Abstract
Context: Shao Fu Zhu Yu decoction (SFD), a well-known Chinese medicine, has been used for the treatment of primary dysmenorrhea in China for more than 200 years.
Objective: A crude water extract and four fractions from SFD were evaluated for their analgesic activities for the purpose of validating the ethnomedical use of SFD.
Material and method: The analgesic activities were studied by measuring nociception using acetic acid-induced abdominal contractions, the hot-plate test, formalin-induced licking and oxytocin-induced writhing in estrogen-treated mouse models. Prostaglandin E2 and nitric oxide production in cultured lipopolysaccharide (LPS)-treated macrophage cells were determined. Chemical components were separated and identified in the SFD analgesic fractions using ultra-performance liquid chromatography tandem mass spectrometry (UPLC-MS).
Results: Oral SFD exerted significant analgesic activities in all nociceptive models except the hot-plate test. The activity-guided fractionation demonstrated that the SFD-40% fraction was the most potent with marked inhibition of pain responses at a dose of 54 mg/kg in vivo, and significantly inhibited prostaglandin E2 and nitric oxide production in LPS-treated mouse peritoneal macrophages in vitro. Further UPLC-MS analysis showed the presence of several chemical components in the SFD-40% fraction, including ferulic acid, paeoniflorin, typhaneoside, and isorhamnetin-3-O-neohesperidoside.
Discussion and conclusion: These results demonstrated that SFD has significant peripheral analgesic activities, mainly attributed to the SFD-40% fraction, and supports the use of SFD in traditional Chinese medicine.
Introduction
Shao Fu Zhu Yu decoction (SFD), a well-known Chinese medicine to improve cold-induced blood stasis, is composed mainly of herbs which warm blood vessels to increase flow. These include Radix Angelica [Angelica sinensis (Oliv.) Diels (Umbelliferae)], Rhizoma Ligusticum [Ligusticum chuanxiong Hort. (Umbelliferae)], Radix Paeonia [Paeonia lactiflora Pall. (Paeoniaceae)], Cortex Cinnamomum [Cinnamomum cassia Presl. (Lauraceae)], Fructus Foeniculum [Foeniculum vulgare Mill. (Umbelliferae)], and Resin Commiphora [Commiphora myrrha Engl. (Burseraceae)] (CitationSu et al., 2007). SFD is believed to be effective in the treatment of painful gynecological disorders such as primary dysmenorrhea, menoxenia and other inflammation (CitationZhang, 1987), and has been used in China for more than 200 years. Non-specified dysmenorrhea is one of the most common gynecological complaints characterized by pain, cramping, and backache occurring during menses in young women. Its etiology has not been clearly elucidated. However, available data show that the pain associated with primary dysmenorrhea is caused by the hypercontractility of the uterine muscle, the subsequent reduction in blood flow and concomitant uterine ischemia (CitationAlkerlund, 1979; CitationDu et al., 2007). In traditional Chinese medicine (TCM), symptoms of dysmenorrhea are considered to be characterized by blood stasis in the uterus (CitationZhang, 1992; CitationLi, 2006). The syndrome of blood stasis induced by cold is one of the most common types of dysmenorrhea in TCM, and could be modulated by SFD, promoting blood circulation, warming of the vessels and alleviation of pain.
It has been demonstrated that the active herbs in the SFD formula have a wide range of biological activities, including anti-inflammatory, analgesic, antioxidant, immunomodulatory, and anticoagulant activities (CitationChien et al., 2008; CitationZhang et al., 2008; CitationDu et al., 2007; CitationLantz et al., 2007; CitationTognolini et al., 2006). However, there are few reports on the pharmacological actions and active components of whole-formula SFD related to primary dysmenorrhea. In a recent study we investigated the effects of SFD on hemorheology in rats treated with an ice water bath in combination with an injection of adrenaline. Our data showed that SFD significantly improved the hemorheological indices of rats in this model of blood stasis (CitationSu et al., 2008). In addition, our data demonstrated that both whole-formula SFD and the SFD-40% fraction inhibited oxytocin-induced uterine contractions and the increase in the contractility of uterine horns in vitro (CitationSu et al., 2007). These results clearly showed that SFD possesses hemorheology-modulating and non-specific antispasmodic functions, and implied that SFD-40% is one of active fractions of SFD to improve symptoms of dysmenorrhea. As pain is the main symptom of dysmenorrhea, we further examined the analgesic effects of SFD and its fractions, obtained by solvent extraction, for the purpose of validating its ethnomedical use.
Methods
Plant material
The ten crude drugs used in this study were purchased from Nanjing Medicinal Material Company and identified by Jinao Duan of Jiangsu Key Laboratory for TCM Formulae Research as Radix Angelica [Angelica sinensis (Oliv.) Diels (Umbelliferae)], Rhizoma Ligusticum [Ligusticum chuanxiong Hort. (Umbelliferae)], Radix Paeonia [Paeonia lactiflora Pall. (Paeoniaceae)], Cortex Cinnamomum [Cinnamomum cassia Presl. (Lauraceae)], Fructus Foeniculum [Foeniculum vulgare Mill. (Umbelliferae)], Resin Commiphora [Commiphora myrrha Engl. (Burseraceae)], Rhizoma Zingiber [Zingiber officinale Roscoe (Zingiberaceae)], Rhizoma corydalis [Corydalis yanhusuo W.T.Wang (Papaveraceae)], Faeces Trogopterpri [Trogopterus xanthipes Milne-Edwards (Sciuridae)] and Typhae Pollen [Typha angustifolia L. (Typhaceae)]. The voucher specimens (No. NJUTCM-20060818∼20060827) were deposited in Nanjing University of Chinese Medicine.
Extraction and isolation
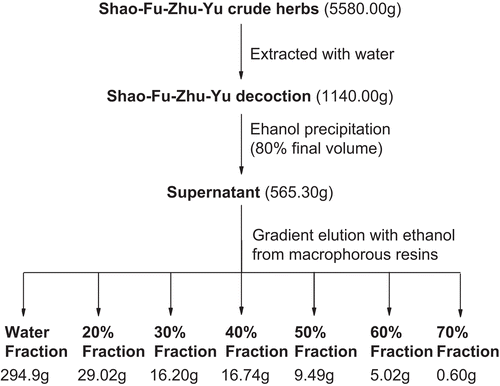
Clinically, a single formula of SFD consists of 10 g Angelica radix, 3 g Ligusticum rhizoma, 6 g Paeonia radix, 6 g Cinnamomum cortex, 1.5 g Foeniculum fructus, 6 g Zingiber rhizoma, 3 g Commiphora resin, 10 g Trogopterori feces, 3 g Typhae pollen and 3 g Corydalis rhizoma. The mixed herbs (5.58 kg) were crushed into small pieces and extracted with 1000 mL boiling water twice (1 h each time), and the filtrate was filtered and concentrated to dryness in a vacuum in order to obtain a dried SFD extraction (yield: 20.4%, dry weight: 1140 g). SFD was partially purified by ethanol precipitation (80% final volume) and the supernatant was concentrated (yield: 10.1%). As shown in , the concentrate (565.3 g) was separated by gradient elution (10% increments) with different concentrations of ethanol from macroporous adsorptive resins D101 to give 29.02 g SFD-20% fraction (yield: 0.51%), 16.2 g SFD-30% fraction (yield: 0.28%), 16.74 g SFD-40% fraction (yield: 0.3%), 9.49 g SFD-50% fraction (yield: 0.17%), 5.02 g SFD-60% fraction (yield: 0.089%) and 0.6 g SFD-70% fraction (yield: 0.011%). Preliminary analysis showed that the chemical constituents of SFD-20% and SFD-70% could be detected in SFD-30% and SFD-60%, respectively. These fractions were further merged into SFD-20∼30% fraction (yield 0.79%) and SFD-60∼70% fraction (yield 0.1%).
The animal dose of SFD was extrapolated from the human daily dose, using the body surface area normalization method. The middle dose (1.84 g/kg) of SFD was approximately equivalent to the adult daily dose (51.5 g) of Shao Fu Zhu Yu crude herbs based on the traditional Chinese medicine prescription. The formula for dose translation was as follows:
The dose of every fraction was equivalent to the dose (3.68 g/kg) of SFD and calculated according to their yields from Shao Fu Zhu Yu crude herbs by the following expression:
SFD and its fractions were freshly prepared with physiological saline at the desired concentrations for the study in vivo. The analgesic fraction of SFD was dissolved in dimethylsulfoxide (0.1% or less) for in vitro study.
Animals
All experiments were performed with Four hundred female ICR mice, weighing 18–22 g, obtained from the experimental animal center of Nanjing University of Chinese Medicine. The mice were kept in plastic cages at 22° ± 2°C with free access to pellet food and water and on a 12 h light/dark cycle. Animal welfare and experimental procedures were approved by the Animal Ethics Committee of Nanjing University of Chinese Medicine, and were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (CitationNational Research Council of USA, 1996) and related ethical regulations of our university.
Acetic acid-induced abdominal contraction
Abdominal contraction, induced by intraperitoneal injection of acetic acid, consisted of a contraction of the abdominal muscle together with a stretching of the hind limbs (CitationHarada et al., 1979). The animals were orally pretreated with aspirin (100 mg/kg) (Citational-Swayeh et al., 2000), used as a positive control, or SFD (0.92, 1.84, 3.68 g/kg) and its fractions (the equivalent dose to 3.68 g/kg of SFD) 1 h before acetic acid injection. After challenge, pairs of mice were placed in separate boxes and the number of abdominal contractions was counted from 5 to 20 min.
Hot-plate test
The test was carried out using a previously described technique (Citationvan Eick, 1967). Female mice were placed on a hot-plate maintained at 55° ± 5°C. The time between placement and shaking or licking hind paws or jumping was recorded as the hot-plate latency. Mice with baseline latencies of <5 s or >30 s were eliminated from the study. After the determination of baseline response latencies, hot-plate latencies were re-determined at 15, 30 and 60 min after oral administration of the tested drugs. Dolantin (15 mg/kg) was administered to mice as a positive control in this experiment.
Formalin-induced paw licking
The procedure used was similar to that described previously (CitationSugimoto et al., 1986). A dose of 2.5% formalin solution (20 µL) was injected under the plantar surface of the right hind paw. The animals were placed in clear cages and observed from 0 to 30 min following formalin injection. The amount of time spent licking the injected paw was timed with a chronometer. The initial nociceptive response normally peaked 5 min after formalin injection (early phase) and 20–30 min after formalin injection (late phase), representing the tonic and inflammatory pain responses, respectively. The animals were orally pretreated with SFD (0.92, 1.84, 3.68 g/kg) and its fractions (the equivalent dose to 3.68 g/kg of SFD), or with celecoxib (60 mg/kg) as a positive control.
Oxytocin-induced writhing response in estrogen-treated mice
The test was carried out using a previously described technique (CitationSun et al., 2002). One hundred thirty-seven female mice were injected subcutaneously with 0.1 mL/10 g body weight of 1 mg/mL estrogen solution for 6 days. SFD (0.92, 1.84, 3.68 g/kg) and its fractions (the equivalent dose to 3.68 g/kg of SFD) were administered orally at day 5 and repeated every 24 h until 72 h. Mice were injected intraperitoneally with 1 mg/mL oxytocin solution in saline at day 7. The time that elapsed until the occurrence of the writhing response was recorded as pain latency. The number of writhing responses occurring between 5 and 40 min after oxytocin injection was recorded. After the writhing test, uterine tissue was isolated and homogenized in 9 volumes of homogenizing medium for 5 min. The homogenate was then centrifuged at 3000 rpm for 15 min. Calcium in the uterine homogenate was measured by methyl thymol blue colorimetry in accordance with the manufacturer’s instructions. Calcium was expressed in mmol/gprot.
Prostaglandin E2 and nitric oxide production in lipopolysaccharide-treated macrophage cells
The test was carried out using a previously described technique (CitationWiener & Levanon, 1968). Highly purified mouse peritoneal macrophages (2.5 × 107 cells) were harvested by peritoneal lavage. The cells were re-suspended in RPMI-1640 medium supplemented with penicillin-streptomycin and 10% fetal bovine serum in 96-well culture plates and incubated for 3 h. The supernatant was removed, washed three times with fresh media, and then incubated in the fresh medium with 10 μg/mL LPS (except the blank vehicle). Test materials were simultaneously added to each well (except control). Plates were incubated at 37°C in 5% CO2 for 48 h (CitationWu et al., 1998). Supernatants were immediately removed and stored at 70°C and prostaglandin E2 (PGE2) was determined by radioimmunoassay using a commercial PGE2 kit (Huaying Biotechnology Research Institute, Beijing). Content of nitric oxide (NO) was detected by Griess reagent at 550 nm and macrophage cell viability was determined by MTT assay at 590 nm in a microplate reader.
UPLC-MS analysis of SFD and SFD-40% fraction
The components of SFD were identified using a previously described method with modification (CitationSu et al., 2007). UPLC-MS analysis was performed using an Acquity UPLC BE-HC18 (2.1 mm × 50 mm, 1.7 μm) column in (+) electrospray ionization mode. The mobile phase was a gradient elution of HCOOH and CH3CN (A) and 0.1% HCOOH commencing with 5%A, rising to 20%A after 4.5 min then to 45%A after 5 min and 90%A after 8 min. The flow rate was 0.4 mL/min. The first-order full-scan mass spectra covered the range 50–1000 m/z. Compounds were identified by MS characterization and UV absorption against external standards of protocatechuic acid, caffeic acid, albiflorin, paeoniflorin, ferulic acid, typhaneoside, tetramethylpyrazine and isorhamnetin-3-O-neohesperidoside. SFD and the most active analgesic fractions were subjected to UPLC-MS analysis.
Statistical analysis
The results were expressed as mean ± SEM. Statistical analysis was performed using two-tailed unpaired Student’s t-test. A p value of less than 0.05 was considered statistically significant and p less than 0.01 being very significant.
Results
Effects of SFD and its fractions on acetic acid-induced abdominal contraction
SFD (1.84, 3.68 g/kg) exhibited a significant decrease in the number of abdominal contractions induced by the injection of an aqueous solution of acetic acid, with inhibition of 47.8% and 61.2%, respectively. The group that received 3.68 g/kg SFD produced a comparable analgesic effect to that of the aspirin group ().
Figure 2. Effect of SFD on the acetic acid-induced writhing response in mice. Data represent mean ± SEM (n = 10–12). **p <0.01 compared with control group.
To determine the active fractions of SFD, several fractions were further screened. The experiments showed that the analgesic activities of several SFD fractions were different at an equivalent dose to that of SFD (). Both SFD-40% and SFD-50% fractions inhibited acetic acid-induced abdominal contraction, with inhibition of 48.2% and 34.1%, respectively. There was no significant inhibition by the other fractions ().
Table 1. Effects of the different fractions from SFD on pain responses induced by acetic acid, formalin and oxytocin in mice.
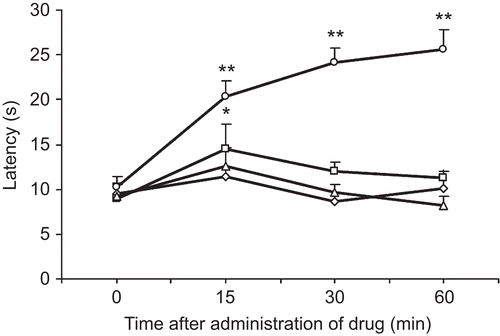
Effect of SFD in the hot-plate test
The results presented in show that SFD at the tested doses did not significantly increase the hot-plate latency of mice at 30 and 60 min after oral treatment, while Dolantin (15 mg/kg) as a positive control markedly increased the hot-plate latency of mice.
Effects of SFD and its fractions on formalin-induced licking
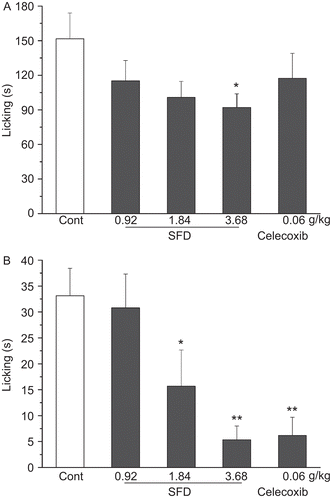
SFD at a dose of 3.68 g/kg produced marked inhibition of the neurogenic pain (early-phase, inhibition of 39.4%) and inflammatory pain (late-phase, inhibition of 83.9%), caused by intraplantar injection of formalin in mice (). The positive control drug, Celecoxib (60 mg/kg), caused significant inhibition (81.3%) in the late phase, but not in the early phase, of formalin-induced nociceptive pain ().
Figure 4. Effect of SFD on formalin-induced nociception in mice. (A) Number of writhing responses recorded in the early-phase (0-5 min). (B) Number of writhing responses recorded in the late-phase (20–30 min). Data represent mean ± SEM (n = 10–12). *p <0.05, **p <0.01 compared with control group.
Both SFD-40% and SFD-50% fractions significantly inhibited early phase pain and late phase pain in this test. There was no significant inhibition by other fractions ().
Effects of SFD and its fractions on the oxytocin-induced writhing response in estrogen-treated mice
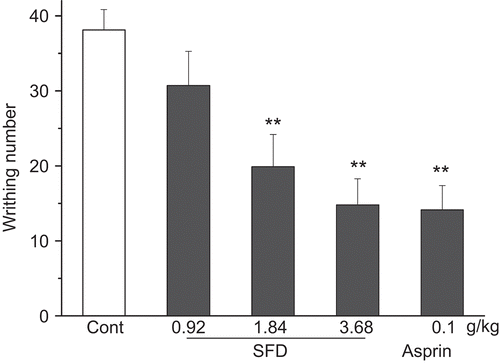
As shown in , obvious pain responses were observed after estrogen-induced enhancement of oxytocin sensitivity in female mice, with 29.5 ± 4.3 writhings in the control group. SFD (0.92–3.68 g/kg) produced marked inhibition on the writhing response. At a dose of 3.68 g/kg of SFD, the writhing response was inhibited by 57%. In addition, SFD (0.92, 1.84, 3.68 g/kg) significantly decreased uterine homogenate Ca2+ levels, from 1.7 ± 0.32 in the control group to 0.9 ± 0.6, 1.1 ± 0.17 and 0.8 ± 0.12 mmol/gprot, respectively, in the SFD groups ().
Figure 5. Effect of SFD on the oxytocin-induced pain response in estrogen-treated mice. (A) Number of writhing responses as pain intensity index recorded for 40 min. (B) Uterine Ca2+ content determined by methyl thymol blue colorimetry. Data represent mean ± SEM (n = 12–15). *p <0.05, **p <0.01 compared with control group.
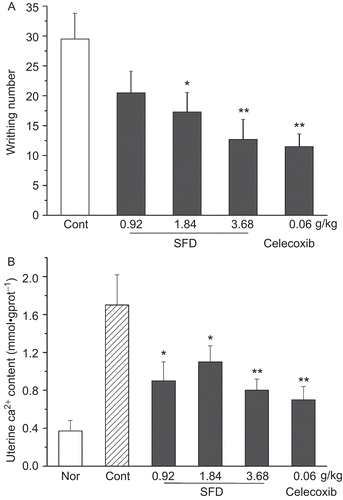
Meanwhile, only the SFD-40% fraction significantly inhibited the writhing response induced by oxytocin and estrogen, and reduced uterine homogenate Ca2+ levels ().
Effect of SFD-40% fraction on PGE2 and NO accumulation in cultured LPS-treated macrophage cells
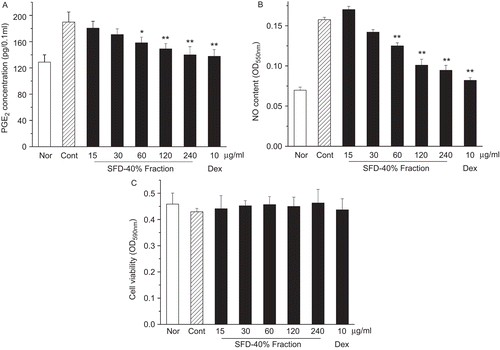
The results of this test are shown in . When incubated with vehicle alone, the cells yielded 128.8 pg/0.1 mL PGE2. Treatment of the cells with 10 μg/mL LPS produced 189.6 pg/0.1 mL PGE2, a 1.5-fold increase compared with vehicle. When the cells were pretreated with 60, 120 and 240 μg/mL of SFD-40% fraction before LPS treatment, PGE2 production was significantly attenuated (). NO production was also attenuated in this test (). In addition, the SFD-40% fraction at these doses was not cytotoxic to the macrophage cells ().
Figure 6. Effect of the SFD-40% fraction on the PGE2 and NO accumulation in LPS-treated macrophage. (A) PGE2 was detected by radioimmunoassay. (B) NO was detected by Griess reagent. (C) Macrophage cell viability was determined by MTT assay. Values are means ± SD (n = 4). *p<0.05, **p<0.01 compared with LPS-stimulated control group.
Chemical components of SFD and the SFD-40% fraction
The chemical components of SFD () and the SFD-40% fraction () were analyzed by UPLC-MS. In total, 11 compounds were identified from SFD in 7 min including protocatechuic acid (m/z 155), tetramethylpyrazine (m/z 137), caffeic acid (m/z 181), albiflorin (m/z 481), paeoniflorin (m/z 481), ferulic acid (m/z 195), typhaneoside (m/z 771), isorhamnetin-3-O-neohesperidoside (m/z 625), senkyunolide I (m/z 225), senkyunolide H (m/z 225), and quercetin (m/z 303).
Figure 7. UPLC-positive ion ESI-MS total ion current (TIC) chromatograph of SFD and the SFD-40% fraction. The mobile phase was a gradient elution of HCOOH and CH3CN. Flow rate was 0.4 mL/min. (A) TIC of mixed standards, (B) TIC of SFD and (C) TIC of the SFD-40% fraction. For peak identification: 1, protocatechuic acid; 2, tetramethylpyrazine; 3, caffeic acid; 4, albiflorin; 5, paeoniflorin; 6, ferulic acid; 7, typhaneoside; 8, isorhamnetin-3-O-neohesperidoside; 9, senkyunolide I; 10, senkyunolide H; 11, quercetin.
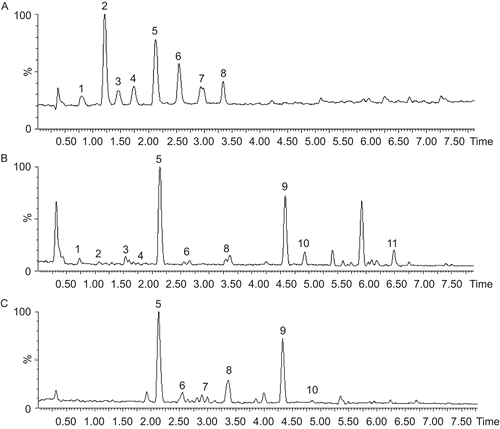
The SFD-40% fraction was found to contain paeoniflorin, ferulic acid, typhaneoside, isohamnetin-3-O-neohesperidoside, senkyunolide I and senkyunolide H (). In this fraction, the highest content compounds were paeoniflorin (12%), isorhamnetin-3-O-neohesperidoside (2.98%) and typhaneoside (2%), while the contents of other components were less than 1%.
Discussion
In the present study we used several mouse models such as acetic acid-induced abdominal constriction, the hot-plate test, formalin-induced licking, and oxytocin-induced writhing, to investigate the analgesic effects of SFD and its various extracted fractions to validate its traditional use in the treatment of menstrual pain. All these animal models, but not the hot-plate test, clearly demonstrated that SFD produced significant analgesic effects.
Acetic acid-induced writhing is a visceral pain model and widely used for investigating peripheral analgesia. It was reported that the writhing response induced by acetic acid was closely related to PG biosynthesis and highly dependent on both peritoneal macrophages and mast cells (CitationDu et al., 2007). In the current study, acetic acid injection was demonstrated to induce a characteristic writhing response in mice. SFD produced a significant analgesic effect on the number of writhing responses induced by acetic acid, suggesting that it exerts a peripheral analgesic effect. The action of SFD may be related to inhibition of PG accumulation in peritoneal macrophages.
Thermal painful stimuli in hot-plate tests are known to be diminished by centrally acting, but not peripherally acting, analgesic drugs (CitationMa et al., 2007). The results of the present study showed that Dolantin (a centrally acting analgesic drug) produced an inhibitory effect on the nociceptive response in the hot-plate test, while SFD treatment had a very weak effect. Therefore, it seems that SFD at the present doses had little central analgesic activity.
The formalin test is a useful model of clinical pain in which the first phase results from direct chemical stimulation of nociceptors whereas the second phase is dependent on peripheral inflammation. Several reports showed that substance P and bradykinin participated in the early phase, while histamine, PGs and bradykinin were involved in the late phase (CitationSayyah et al., 2004). Our results showed that SFD exhibited more potent inhibition on the second phase inflammatory response than that on the first phase neurogenic response. It is well demonstrated in the review literature that non-steroidal anti-inflammatory drugs (NSAIDs) can attenuate, in a dose-related manner, late-phase licking, but are largely ineffective or cause very weak inhibition against early-phase licking in the formalin test (CitationMalmberg & Yaksh, 1992). Celecoxib, a selective COX-2 inhibitor, was ineffective against early-phase licking in this test, which correlated with previous reports (CitationSantos et al., 1998). These data suggested that the antinociception caused by SFD mainly results from its peripheral actions (anti-inflammatory effects).
Oxytocin-induced writhing test in estrogen-treated mice, a reliable model relevant to primary dysmenorrhea, was further utilized for the assessment of the analgesic effects of SFD. This model reflects the clinical features and pathogenesis of menstrual pain (CitationSun et al., 2002), such as severe pain, uterine contraction, Ca2+ influx and prostaglandin synthesis. It is well demonstrated in the literature that uterine contraction induced by oxytocin or PGF-2α is associated with the external Ca2+ influx into myometrial cells (CitationTasaka et al., 1991; CitationRuttner et al., 2002; CitationHsia et al., 2008) and controlled by sex steroids such as estrogen and progesterone (CitationMesiano & Welsh, 2007). Estrogen could increase the number of uterus oxytocin receptors and result in an increased uterine response to contractile agents (CitationNissenson et al., 1978). In this study, it was found that oxytocin induced an obvious writhing response because of uterine contraction after administration of estrogen in mice, and SFD treatment significantly attenuated such pain behavior associated with reduced uterine Ca2+ levels. Our previous study showed that SFD inhibited uterine contractions induced by oxytocin or PGF-2α in vitro (CitationSu et al., 2007). These results, plus the findings obtained from the present study, implied that SFD exerted its analgesic effects partly by reducing uterine hypercontractility in dysmenorrhea.
Of the 4 SFD fractions, the SFD-40% fraction had the greatest potential to decrease pain responses induced by acetic acid, formalin and oxytocin, while the other fractions showed less or no analgesic activity at the tested doses. This implied that SFD-40% was the key fraction of SFD in the overall analgesic effect. In this study, it was also observed that SFD-50% fraction had inhibitory effects on acid- or formalin-induced pain, though the effects were weaker than those of SFD-40%. Thus, the therapeutic effect of SFD may result from a combination of active materials rather than the SFD-40% fraction only. In general, however, the observations support the conclusion that the main analgesic effects of whole-formula SFD could be attributed to the SFD-40% fraction.
Given the important role of the SFD-40% fraction in whole-formula SFD in alleviating pain, we further investigated the effect of the SFD-40% fraction on PGE2 production in LPS-stimulated macrophages in vitro. Many reports demonstrated that dysmenorrhea leads to increased PG production, which could cause contraction of the blood vessels and myometrium, and insufficient blood flow to the endometrium (CitationDawood & Khan-Dawood, 2007; CitationLumsden et al., 1983), and NSAIDs (cyclooxygenase inhibitors) are used to treat primary dysmenorrhea in western medicine (CitationRainsford, 2006). In this study our results showed that the SFD-40% fraction significantly reduces PGE2 and NO accumulation in LPS-treated macrophage cells, suggesting that this fraction may induce peripheral analgesia partly by inhibiting PG production. In addition, we identified several chemical components from the SFD-40% fraction by UPLC-MS, including paeoniflorin, ferulic acid, typhaneoside, isorhamnetin-3-O-neohesperidoside, senkyunolide I and senkyunolide H. Paeoniflorin, a major component with 12% content (w/w) in the SFD-40% fraction, has analgesic effects (CitationZhang et al., 2008); recently it has been reported to inhibit COX-2 expression and activity in LPS-stimulated RAW264.7 (CitationSun et al., 2009), and could also inhibit the L-type Ca2+ current and Ca2+ influx in neuronal cells (CitationTsai et al., 2005). However, it is currently unknown whether isorhamnetin-3-O-neohesperidoside and typhaneoside, the other two compounds with a content of 2.98% and 2% (w/w), respectively, in the SFD-40% fraction, play a role in inhibition of COX-2 or Ca2+ influx. The results suggest that the SFD-40% fraction mainly accounts for the analgesic effects of SFD, partly by inhibition of prostaglandin production, and further studies may elucidate the role of several different compounds in this fraction.
Conclusions
The present data indicate that SFD, a well-known Chinese formula for cold-induced blood stasis syndrome, has peripheral analgesic effects, thus partially explaining the beneficial effects of SFD on primary dysmenorrhea. Meanwhile, activity-guided fractionation demonstrated that the SFD-40% fraction, mainly containing paeoniflorin, isorhamnetin-3-O-neohesperidoside and typhaneoside, is an important fraction responsible for the analgesic activities of SFD. The findings of this study, in addition to the data we reported recently on its antispasmodic effect on the uterus, suggest that SFD may be considered as a feasible alternative therapeutic agent for dysmenorrhea.
Acknowledgements
We would like to thank Li Liu, Junfeng Zhang, Fang Wang and Jianfei Song for their assistance in experiments.
Declaration of interest
This work was supported by the National Natural Science Fund of China (30973885), the National Science Fund for Distinguished Young Scholars (30901894), the Research Fund for the Doctoral Program of Higher Education of China (20093237120013), the Science Fund of Jiangsu Administration of TCM (LZ09017), and the Program for New Century Excellent Talents by the Ministry of Education (NCET-09-0163). The authors alone are responsible for the content and writing of the paper.
References
- Alkerlund M. (1979). Pathophysiology of dysmenorrhea. Acta Obstet Gynecol Scand, 87, 27–32.
- al-Swayeh OA, Clifford RH, del Soldato P, Moore PK. (2000). A comparison of the anti-inflammatory and anti-nociceptive activity of nitroaspirin and aspirin. Br J Pharmacol, 129, 343–350.
- Chien TY, Chen LG, Lee CJ, Lee FY, Wang CC. (2008). Anti-inflammatory constituents of Zingiber zerumbet. Food Chem, 110, 584–589.
- Dawood MY, Khan-Dawood FS. (2007). Differential suppression of menstrual fluid prostaglandin F2a, prostaglandin E2, 6-keto prostaglandin F1a and thromboxane B2 by suprofen in women with primary dysmenorrhea. Prostaglandins Other Lipid Mediat, 83, 146–153.
- Du JR, Yu Y, Ke Y, Wang CY, Zhu L, Qian ZM. (2007). Ligustilide attenuates pain behavior induced by acetic acid or formalin. J Ethnopharmacol, 112, 211–214.
- Harada T, Takahashi H, Kaya H, Inoki R. (1979). A test for analgesics as an indicator of locomotor activity in writhing mice. Arch Int Pharmacodyn Ther, 242, 273–284.
- Hsia SM, Kuo YH, Chiang W, Wang PS. (2008). Effects of adlay hull extracts on uterine contraction and Ca2+ mobilization in the rat. Am J Physiol Endocrinol Metab, 295, 719–726.
- Lantz RC, Chen GJ, Sarihan M. (2007). The effect of extracts from ginger rhizome on inflammatory mediator production. Phytomedicine, 14, 123–128.
- Li QZ. (2006). An analysis on the main type of syndrome in TCM and the factors affecting primary dysmenorrheal in adolescent girls. J Emerg TCM, 15, 996–997.
- Lumsden MA, Kelly RW, Baird DT. (1983). Primary dysmenorrhoea: The importance of both prostaglandins E2 and F2 alpha. Br J Obstet Gynaecol, 90, 1135–1140.
- Ma HY, Kou JP, Wang JR, Yu BY. (2007). Evaluation of the anti-inflammatory and analgesic activities of Liu-Shen-Wan and its individual fractions. J Ethnopharmacol, 112, 108–114.
- Malmberg AB, Yaksh TL. (1992). Antinociceptive actions of spinal nonsteroidal anti-inflammatory agents on the formalin test in the rat. J Pharmacol Exp Ther, 263, 136–146.
- Mesiano S, Welsh TN. (2007). Steroid hormone control of myometrial contractility and parturition. Semin Cell Dev Biol, 18, 321–331.
- National Research Council. (1996). Guide for the Care and Use of Laboratory Animals. Washington DC, USA: National Academy Press.
- Nissenson R, Flouret G, Hechter O. (1978). Physiological sciences opposing effects of estradiol and progesterone on oxytocin receptors in rabbit uterus. Proc Natl Acad Sci USA, 4, 2044–2048.
- Rainsford KD. (2006). Current status of the therapeutic uses and actions of the preferential cyclo-oxygenase-2 NSAID, nimesulide. Inflammopharmacology, 14, 120–137.
- Ruttner Z, Ivanics T, Slaaf DW, Reneman RS, Toth A, Ligeti L. (2002). In vivo monitoring of intracellular free calcium changes during uterine activation by prostaglandin F2 alpha and oxytocin. J Soc Gynecol Investig, 9, 294–298.
- Santos AR, Vedana EM, De Freitas GA. (1998). Antinociceptive effect of meloxicam in neurogenic and inflammatory nociceptive models in mice. Inflamm Res, 47, 302–307.
- Sayyah M, Hadidi N, Kamalinejad M. (2004). Analgesic and anti-inflammatory activity of Lactuca sativa seed extract in rats. J Ethnopharmacol, 92, 325–329.
- Su SL, Hua YQ, Duan JA, Tang YP, Lu Y, Ding AW. (2007). In vitro inhibition on the contraction of isolated mouse uterine and chemical components of Shaofu Zhuyu decoction. J China Pharm Univ, 38, 544–548.
- Su SL, Duan JA,Wang TJ, Yu L, Hua YQ, Tang YP. (2008). Evaluating the effects of Shaofu Zhuyu decoction on hemorheology and ovarian function in rat model of Han-ning blood stasis. Ch J Exp Trad Med Form, 14, 41–43.
- Sugimoto M, Kuraishi Y, Satoh M, Takagi H. (1986). Involvement of medullary opioid-peptidergic and spinal noradrenergic systems in the regulation of formalin-induced persistent pain. Neuropharmacology, 25, 481–485.
- Sun HY, Cao YX, Liu J, Gao JW, Ma M. (2002). The establishment of the dysmenorrhea model in mice. Ch Pharm Bull, 18, 233–236.
- Sun Y, Dong Y, Jiang HJ, Cai TT, Chen L, Zhou X, Chen T, Xu Q. (2009). Dissection of the role of paeoniflorin in the traditional Chinese medicinal formula Si-Ni-San against contact dermatitis in mice. Life Sci, 84, 337–344.
- Tasaka K, Masumoto N, Miyake A, Tanizawa O. (1991). Direct measurement of intracellular free calcium in cultured human puerperal myometrial cells stimulated by oxytocin: Effects of extracellular calcium and calcium channel blockers. Obstet Gynecol, 77, 101–106.
- Tognolini M, Barocelli E, Ballabeni V, Bruni R, Bianch A. (2006). Comparative screening of plant essential oils: Phenylpropanoid moiety as basic core for antiplatelet activity. Life Sci, 78, 1419–1432.
- Tsai TY, Wu SN, Liu YC, Wu AZ, Tsai YC. (2005). Inhibitory action of L-type Ca2+ current by paeoniflorin, a major constituent of peony root, in NG108-15 neuronal cells. Eur J Pharmacol, 523, 16–24.
- van Eick AJ. (1967). A change in the response of the mouse in the “hot plate” analgesia-test, owing to a central action of atropine and related compounds. Acta Physiol Pharmacol Neerl, 14, 499–500.
- Wiener E, Levanon D. (1968). The in vitro interaction between bacterial lipopolysaccharide and differentiating monocytes. Lab Invest, 19, 584–590.
- Wu D, Mura C, Beharka AA, Han SN, Paulson KE, Hwang D, Meydani SN. (1998). Age-associated increase in PGE2 synthesis and COX activity in murine macrophages is reversed by vitamin E. Am J Physiol, 275, 661–668.
- Zhang XJ, Li Z, Leung WM, Liu L, Xu HX, Bian ZX. (2008). Analgesic effect of paeoniflorin on maternal separation induced visceral hyperalgesia in rats. J Pain, 9, 497–505.
- Zhang XL. (1987). Clinic applications of Shao-Fu-Zhu-Yu decoction. Hebei J TCM, 3, 18.
- Zhang XM. (1992). Progress of the treatment in dysmenorrhoea with traditional Chinese medicine. Ch J Pract Ch Mod Med, 5, 423–425.