Abstract
Context: Pseudorabies herpesvirus (PrV) belongs to the Alphaherpesvirinae. Piglets infected with PrV die within a few days. Development of effective antiviral agents is one alternative or complementary method to prevent PrV infection. Houttuynia cordata Thunb. (Saururaceae), H. cordata, a traditional Chinese medicine, is often used to relieve lung abnormal symptoms, infectious disease, refractory hemoptysis and malignant pleural effusion in China.
Objective: The present study aimed to investigate the effect of H. cordata injection on cell infection by PrV using Vero cells (a monkey kidney cell line) and swine testis cells (ST) as models.
Materials and methods: The infectivity of PrV was determined by plaque assays when H. cordata was applied to the virus, to the virus infected cells, and to the cells prior to infection. The genomic DNA copies post-drug treatment were confirmed by PCR and reverse transcription PCR. The cell apoptosis caused by the virus was analyzed.
Results: H. cordata efficiently inhibited cell infection after incubating the drug with PrV. Nevertheless, H. cordata was more efficient in Vero cells than in ST cells in terms of its inhibitory effect. Low-dosage drug inhibited cell apoptosis induced by PrV; nevertheless, high-dosage drug alone resulted in cell apoptosis.
Discussion and conclusion: H. cordata has a direct inhibitory activity against PrV in vitro. H. cordata may be used as an anti-PrV agent or combined with other anti-PrV agents. PrV infection induces cell apoptosis and H. cordata inhibits cell infection. The optimal administration dosage of H. cordata should be taken into account in the future, because high-dosage H. cordata alone causes cell apoptosis.
Keywords::
Introduction
Pseudorabies virus (PrV) is a major porcine pathogen that belongs to the Alphaherpesvirinae (CitationAoki et al., 1999; CitationNewby et al., 2002; CitationViejo-Borbolla et al., 2010; CitationCuranovic & Enquist, 2009). PrV can infect most mammals and some avian species; nevertheless, pig is the natural reservoir for the virus (CitationGerdts et al., 1999). Piglets infected with PrV die within a few days with acute symptoms. Some types of vaccines against PrV infection are used in China (CitationKong, 2000), which decrease the occurrence of the disease to some extent, but the application of either live or inactivated vaccines may have some inherent drawbacks, such as recovery of virulence, spread of viruses and failure to protect against new virus variants. In recent years, there are still reports regarding the occurrence of PrV infection in China (CitationCui et al., 2005; CitationZhou et al., 2007; CitationLang et al., 2008; CitationZhao, 2009). Development of effective antiviral agents is one alternative to try to prevent PrV infection.
Houttuynia cordata Thunb. (Saururaceae) is a herbaceous perennial flowering plant native to southeast Asia including China, Korea and Japan. As a traditional Chinese medicine, H. cordata has been used for centuries to relieve abnormal lung symptoms such as lung abscess, phlegm, cough and dyspnea and to cure pneumonia, infectious disease, refractory hemoptysis and malignant pleural effusion (CitationZheng et al., 1998; CitationState Pharmacopoeia Commission of People’s Republic of China, 2005; CitationLu et al., 2006). The major functional component of H. cordata is the essential oil from the Houttuynia cordata sold in China, and it has one trade name ‘Yuxingcao’, obtained by hydrodistillation. It has been reported that H. cordata has anti-microbial, immunostimulatory, diuretic, anticancer, sedative, anti-inflammatory, anti-allergic and antitussive effects (CitationPark et al., 2005; CitationLi et al., 2005; CitationLu et al., 2006; CitationKim et al., 2007). H. cordata has antiviral activities against herpes simplex virus type 1 (HSV-1), influenza virus and HIV as well as SARS-CoV (CitationHayashi et al., 1995; CitationChiang et al., 2003; CitationOno et al., 2006; CitationLau et al., 2008). Because H. cordata is very cheap and easily available in China and it may be one prospective natural products that can be used for treating PrV infection. In fact, Houttuynia cordata injection (HCI), the physical salt solution of the steam distillate from plants of H. cordata has been used in the treatment of various ailments (CitationLu et al., 2006). The aim of the current study was to evaluate the antiviral effect of HCI on PrV infection in vitro and to analyze its mode of action.
Materials and methods
Houttuynia cordata injection preparation
HCI was purchased from Shanxi Kelong Pharmacy (No. 040195211, Ruicheng, Shanxi, China). The component of the HCI was the essential oil prepared according to the standard extracting method in the Pharmacopoeia of the People’s Republic of China (CitationState Pharmacopoeia Commission of the People’s Republic of China, 2005). Briefly, the aerial parts of the fresh plant picked from the field were washed and steam distilled to obtain the essential oil. The essential oil was mixed with an aqueous solution of sodium chloride, then filtrated and sterilized, to yield HCI in the factory (CitationLu et al., 2006). The stock concentration of H. cordata was dissolved in 0.9% physiological salt solution at concentration of 20 g/10 mL. The authentication of the product was confirmed by thin layer chromatography and visualized with staining solution of dinitrophenylhydrazine against methyl nonyl ketone, the main compound in the essential oil (National Institute for the Control Pharmaceuticals and Biological Products, Beijing) as the chemical marker in accordance with the Pharmacopoeia (CitationState Pharmacopoeia Commission of People’s Republic of China, 2005).
Cell lines and virus
African green monkey kidney cell line (Vero) and swine testis (ST) cell line were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% newborn calf serum (ExCell Bio, Shanghai, China) and 5% CO2 in air. PrV strain Bartha K-61 was used in the study.
Cytotoxic assay
The cytotoxic activity of HCI was firstly evaluated by checking the morphological change of cells incubated with drugs at different concentrations. Mock-treated cells were used as control. The cells were cultured at 37°C for 48-72 h prior to observation through a light microscope. To analyze the cell proliferation, cells in 96-well plates were incubated with the drug as above. Then, conventional MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] cell viability assays were performed and the optical density (OD) of the wells was determined using a plate reader at a test wavelength of 570 nm (CitationSui et al., 2010). Cell survival rate was calculated as drug average OD value/control average OD value and 50% above cell survival rate was regarded as a non-toxic concentration of drug. The viability of cells was confirmed by trypan blue staining. Trypan blue mixture (0.5% w/v in PBS) was incubated with the cells at room temperature for 5 min prior to washing with PBS. The percentage of viable cells was determined by dividing the number of unstained cells by the total number of cells and multiplying by 100.
Virus titration and infection
Virus titration and infectivity analysis were performed according to established methods with modifications (CitationRen et al., 2008; CitationLi et al., 2009; CitationSui et al., 2010). Briefly, cells seeded in 24-well culture plates were inoculated with serially diluted viruses at 37°C for 1 h, after which they were washed three times with PBS. The inocula were replaced with 1% (w/v) methylcellulose in DMEM and incubated at 37°C for 48 h. After the overlaid medium was removed, the cells were fixed with 3% formalin in PBS for 30 min at room temperature. Then, 1% crystal violet (w/v) diluted in 5% ethanol in PBS was used to stain the cells for 20 min. The plaque number of the plates was counted and the virus titer in plaque-forming units (pfu) was calculated. The experiment was done in triplicate.
Cell infection by PrV treated with drug
Virus (1 × 106 pfu/mL) was incubated with serially diluted drugs at 37°C for 1 h and then these viruses at a multiplicity of infection (MOI) of 1 were used to infect the cells in a 24-well plate. At 24 h post-infection (hpi), the infectivity of the virus was analyzed using plaque assays as above.
Treatment of cells with drug prior to virus infection
To analyze the effect of the drug on cells, the cells were treated with H. cordata at different concentrations at 37°C for 1 h. The cells were then infected with PrV and subjected to plaque assays as above.
Effect of drug on PrV-infected cells
To evaluate the effect of H. cordata on PrV-infected cells, the cells in 24-well plates were infected with PrV at an MOI of 1 at 37°C for 1 h. The cells were treated with different concentrations of the drug at 37°C for 24-36 h. The cell infection by the viruses was analyzed as above.
PrV gD gene amplified by PCR and RT-PCR
To analyze the impact of H. cordata on the viral genome load, the amplification of PrV gD gene, a major structural gene of PrV (approximately 1200 bp in length) was performed by PCR and reverse transcription (RT)-PCR according to CitationSui et al. (2010). Briefly, virus-infected cells were subjected to DNA extraction with a commercial kit (Qiagen, Hilden, Germany). A pair of primers composed of forward primer 5′-CCCGAATTCATGCTGCTCGCAGCGC-3′ and reverse primer 5′- GGGCTCGAGACGGACCGGGCTGCGC-3′ were used to amplify the gD gene. The PCR system included 2 μL DNA template, 12.5 μL 2× GC PCR buffer, 0.5 μL each primer, 4 μL dNTP, 0.25 μL rTaq polymerase, 5.25 μL sterile H2O. The PCR parameters included 94°C for 1 min and 30 cycles of 94°C for 30 s, 60°C for 30 s, 72°C for 2 min. A final extension was done at 72°C for 10 min. RT-PCR was performed using a RT-PCR kit (TaKaRa, Dalian, China). The reverse primer for amplifying gD gene was used for RT, and the subsequent PCR procedure was performed as above.
Cell apoptosis analysis
Cell apoptosis was analyzed with Annexin V-FITC kit (Nanjing KeyGen, China). Briefly, 50 µL fluorescein isothiocyanate (FITC)-conjugated Annexin V and 50 µL propidium iodide (PI) were added into each cell-containing well of 24-well plates after the cells were infected by drug-treated viruses. The plates were then incubated at room temperature for 15 min in darkness. The fluorescence analysis was performed by fluorescence microscope (Leica, Wetzlar, Germany) at 12 hpi.
Statistical analysis
The data were analyzed using Student’s t-test, and were expressed as mean value ± standard error of the mean (SEM). A P value of less than 0.05 was considered statistically significant.
Results
Maximum nontoxic concentration of drug
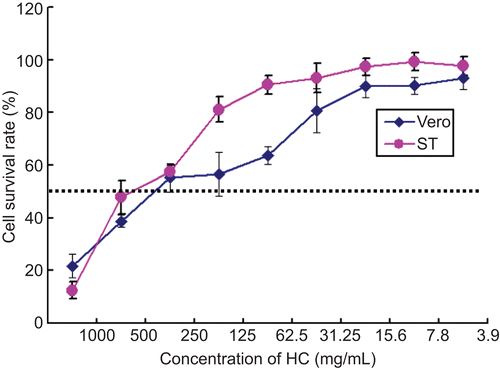
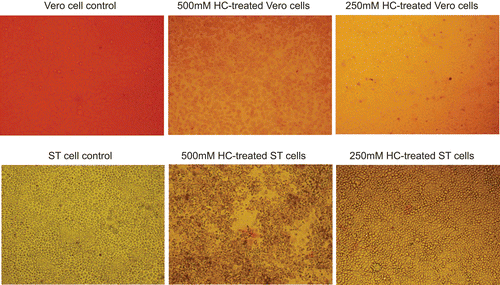
The maximum non-toxic concentrations of H. cordata diluted in DMEM medium were 250 mg/mL. Below that concentration, there was no difference between the drug-treated cells and mock-treated cells in terms of cell morphology (data not shown). MTT assay indicated that the cell proliferation was not significantly affected below the non-toxic concentrations of the drug (). Trypan blue staining showed that the both Vero and ST cells were 100% viable below the above-mentioned concentrations ().
Inhibitory effect of H. cordata on cell infection by PrV
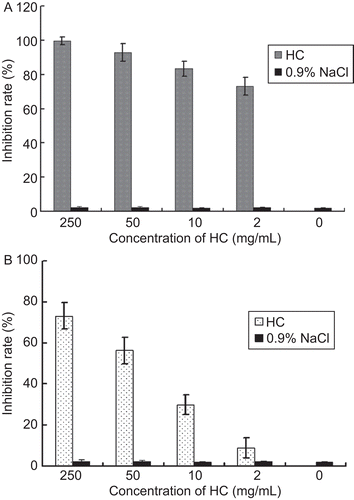
After incubation of the viruses with different concentrations of H. cordata, the cells were infected with the drug-treated viruses. As far as Vero cell infection by PrV was concerned, H. cordata at 2 mg/mL had a partial inhibition of virus infectivity (approximately 70% inhibition rate), H. cordata at 50 mg/mL had a significant inhibition and H. cordata at 250 mg/mL gave rise to complete inhibition (). When ST cells were tested, the virus infection inhibition was lower than that in Vero cells (). The solvent, 0.9% NaCl was used as a control, and only negligible effect of the solvent on virus infectivity was observed in both cell lines.
Figure 3. PrV incubated with H. cordata decreased its infectivity in cell culture. Different concentrations of H. cordata were incubated with PrV for 1 h prior to cell infection. The cell infection was analyzed by plaque assays. The plaque inhibition rate of H. cordata to the viruses in Vero cells (A), and ST cells (B), respectively, is shown.
H. cordata had no inhibitory activity against virus-infected cells and cells alone
When the effect of H. cordata on cells alone and PrV-infected cell was analyzed, no inhibitory effect was observed in either case (data not shown).
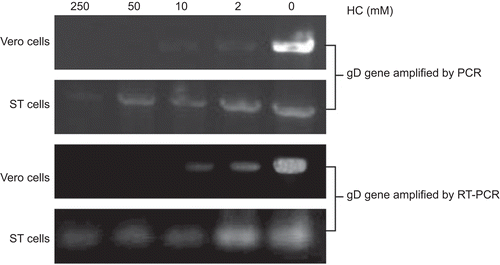
PCR and RT-PCR confirmed inhibitory effect of H. cordata on cell infection by PrV
The cells were infected with PrV after they were treated with different concentrations of H. cordata. Then the cell samples were subjected to DNA and RNA extraction followed by PCR and RT-PCR. As shown in , the genomic DNA copies decreased with the increased concentrations of the drug, which was consistent with our infection analysis. The results also confirmed the inactivation of viruses by the drug.
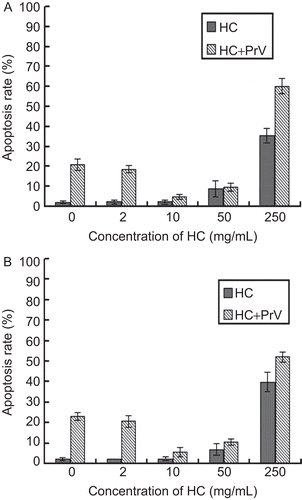
H. cordata decreased the number of apoptotic cells induced by PrV
After the cells were infected with H. cordata-treated viruses, the PrV-induced cell apoptosis was analyzed. At 12 hpi, the number of apoptosis cells decreased as the concentrations of H. cordata increased; however, when the concentration of H. cordata was higher than 50 mg/mL, the number of apoptosis cells increased (). To analyze the effect of the drug on cells, the H. cordata alone was used to treat both cell lines, and as shown in , the higher concentrations (more than 50 mg/mL) still resulted in more apoptotic cells than mock-treated cells.
Discussion
PrV infection results in serious economic loss in agriculture. In addition to vaccination, application of effective antiviral may be a complementary strategy in the future. H. cordata is a traditional medicinal plant used in China for many years for the treatment of cough, leucorrhea and urethritis. In particular, there are reports regarding its therapeutic effect in anti-inflammation, antibacterial and antiviral research. In our study we were interested in knowing the inhibitory effect of H. cordata on PrV infection in vitro. The first concern is the toxicity of the drug, therefore cell morphology observation, MTT assay and Trypan blue staining were performed to analyze the non-toxic concentration of H. cordata. These results showed that even at a relatively high concentration, there was no difference between the mock-treated and drug-treated cells, supporting the conclusion that H. cordata is a very safe and low-cost drug. It is a good prospective antiviral candidate.
To further analyze its effect on cell infection by PrV and its mode of action, the cells were infected with the drug-treated viruses. In our study, both porcine-derived cells, (ST) and monkey-derived cells (Vero) were used for comparative analysis. The results indicated the dose-dependent inhibitory effects of the drug on the infectivity of PrV and the drug had a more significant inhibition activity for the virus in Vero cells than that in ST cells. Although Vero cells are very suitable for propagating viruses including PrV, the cell line is derived from monkey, rather than pig. Therefore, the susceptibility between Vero and ST might be different. Nevertheless, in our experience, Vero cells were more susceptible to PrV infection in vitro, producing more significant cytopathic effect. The current study indicates that H. cordata does exert a direct virucidal effect and the degree of its inhibitory effect on monkey and pig cell lines is dissimilar. These findings may have implications that there are different components between Vero and ST cells mediating the interaction of PrV to cell.
In our study we found that H. cordata had no inhibitory activity against virus-infected cells and cells alone. Nevertheless, although we have no direct evidence showing the inhibitory effect of H. cordata on virus replication, we cannot exclude the possibility that the drug has effects on cell signaling which could easily lead to changes in virus replication or other steps of virus cycle. In addition, we found different susceptibility between Vero and ST cells, therefore, the effect of H. cordata on several known cellular factors such as Nectin-1, an alphaherpesvirus receptor that binds to virion glycoprotein D (gD) and mediates entry of PrV, should be analyzed in the future.
PCR analysis indicated that PrV genomic DNA copies decreased with the increased concentrations of the drug, which was consistent with our infection analysis. At the same time, RT-PCR amplifying the gD gave rise to similar results, confirming the inhibitory effect of H. cordata. The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal reference for our PCR analysis, and the housekeeping gene did not vary as previously reported (CitationSui et al., 2010). In this study we firstly selected PrV gD gene as a target gene because it is active during cell adhesion and the interaction of gD with its cellular receptor mediates the binding of PrV infected cells to uninfected cells (CitationHanssens et al., 1995). Nevertheless, there are ten more structural proteins on the surface of PrV (CitationHo et al., 1998), therefore, the effect of the drug on other genes of PrV remains unclear.
PrV is not only a vital animal pathogen, but also a useful model virus in herpes virus biology. Therefore, the virus was used as a model to analyze cell apoptosis in the study. We observed that low-dose H. cordata inhibited PrV-induced cell apoptosis, however at high-doses of H. cordata, the drug alone resulted in more apoptotic cells than mock-treated cells. The finding suggests that there is a limited dose of H. cordata application. It has been demonstrated that, during PrV replication, the host cell was induced to undergo apoptosis (CitationCheung et al., 2000) and the results of the current study confirmed that PrV infection was correlated with cell apoptosis. Also, the increased number of apoptotic cells was observed at 24 hpi in our study (data not shown). However, it would be interesting to know whether the activity of H. cordata on cell apoptosis is a possible mechanism for preventing virus infection in the future.
Conclusions
H. cordata has a direct inhibitory activity against PrV in vitro. PrV infection induces cell apoptosis and H. cordata inhibits cell infection. High-dosage H. cordata alone causes cell apoptosis.
Declaration of interest
The National Natural Science Foundations of China (30700590; 30700591; 30972195) and funding supported by the Program for New Century Excellent Talents In Heilongjiang Provincial University (1155–NCET–005) are acknowledged. The authors alone are responsible for the content and writing of the paper.
References
- Aoki H, Sakoda Y, Jukuroki K, Takada A, Kida H, Fukusho A. (1999). Induction of antibodies in mice by a recombinant baculovirus expressing pseudorabies virus glycoprotein B in mammalian cells. Vet Microbiol, 68, 197–207.
- Cheung AK, Chen Z, Sun Z, McCullough D. (2000). Pseudorabies virus induces apoptosis in tissue culture cells. Arch Virol, 145, 2193–2200.
- Chiang LC, Chang JS, Chen CC, Ng LT, Lin CC. (2003). Anti-Herpes simplex virus activity of Bidens pilosa and Houttuynia cordata. Am J Chinese Med, 31, 355–362.
- Cui S, Wei X, Song C. (2005). Analysis on the prevalence of psedorabies in China. Animal Sci Vet Med, 22, 14–15 (in Chinese).
- Curanovic D, Enquist L. (2009). Directional transneuronal spread of alpha-herpesvirus infection. Future Virol, 4, 591–603.
- Gerdts V, Jöns A, Mettenleiter TC. (1999). Potency of an experimental DNA vaccine against Aujeszky’s disease in pigs. Vet Microbiol, 66, 1–13.
- Hanssens FP, Nauwynck HJ, Mettenlieter TC. (1995). Role of glycoprotein gD in the adhesion of pseudorabies virus infected cells and subsequent cell-associated virus spread. Arch Virol, 140, 1855–1862.
- Hayashi K, Kamiya M, Hayashi T. (1995). Virucidal effects of the steam distillate from Houttuynia cordata and its components on HSV-1, influenza virus, and HIV. Planta Med, 61, 237–241.
- Ho TY, Hsiang CY, Hsiang CH, Chang TJ. (1998). DNA vaccination induces a long-term antibody response and protective immunity against pseudorabies virus in mice. Arch Virol, 143, 115–125.
- Jin S, Chen H, Xiong F. (2002). Research progress on pseudorabies gene-deleted vaccine. Sci Agr Sin, 35, 89–93 (in Chinese).
- Kim IS, Kim JH, Kim JS, Yun CY, Kim DH, Lee JS. (2007). The inhibitory effect of Houttuynia cordata extract on stem cell factor-induced HMC-1 cell migration. J Ethnopharmacol, 112, 90–95.
- Kong L. (2000). The current status of pseudorabies and the application of pseudorabies vaccines in China. Swine Production, 1, 39–40 (in Chinese).
- Lang X, Zhang J, Li Z, Li L. (2008). Diagnosis and therapy on the mixed infection of swine fever virus, porcine reproductive and respiratory syndrome virus and pseudorabies virus. Zhejiang J Animal Sci Vet Med, 33, 35–35 (in Chinese).
- Lau KM, Lee KM, Koon CM, Cheung CS, Lau CP, Ho HM, Lee MY, Au SW, Cheng CH, Lau CB, Tsui SK, Wan DC, Waye MM, Wong KB, Wong CK, Lam CW, Leung PC Fung KP. (2008). Immunomodulatory and anti-SARS activities of Houttuynia cordata. J Ethnopharmacol, 118, 79–85.
- Li GZ, Chai OH, Lee MS, Han EH, Kim HT, Song CH. (2005). Inhibitory effects of Houttuynia cordata water extracts on anaphylactic reaction and mast cell activation. Biol & Pharm Bull, 28, 1864–1868.
- Li J, Yin J, Sui X, Li G, Ren X. (2009). Comparative analysis on the effect of glycyrrhizin diammonium and lithium chloride on infectious bronchitis virus infection in vitro. Avian Pathol, 38, 215–221.
- Lu HM, Liang YZ, Yi LZ, Wu XJ. (2006). Anti-inflammatory effect of Houttuynia cordata injection. J Ethnopharm, 104, 245–249.
- Newby TJ, Carter DP, Yoon KJ, Jackwood MW, Hawkins PA. (2002). Assessment of replication and virulence of attenuated pseudorabies virus in swine. J Vet Sci, 3, 61–66.
- Ono E, Tomioka Y, Watanabe Y, Amagai K, Taharaguchi S, Glenisson J, Cherel P. (2006). The first immunoglobulin-like domain of porcine nectin-1 is sufficient to confer resistance to pseudorabies virus infection in transgenic mice. Arch Virol, 151, 1827–1839.
- Park E, Kum S, Wang C, Park SY, Kim BS, Schuller-Levis G. (2005). Antiinflammatory activity of herbal medicines: inhibition of nitric oxide production and tumor necrosis factor-alpha secretion in an activated macrophage-like cell line. American J Chinese Med, 33, 415–424.
- Ren X, Glende J, Yin J, Schwegmann-Wessels C, Herrler G. (2008). Importance of cholesterol for infection of cells by transmissible gastroenteritis virus. Virus Res, 137, 220–224.
- State Pharmacopoeia Commission of the People’s Republic of China. (2005). Pharmacopoeia of the People’s Republic of China, Vol. I. Beijing: Chemical Industry Press, pp. 155.
- Sui X, Yin J, Ren X. (2010). Antiviral effect of diammonium glycyrrhizinate and lithium chloride on cell infection by pseudorabies herpesvirus. Antiviral Res, 85, 346–353.
- Viejo-Borbolla A, Muñoz A, Tabarés E, Alcamí A. (2010). Glycoprotein G from pseudorabies virus binds to chemokines with high affinity and inhibits their function. J Gen Virol, 91, 23–31.
- Zhao X. (2009). Diagnosis and therapy on mixed infection of swine fever, pseudorabies, and Eperythrozoon Suis. Shanghai J Animal Hus Vet Med, 3, 98–99 (in Chinese).
- Zheng HZ, Dong ZH, She J. (1998). Houttuynia cordata. In: Zheng HZ, Dong ZH, She J, eds, Modern Study of Traditional Chinese Medicine, Vol. 3. Xue Yuan Press, Beijing, pp. 2983–3003.
- Zeng Z, Tang D, Guo W. (2007). Research on pseudorabies vaccines. World Agriculture, 10, 66–69 (in Chinese).
- Zhou X, Li G, Li J, Qin Y. (2007). Strengthening immunization and surveillance to eradicate pseudorabies-investigation on the prevalence of pseudorabies field isolates among nine provinces of China in 2006. Swine Industry Sci, 24, 78–80 (in Chinese).