, Sonja MD, Gordana SC, Vesna TT. (2004). Free-radical scavenging activity of wormwood (Artemisia absinthium) extracts. J Sci Food Agr, 85, 265–272.
- Jin H, Yan-Ling Z, Li-Mei S, Feng-Jiao H, Xiao-He X. (2005). An experiment on standardized cell culture assay in assessing the activities of Composite Artemisia capillaries Tablets against hepatitis B virus replication in vitro. Chinese J Integr Med, 1, 54–56.
- Juteau F, Jerkovic I, Masotti V, Milos M, Mastelic J, Bessiere JM, Viano J. (2003). Composition and antimicrobial activity of the essential oil of Artemisia absinthium from Croatia and France. Planta Med, 69, 158–161.
- Kaczmarek F, Malek B. (1955). Santonin extracted from indigenous Artemisia maritima. Acta Pol Pharm, 12, 173–177.
- Kalemba D, Kusewicz D, Swiader K. (2002). Antimicrobial properties of the essential oil of Artemisia asiatica Nakai. Phytother Res, 16, 288–291.
- Kim EK, Kwon KB, Han MJ, Song MY, Lee JH, Lv N, Choi KB, Ryu DG, Kim KS, Park JW, Park BH. (2007). Inhibitory effect of Artemisia capillaris extract on cytokine-induced nitric oxide formation and cytotoxicity of RINm5F cells. Int J Mole Med, 19, 535–540.
- Kim YS, Bahn KN, Hah CK, Gang HI, Ha YL. (2008). Inhibition of 7,12-dimethylbenz[a]anthracene induced mouse skin carcinogenesis by Artemisia capillaries. J Food Sci, 73, T16–20.
- Kiso Y, Ogasawara S, Hirota K, Watanabe N, Oshima Y, Konno C, Hikino H. (1984). Antihepatotoxic principles of Artemisia capillaris buds. Planta Med, 50, 81–85.
- Kolodziejski J, Stanislaw G, Surewicz H. (1959). Chemical composition of Artemisia abrotanum. Dissertationes Pharmaceuticae, 11, 67–74.
- Kordali S, Cakir A, Mavi A, Kilic H, Yildirim A. (2005). Screening of chemical composition and antifungal and antioxidant activities of the essential oils from three Turkish Artemisia species. J Agr Food Chem, 53, 1408–1416.
- Kotnis MS, Patel P, Menon SN, Sane RT. (2004). Reneprotective effect of Hemisdesmus indicus, a herbal drug used in gentomicin-induced renal toxicity. Nephrology, 3, 142–152.
- Kranen-Fiedler U. (1956). Some ingredients of Artemisia abrotanum. Arzneimittel-Forsch, 6, 475–479.
- Kundan SB, Anupam S. (2010). Neuroprotective effect of Artemisia absinthium L. on focal ischemia and reperfusion-induced cerebral injury. J Ethnopharmacol, 129, 1–7.
- Leung AY. (1980). Encyclopedia of Common Natural Ingredients Used in Food, Drugs, and Cosmetics. New York: Wiley.
- Lim BO, Chung HG, Lee WH, Lee HW, Suk K. (2008). Inhibition of microglial neurotoxicity by ethanol extract of Artemisia asiatica Nakai. Phytother Res, 22, 279–282.
- Lin S, Yong-Qing X, Qi-Wei Z, Ning-Ning Z. (2004). Studies on chemical constituents in bud of Artemisia scoparia (II). Zhongguo Zhongyao Zazhi, 29, 152–154.
- Liu CZ, Murch SJ, El-Demerdash M, Saxena PK. (2004). Artemisia judaica L., micropropagation and antioxidant activity. J Biotechnol, 110, 63–71.
- Liu NQ, Van der Kooy F, Verpoorte R. (2009). Artemisia afra, A potential flagship for African medicinal plants? S Afr J Bot, 75, 185–195.
- Macchioni F, Perrucci S, Flamini G, Cioni PL, Morelli I. (1999). Antimycotic activity against Saprolegnia ferax of extracts of Artemisia verlotorum and Santolina etrusca. Phytother Res, 13, 242–244.
- Maksudov NK, Pogorelko IP, Yuldashev PKA. (2003). Chemical investigation of Artemisia scoparia. Usbeksk Khim Zh (1962), 6, 84–86.
- Mashimo K, Shimizu K, Chihara G. (1963). Cholagogues. The cholagogic action of Artemisia capillaries. Saishin Igaku, 18, 1430–1434.
- Mazur Y, Meisels A. (1955). The isolation of 5-hydroxy-3,6,7,3′,4′-pentamethoxyflavone from Artemisia arborescens. B Res Coun Israel, 5A, 67–69.
- Mehrdad I, Seyed EA, Meysam MS. (2007). Detection of sesquiterpene lactones in ten Artemisia species population of Khorasan provinces. Iran J Basic Medi Sci, 10, 183–188.
- Min-Jung K, Do-Hee K, Hye-Kyung N, Tae-Young O, Chang-Yell S, Young-Joon S. (2005). Eupatilin, a pharmacologically active flavone derived from Artemisia plants, induces apoptosis in human gastric cancer cells. J Environ Pathol Tox, 24, 261–269.
- Moerman D. (1998). Native American Ethnobotany. Portland: Timber Press.
- Mucciarelli M, Maffel M. (2002). Introduction to the Genus. In: Wright, C.W., ed. Artemisia. Medicinal and Aromatic Plants – Industrial Profiles, Vol. 18. London: Taylor and Francis, 1–50.
- Mueller MS, Karhagomba IB, Hirt HM, Wemakor E. (2000). The potential of Artemisia annua as a locally produced remedy for malaria in the tropics, agricultural, chemical and clinical aspects. J Ethnopharmacol, 73, 487–493.
- Murray RDH, Stefanovic M. (1986). 6-Methoxy-7,8-methylenedioxycoumarin from Artemisia dracunculoides and Artemisia vulgaris. J Nat Prod, 49, 550–551.
- Muyima NYO, Zulu G, Bhengu T, Popplewell D. (2002). The potential application of some novel essential oils as natural cosmetic preservatives in an aqueous cream formulation. Flavour Frag J, 17, 258–266.
- Negahban M, Moharramipour S, Sefidkon F. (2006). Chemical composition and insecticidal activity of Artemisia scoparia essential oil against three coleopteran stored-product insects. J Asia-Pac Entomol, 9, 381–388.
- Oswiecimska M, Polak A, Seidl O, Sendra J. (1965). Comparative study of chromatograms of the flavonoid fractions from herbs of some species of the genus Artemisia. Dissertationes Pharmaceuticae, 17, 503–511.
- Parihar DB, Dutt S. (1950). Chemical examination of the fixed oil of Artemisia scoparia. Indian Soap J, 15, 161–165.
- Park EJ, Nan JX, Kim JY, Kang HC, Choi JH, Lee SJ, Kim YC, Sohn DH. (2000). The ethanol-soluble part of a hot-water extract from Artemisia iwayomogi inhibits liver fibrosis induced by carbon tetrachloride in rats. J Pharmacy Pharmacol, 52, 875–881.
- He SP, Tan GY, Li G, Tan WM, Nan TG, Wang BM, Li ZH, Li QX. (2009). Development of a sensitive monoclonal antibody-based enzyme-linked immunosorbent assay for the antimalaria active ingredient artemisinin in the Chinese herb Artemisia annua. Anal Bioanal Chem, 393, 1297–1303.
- Pires JM, Mendes FR, Negri G, Duarte-Almeida JM, Carlini EA. (2009). Antinociceptive peripheral effect of Achillea millefolium L. and Artemisia vulgaris L., both plants known popularly by brand names of analgesic drugs. Phytother Res, 23, 212–119.
- Polyakov P, Shishkin BK. (1995). Artemisia in Flora of the USSR. Koeringstein Germany: Bisnen Singh Scientific Books, 488–489.
- Qi-Wei Z, Yong-Xi Z, Ying Z, Yong-Qing X, Zhi-Min W. (2002). Studies on chemical constituents in buds of Artemisia scoparia. Zhongguo Zhongyao Zazhi, 27, 202–204.
- Ragasa CY, de Jesus JP, Apuada MJ, Rideout JA. (2008). A new sesquiterpene from Artemisia vulgaris. Nature Med, 62, 461–463.
- Rattu A, Maccioni A. (1953). Essential oils of Sardinian aromatic plants. IV. Essence from Artemisia arborescens. Rend Seminar Fac Sci Univ Cagliari, 23, 91–96.
- Reddy AM, Lee JY, Seo JH, Kim BH, Chung EY, Ryu SY, Kim YS, Lee CK, Min KR, Kim Y. (2006). Artemisolide from Artemisia asiatica, nuclear factor-kappaB inhibitor suppressing prostaglandin E2 and nitric oxide production in macrophages. Archiv Pharmacol Res, 29, 591–597.
- Remberg P, Bjork L, Hedner T, Sterner O. (2004). Characteristics, clinical effect profile and tolerability of a nasal spray preparation of Artemisia abrotanum for allergic rhinitis. Int J Phytother Phytopharmacol, 11, 36–42.
- Ribnicky DM, Poulev A, Watford M, Cefalu WT, Raskin I. (2006). Antihyperglycemic activity of Tarralin TM, an ethanol extract of Artemisia dracunculus. Phytomedicine, 13, 550–557.
- Ryu BK, Ahn BO, Oh TY, Kim SH, Kim WB, Lee EB. (1998). Studies on protective effect of DA-9601), Artemisia asiatica extract, on acetaminophen- and CCl4-induced liver damage in rats. Arch Pharmacol Res, 21, 508–513.
- Saber AH, Khafagy SM. (1958a). The study of Artemisia judaica. Egypt Pharm Bull, 40, 91–95.
- Saber AH, Khafagy SM. (1958b). Artemisia judaica. Egypt Pharm Bull, 40, 37–43.
- Saddi M, Sanna A, Cottiglia F, Chisu L, Bonsignore L, De Logu A. (2007). Antiherpevirus activity of Artemisia arborescens essential oil and inhibition of lateral diffusion in Vero cells. Ann Clin Microbiol Antimicrob, 6, 10.
- Saratikov AS, Prishchep TP, Vengerovskii AI, Taran DD, Beresovskaya TP, Kalinkina G, Serykh EA. (1986). Anti-inflammatory properties of essential oils of Achillea asiatica and some species of Artemisia. Khimiko-Farmatsevticheskii Zhurnal, 20, 585–588.
- Shahriyary L, Yazdanparast R. (2007). Inhibition of blood platelet adhesion, aggregation and secretion by Artemisia dracunculus leaves extracts. J Ethnopharmacol, 114, 194–198.
- Sharaf A, Fahmy IR, Ahmed ZF, Moneim FA. (1959). Pharmacological study of Artemisia monospermal. Egypt Pharml Bull, 41, 47–52.
- Singh HP, Kaur S, Mittal S, Batish DR, Kohli RK. (2008). Phytotoxicity of major constituents of the volatile oil from leaves of Artemisia scoparia Waldst. & Kit. J Biosci, 63, 663–666.
- Singh HP, Kaur S, Mittal S, Batish DR, Kohli RK. (2009). Essential oil of Artemisia scoparia inhibits plant growth by generating reactive oxygen species and causing oxidative damage. J Chem Ecol, 35, 154–162.
- Sinitsyn GS, Sinitsyn GS. (1960). Dynamics of accumulation of santonins in cultivated Artemisia. Izvest Akad Nauk Kazakh SSR, 3, 86–93.
- Slepetys J. (1975). Biology and biochemistry of wormwood. Accumulation dynamics of tannins, ascorbic acid, and carotene. Liet TSR Mokslu Akad Darb Ser C, 1, 43–148.
- Stavri M, Ford CHJ, Bucar F, Streit B, Hall ML, Williamson RT, Mathew KT, Gibbons S. (2005). Bioactive constituents of Artemisia monospermal. Phytochemistry, 66, 233–239.
- Swanston-Flatt SK, Flatt PR, Day C, Bailey CJ. (1991). Traditional dietary adjuncts for the treatment of diabetes mellitus. Proc Nutr Soc, 50, 641–651.
- Tan RX, Lu H, Wolfender JL, Yu TT, Zheng WF, Yang L, Gafner S, Hostettmann K. (1999). Mono- and sesquiterpenes and antifungal constituents from Artemisia species. Planta Med, 65, 64–67.
- Tan RX, Tang HQ, Hu J, Shuai B. (1998). Lignans and sesquiterpene lactones from Artemisia sieversiana and Inula racemosa. Phytochemistry, 49, 157–161.
- Tanaka K. (1961). Antimicrobial activity of capillin, a component of the essential oil of Artemisia capillaris and of its derivatives. Seikagaku, 33, 399–409.
- Tariq KA, Chishti MZ, Ahmad F, Shawl AS. (2009). Anthelmintic activity of extracts of Artemisia absinthium against ovine nematodes. Vet Parasitol, 160, 83–88.
- Temraz A, El-Tantawy WH. (2008). Characterization of antioxidant activity of extract from Artemisia vulgaris. Pakistan J Pharm Sci, 21, 321–326.
- Thring TSA, Weitz FM. (2006). Medicinal plant use in the Bredasdorp/Elim region of the Southern Overberg in the Western Cape Province of South Africa. J Ethnopharmacol, 103, 261–275.
- Tigno XT, de Guzman F, Flora AM. (2000). Phytochemical analysis and hemodynamic actions of Artemisia vulgaris L. Clin Hemorheol Micro, 23, 167–175.
- Trumpowskaand M, Olszewski Z. (1968). Chromatographic analysis of the essential oil from Artemisia vulgaris harvested in various seasons. Acta Pol Pharm, 25, 319–328.
- Tunon H, Thorsell W, Mikiver A, Malander I. (2006). Arthropod repellency, especially tick (Ixodes ricinus), exerted by extract from Artemisia abrotanum and essential oil from flowers of Dianthus caryophyllum. Fitoterapia, 77, 257–261.
- Wake G, Court J, Pickering A, Lewis R, Wilkins R, Perry E. (2000). CNS acetylcholine receptor activity in European medicinal plants traditionally used to improve failing memory. J Ethnopharmacol, 69, 105–114.
- Watson LE, Bates PL, Evans TM, Unwin MM, Estes JR. (2002). Molecular phylogeny of Subtribe Artemisiinae (Asteraceae), including Artemisia and its allied and segregate genera. BMC Evol Biol, 2, 17.
- Wei ZX, Pan JP, Li Y. (1992). Artemisinin G, a sesquiterpene from Artemisia annua. Planta Med, 58, 300.
- Weyerstahl P, Kaul VK, Weirauch M, Marschall WH. (1987). Volatile constituents of Artemisia vestita oil. Planta Med, 53, 66–72.
- Woerdenbag HJ, Pras N, Chan NG, Bang BT, Bos R, Van Uden W, Van YP, Van Boi N, Batterman S, Lugt CB. (1994). Artemisinin, related sesquiterpenes and essential oil in Artemisia annua during a vegetation period in Vietnam. Planta Med, 60, 272–275.
- Wu TS, Tsang ZJ, Wu PL, Lin FW, Li CY, Teng CM, Leec KH. (2001). New constituents and antiplatelet aggregation and anti-HIV principles of Artemisia capillaris. Bioorg Med Chem, 9, 77–83.
- Xie G, Schepetkin IA, Siemsen DW, Kirpotina LN, Wiley JA, Quinn MT. (2008). Fractionation and characterization of biologically active polysaccharides from Artemisia tripartita. Phytochemistry, 69, 1359–1371.
- Ye Y, Fang-Yuan G, Xing-Xin W, Yang S, Yi-Hua L, Ting C. (2008). Anti-inflammatory and immunosuppressive effect of flavones isolated from Artemisia vestita. J Ethnopharmacol, 120, 1–6.
- Yeung HC. (1985). Handbook of Chinese Herbs and Formulas. Los Angeles: Institute of Chinese Medicine, 455.
- Zhang Y, Zhang J, Yao J, Yang YL, Wang L, Dong LN. (2005). Studies on the chemical constituents of the essential oil of Artemisia dracunculus. Chinese Materia Med, 30, 594–596.
- Zheng WF, Tan RX, Yang L, Liu ZL. (1996). Two flavones from Artemisia giraldii and their antimicrobial activity. Planta Medica, 62, 160–162.