Abstract
Context: Extraction techniques may alter the antibacterial activity of Coptidis Rhizoma (C. Rhizoma), which is regarded as characteristic of this herb.
Objective: To explore the best extraction techniques of C. Rhizoma and the fraction(s) with the strongest antibacterial activity.
Methods: Using microcalorimetry, the influence of different extraction fractions of C. Rhizoma obtained by decoction, reflux, and ultrasound techniques on Escherichia coli growth was investigated by analyzing the power–time curves and some thermokinetic parameters. Then the antibacterial activities of each fraction of C. Rhizoma were compared by analysis of variance (ANOVA). Meanwhile, the minimum inhibitory concentration (MIC) of the strongest antibacterial fraction was determined by 2-fold dilution method.
Results and conclusion: The petroleum ether (PE), chloroform (CHCl3), ethyl acetate (EtOAc), and n-butyl alcohol (n-BuOH) fraction, water extract, and the residue after extraction all inhibited the growth of E. coli. The potency of the inhibitory effects was as follows: n-BuOH fraction > EtOAc fraction > CHCl3 fraction > PE fraction > residue after extraction > water extract. The decoction technique was regarded as the optimum extraction technique. The n-BuOH fraction from the decoction technique was observed to have the strongest antibacterial fraction with half-inhibitory concentration IC50 of 1.68 mg/mL and MIC of 200 μg/mL.
Introduction
Coptidis Rhizoma (C. Rhizoma, Huanglian in Chinese), the dried rhizome of Ranunculaceae plants such as Coptis chinensis Franch. and Chaenomeles japonica Makino, is officially listed in the Chinese Pharmacopoeia (China Pharmacopoeia Committee, 2005). It possesses anti-inflammatory and antimicrobial activity as well as desiccatory effects (CitationMou & Paul, 1987; CitationLee et al., 2003; CitationYu et al., 2005). Studies have shown that many pharmacologically active compounds have been isolated from C. Rhizoma (CitationWei et al., 2007; CitationXu & Liu, 2007; CitationYing et al., 2007; CitationYu et al., 2007). The water extracts have been reported to possess strong antimicrobial activities (CitationXu et al., 2007; CitationKong et al., 2009a). The organic solvent extracts with antibacterial activity have been used in treating dysentery, cholera, diabetes, and lung cancer diseases (CitationDing, 1999). However, there is no report of searching for the best extraction techniques and organic solvent fraction with the strongest antibacterial activity of C. Rhizoma.
In any living system, various metabolic events following heat-producing reactions occur. Therefore, the thermogenic curve reflecting the metabolic processes of living cells can be measured by sensitive calorimeters. Microcalorimetry is a nondestructive and noninvasive technique with high sensitivity. Using this microcalorimetric method, the whole metabolic processes of the living system affected by other substances, such as drugs, heavy metal, and so on (CitationLi et al., 2002; CitationKong et al., 2008a–c; CitationYao et al., 2008), can be recorded automatically and continuously. More useful information about the metabolism can be revealed by this method than the existing methods (CitationMcGulnness & Barisas, 1991). It has been widely used in biological experiments and different fields of life sciences (CitationIngemar, 2002; CitationYao et al., 2003; CitationYang et al., 2004 CitationZheng et al., 2006; CitationKong et al., 2008a,Citationb), and can determine the energy transfer and caloric change during the metabolic processes of organisms such as Escherichia coli B growth affected by other materials (CitationLi et al., 2003; CitationYu et al., 2004).
So in this study microcalorimetry was applied to investigate the effects of different extraction fractions of C. Rhizoma on E. coli growth to screen for the best extraction techniques of this herb and fraction(s) with the highest bioactivity, moreover to find the minimum inhibitory concentration (MIC) of the strongest bioactive fraction. The work provides a useful tool to study the interaction of drugs and microbe and advances idea of searching for the best extraction technique and bioactive fraction of traditional Chinese medicines (TCM).
Materials and methods
Apparatus
A 3114/3236 Thermal Activity Monitor (TAM) air isothermal calorimeter (Thermometric AB, Sweden) was used to determine the metabolism of cells. As an eight-channel twin instrument, it can continuously monitor a wide variety of processes and complex systems in a temperature range of 5°C–60°C with the detection limitation of 2 µW, and baseline stability (over a period of 24 h) is 6 µW. Each measuring cylinder contains a sample and a reference in separate measuring cups (twin system). The heat output from the sample flows via the thermoelectric detector to a large heat sink (in close contact with the water bath). Picolog software (Pico Technology, USA) is used to process the data. The detailed description about this apparatus has been reported in CitationWadsö (2002) and CitationKong et al. (2008b).
Plant material and bacterial strain
C. Rhizoma (No. 070605), the rhizome of C. chinensis Franch. (Ranunculaceae), was collected in June 2007 from Anguo city, Hebei province, China, and was identified by Xiao-he Xiao, plant taxonomist, Institute of Chinese Materia Medica, Beijing. Other chemicals used in the experiments were of analytical grade, and double-distilled water was used to prepare all solutions.
The E. coli strain (E. coli CMCC B44103) was provided by the Chinese Center for Type Culture Collections, NICPBP, Beijing. They were inoculated in Luria-Bertani (LB) culture medium and preserved at 4°C. LB culture medium, a solution (pH 7.0–7.2) containing 10 g/L tryptone, 5 g/L yeast extract, and 5 g/L NaCl, was sterilized in high-pressure steam at 121°C for 30 min before use.
Extraction of samples
Extraction by decoction technique
C. Rhizoma were crushed into powder and filtered through an 80-gauge screen mesh. The powder (300 g) was extracted with 3000 mL double-distilled water and decocted in a saucepan. The water decoction was further extracted by 500 mL of each solvent in sequence to get petroleum ether (PE), chloroform (CHCl3), ethyl acetate (EtOAc), n-butyl alcohol (n-BuOH), and residual fractions.
Extraction by refluxing technique
C. Rhizoma were crushed into powder and filtered through an 80-gauge screen mesh. The powder (300 g) was extracted with 3000 mL double-distilled water using a round-bottom flask. The extraction was further extracted by the organic solvents in sequence to get different extraction fractions.
Extraction by ultrasound
C. Rhizoma were crushed into powder and filtered through an 80-gauge screen mesh. The powder (300 g) was extracted with 3000 mL double-distilled water using an ultrasonic washer. The extraction was further extracted by the organic solvents in sequence to get different extraction fractions.
Determination of the thermogenic curves of E. coli growth
The metabolic thermogenic curves of E. coli growth without and with different fractions were determined by microcalorimetry using the ampoule method. Initially, E. coli were cultivated in the incubator at 37°C. They were inoculated in the prepared 5 mL culture medium using a 20-mL glass ampoule, and the initial population density was 1 × 106 colony-forming units (CFU)/mL. The freshly prepared solution of each extraction fraction was added into the ampoule. The glass ampoule was then sealed with a cap and put into the microcalorimeter. The growth process of E. coli affected by different fractions was monitored continuously by this microcalorimeter and the thermogenic power–time curve was determined. All the experiments were carried out under aseptic condition (CitationZhu et al., 2006; CitationKong et al., 2009b).
Statistical analysis
The analysis of variance (ANOVA) followed by Dunnett’s many-to-one multiple comparison tests were applied appropriately to observe whether there were significant differences in the antibacterial activities of these fractions obtained by different extraction techniques. The Wilks’s lambda statistic was preferred to obtain P-values in this analysis and P < 0.05 was considered statistically significant between groups (CitationDuncan, 1957). For this ANOVA, SAS versions 6.12 and 8.0 software for Windows (SAS, Cary, NC) was employed.
Determination of MIC
MIC of n-BuOH fraction on E. coli growth was determined by 2-fold dilution of solution of n-BuOH fraction (0–500.0 μg/mL) in test tubes using fresh LB culture medium. Each test tube was inoculated with bacterial suspension containing 1 × 105 CFU/mL cells and incubated at 37°C for 24 h. The lowest concentration that visibly showed no growth compared with drug-free culture medium inoculated with microbial suspension was considered the MIC (CitationHindler & Isenberg, 1992).
Results
Growth rate constant (k) of E. coli
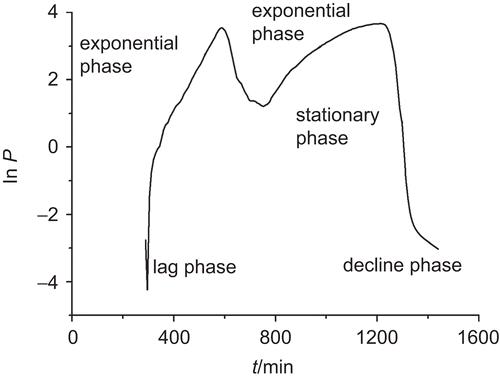
The metabolic curve of E. coli growth in culture medium at 37°C without drug was recorded and shown in . This power–time curve was a typical growth characteristic of E. coli and could be divided into five phases: a lag phase, the first exponential phase, the second exponential phase, a stationary phase, and a decline phase. The peaks and trough are characteristic of organism growth on LB culture medium and with restricted (only the head space within the ampoule) oxygen availability. It is plausible that the first exponential phase seen represents aerobic metabolism, which is then followed by a change to anaerobic metabolism, that is, the second exponential phase (CitationMichael et al., 2003). In the exponential phase, the cell growth is an exponential mode. If the cell number is n0 at time 0, and nt at time t, then
Figure 1. The power–time curve of E. coli growth at 37°C without any substance. E. coli was cultured in LB culture medium and monitored using a TAM air isothermal calorimeter, ampoule method at 37°C.
If the heat power output of each cell is w, then
P0 = n0w and Pt = ntw, giving Pt = P0 exp(kt), or
where P0 represents the heat-output power at time t = 0 and Pt at time t. The exponential phase of E. coli growth corresponded to Equation (3). Using this equation, the growth rate constant k () was calculated by fitting ln Pt and t to a linear equation.
Table 1. Growth rate constants k1 and k2 of E. coli growth at 37°C.
shows k1 = (0.112 ± 0.00021)/min and R all exceeded 0.997, k2 = (0.00887 ± 0.00036)/min and R all exceeded 0.998, indicating good reproducibility and stability of this microcalorimetric method. Subsequent k1 and k2 were the growth rate constants of the first and second exponential phases, respectively. The second exponential phase could be regarded as stable growth stage of the bacteria because k2 was much less than k1 (CitationLiu et al., 2003).
The power–time curves and thermokinetic parameters of E. coli growth affected by each fraction
The power–time curves of E. coli growth affected by each fraction of C. Rhizoma were shown in , and the corresponding thermokinetic parameters were listed in . The final concentration of each fraction in the LB culture medium was 2 mg/mL.
Table 2. The thermokinetic parameters of E. coli growth at 37°C affected by C. Rhizoma extractions at 2 mg/mL.
Figure 2. The power–time curves of E. coli growth at 37°C affected by C. Rhizoma fractions obtained from (A) decoction, (B) refluxing, and (C) ultrasound techniques. The various factions were (a) control, (b) water extraction, (c) residue, (d) PE fraction, (e) CHCl3 fraction, (f) EtOAc fraction, and (g) n-BuOH fraction with the final concentration of 2.00 mg/mL in the LB culture medium.
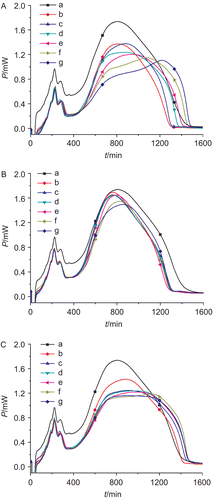
shows that the profiles of power–time curves of E. coli growth changed with the addition of each C. Rhizoma fraction, illustrating that the fractions all had influences on E. coli growth. showed that in comparison with the control (without drug), the lag and exponential phases became longer, k1 and k2 and the maximum heat-output power (the height of the highest peak) Pm decreased, while the appearance time of Pm increased, which indicated that the bacterial culture took longer to produce a sufficient number of cells for a detectable signal, and excess drugs inhibited the growth of E. coli or killed the bacterial cells. These data illustrated that each fraction of C. Rhizoma had anti-E. coli activity.
The data in were input into the SAS software for ANOVA and the results are shown in . From this table, it is apparent that the F-values for optional two groups among decoction, reflux, and ultrasound extractions were all greater than F0.05,4,7 = 4.12 with corresponding P-value smaller than 0.05, showing that there were significant differences for the antibacterial activities of the fractions of these three extraction techniques.
Table 3. ANOVA results for three extraction groups.
It has been reported that the smaller the values of k1, k2, and Pm and the bigger the values of tm were, the stronger the antibacterial activity the drug possesses (CitationLi et al., 2003). General comparison of the parameters in for each extraction group, it could be found that the values of k1, k2, and Pm of the decoction extraction group were the smallest, and tm were the biggest among the three extraction groups, showing that the antibacterial activities of these fractions obtained from decoction techniques were stronger than those obtained by the other two extraction techniques. These also illustrated that the active components in these fractions obtained by decoction techniques were much more sufficient than the other two extraction techniques. Further, it could be concluded that the fractions obtained from decoction could well reflect the antibacterial characteristics of C. Rhizoma. So decoction was selected as the optimum extraction method of C. Rhizoma.
Returning to the values in , we found that the potency of antibacterial activity of the fractions was as follows: n-BuOH fraction > EtOAc fraction > CHCl3 fraction > PE fraction > residue after extraction > water extract. The n-BuOH fraction with the strongest antibacterial activities was selected as the strongest antibacterial fraction of C. Rhizoma. The antibacterial activities of n-BuOH fraction are evaluated specifically in the next section.
The antibacterial activity of n-BuOH fraction
The experimental procedure was the same as above. The final concentration of n-BuOH fraction in each ampoule was 0, 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5 mg/mL. The power–time curves of E. coli growth at 37°C affected by n-BuOH fraction got from decoction technique were recorded and shown in and the corresponding thermokinetic parameters were listed in .
Table 4. The thermokinetic parameters of E. coli growth at 37°C affected by n-BuOH fraction of C. Rhizoma.
Figure 3. The power–time curves of E. coli growth at 37°C affected by n-BuOH fractions of C. Rhizoma got obtained by decoction techniques at concentrations of (a) 0, (b) 0.5, (c) 1.0, (d) 1.5, (e) 2.0, (f) 2.5, (g) 3.0, and (h) 3.5 mg/mL.
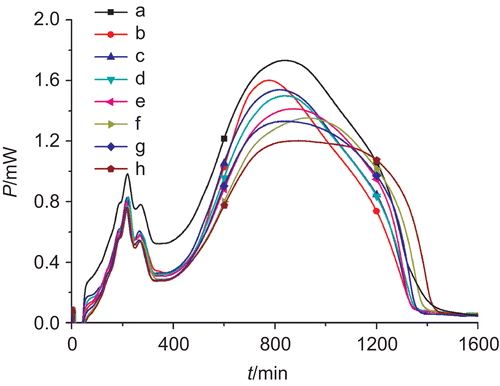
The power–time curves in showed that the highest peaks were lowered with increasing concentrations of n-BuOH fraction, showing that this fraction had strong inhibitory activities on E. coli, and the inhibitory extent was strengthened with the increased concentration. The values of thermokinetic parameters in further illustrate the above results: the values of k1, k2, and Pm decreased and tm increased gradually with increasing the concentration of n-BuOH fraction, showing that the antibacterial activities were enhanced.
Growth inhibition ratio I
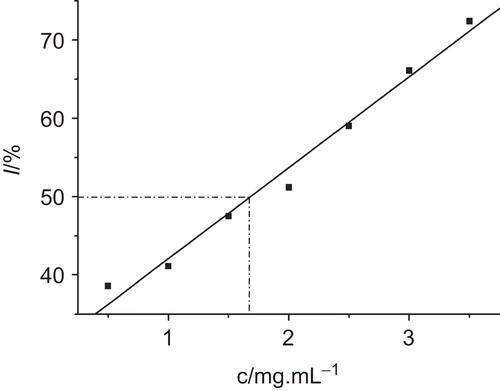
I was calculated on the basis of growth rate constant and could be defined as:
where k0 and kc were the growth rate constants of E. coli in the absence of drug and inhibited at inhibitor concentration c, respectively. When I is 50%, the corresponding inhibitory concentration is called half-inhibitory concentration, IC50. IC50 can be regarded as the inhibitory concentration that produced a 50% decrease in growth rate constant. From the relationship between I and c in , the value of IC50 (1.68 mg/mL) was calculated and also listed in .
MIC (200.0 μg/mL) of n-BuOH fraction on E. coli growth also showed the strong antibacterial activity of this fraction.
Discussion and conclusion
In this study, we proposed a useful tool—microcalorimetry—to screen the extraction technique and bioactive fraction of TCM. This tool can determine the metabolic curve of microbes such as E. coli at a constant temperature of 37°C affected by other substances, and can obtain thermokinetic parameters reflecting the influence of these substances on the growth of the microbe. Microcalorimetry not only supplies a new point of view for the evaluation of the bioactivity of drugs but also provides more information about the microbial growth. All this information could describe the microbe–drug reaction when drugs are added into the culture medium, providing some experimental foundations for deeply studying the pharmacodynamic actions of these TCMs.
The antibacterial activities of the extraction fractions of C. Rhizoma obtained from three extraction methods (decoction, reflux, and ultrasound techniques) were investigated by microcalorimetry in this study. The fractions obtained from decoction had much stronger antibacterial activities than those from the other two extraction methods and decoction was screened as the optimal extraction method of this herb. The n-BuOH fraction with the strongest antibacterial activity among the organic solvent fractions was selected as the bioactive fraction of this herb. All these were suitable for the clinical medication principle and provided some references for the extraction of other commonly used TCM herb. The active components were extracted more completely from C. Rhizoma by decoction, fully expressing the pharmacodynamic actions of this herb. However, some problems still existed; for example, the active components and their structures in this herb obtained from decoction might be destroyed or lost through volatilization because of the high temperature of this method; some pharmaceutical actions might be weakened. All these problems must be addressed in a future study.
Declaration of interest
The authors are grateful for the support of the National Basic Research Program of China (973 project) (2007CB512607), the National Youth Science Foundation (30625042), and the National Natural Science Foundation (30772740).
References
- China Pharmacopoeia Committee. (2005). Pharmacopoeia of the People’s Republic of China, 1st Div. Beijing: China Chemical Industry Press, pp. 213–214.
- Ding DM. (1999). Pharmacodynamic Action and Clinic of Chinese Medicinal Materials. Beijing: China Medicine Technology Press, pp. 54–56.
- Duncan BD. (1957).Multiple range tests for correlated and heteroscedastic means. Biometrics 13:359–364.
- Hindler J, Isenberg HD. (1992).Clinical Microbiology Procedures Handbook. Washington, DC: American Society of Microbiology.
- Ingemar W. (2002).Isothermal microcalorimetry in applied biology. Thermochim Acta 6:394–398.
- Kong WJ, Wang JB, Jin C, Zhao YL, Dai CM, Xiao XH, Li ZL. (2009).Effect of emodin on Candida albicans growth investigated by microcalorimetry combined with chemometric analysis. Appl Microbiol Biotechnol 83:1183–1190.
- Kong WJ, Zhao YL, Shan LM, Xiao XH, Guo WY. (2008a).Investigation of the effect of four organic acids in Radix Isatidis on E. coli growth by microcalorimetry. Chin J Chem 26:113–115.
- Kong WJ, Zhao YL, Shan LM, Xiao XH, Guo WY. (2008b).Microcalorimetric studies of the action on four organic acids in Radix Isatidis on the growth of microorganisms. Chin J Biotech 24:646–650.
- Kong WJ, Zhao YL, Shan LM, Xiao XH, Guo WY. (2008c).Investigation on the spectrum–effect relationships of EtOAc extract from Radix Isatidis based on HPLC fingerprints and microcalorimetry. J Chromatogr B Analyt Technol Biomed Life Sci 871:109–114.
- Kong WJ, Zhao YL, Xiao XH, Wang JB, Li HB, Li ZL, Jin C, Liu Y. (2009).Spectrum–effect relationships between ultra performance liquid chromatography fingerprints and anti-bacterial activities of Rhizoma Coptidis. Anal Chim Acta 634:279–285.
- Lee DU, Kang YJ, Park MK, Lee YS, Seo HG, Kim TS, Kim CH, Chang KC. (2003).Effects of 13-alkyl-substituted berberine alkaloids on the expression of COX-II, TNF-alpha, iNOS, and IL-12 production in LPS-stimulated macrophages. Life Sci 73:1401–1412.
- LiGS, Liu Y, Chen XD, Liu P, Shen P, Qu SS. (2003).Study on interaction between T4 phage and Escherichia coli B by microcalorimetric method. J Virol Methods 112:137–143.
- Li X, Liu Y, Wu J, Liang HG, Qu SS. (2002).Microcalorimetric study of Staphylococcus aureus growth affected by selenium compounds. Thermochim Acta 387:57–61.
- Liu GS, Liu Y, Chen XD, Liu P, Shen P, Qu SS. (2003).Study on interaction between T4 phage and Escherichia coli B by microcalorimetric method. J Virol Methods 112:137–143.
- McGulnness MS, Barisas BG. (1991).Acute toxicity measurements on aquatic pollutants using microcalorimetry on tissue-cultured cells. Environ Sci Technol 25:921.
- Michael AAO, George JV, Anthony E, Alistair HB, Jonathan H, Chloé L, Michael W, Phillip GB. (2003).Antimicrobial properties of silver-containing wound dressings: a microcalorimetric study. Int J Pharm 263:61–68.
- Mou HC, Paul PH. (1987).Pharmacology and Applications of Chinese Materia Medica, Vol. II. World Scientific, Beijing pp. 1061 ff.
- Wadsö I. (2002).Isothermal microcalorimetry in applied biology. Thermochim Acta 394:305–311.
- Wei JQ, Jiang WZ, Jiang SY, Jiang YS, LiQL, Ling L. (2007).Determination of the content of dehydrocavidine in Corydalis saxicola bunting by RP-HPLC. Lishizhen Med Mater Med Res 18:2077–2079.
- Xu Y, Liu HX. (2007).Study on the extraction of berberine from Coptis chinensis by microwave. Lishizhen Med Mater Med Res 18:2231–2232.
- Xu Y, Zhou SW, Tang JL, Huang YP, Chen S. (2007).Protection of renal function by extraction total alkaloids from Rhizoma Coptidis on experimental diabetic nephropathy rats. Chongqing Med 36:526–531.
- Yang Y, Liu Y, Zhu J, Qu SS. (2004).Microcalorimetric study on the transcription start site mutagenesis. J Therm Anal Calorim 3:293–295.
- Yao J, Liu Y, Tuo Y, Liu JB, Chen X, Zhou Q, Dong JX, Qu SS, Yu ZN. (2003).Action of Cu2+ on Bacillus thuringiensis growth investigated by microcalorimetry. Appl Biochem Microbiol 39:656–660.
- Yao J, Tian L, Wang F, Chen HL, Xu CQ, Su CL, Cai MF, Maskow T, Zaray G, Wang YX. (2008).Microcalorimetric study on effect of chromium (III) and chromium (VI) species on the growth of Escherichia coli. Chin J Chem 26:101–106.
- Ying Y, He ZH, Zhou SW, Tang JL, Yang X, Wang L. (2007).Determination of the alkaloids in Rhizoma Coptidis by HPLC. Acta Academic Med Militaris Tert 29:843–845.
- Yu HH, Kim KJ, Cha JD, Kim HK, Lee YE, Choi NY, You YO. (2005).Antimicrobial activity of berberine alone and in combination with ampicillin or oxacillin against methicillin-resistant Staphylococcus aureus. J Med Food 8:454–461.
- Yu S, Pang XY, Deng YX, Liu L, Liang Y, Liu XD, Xie L, Wang GJ, Wang XT. (2007).A sensitive and specific liquid chromatography mass spectrometry method for simultaneous determination of berberine, palmatine, coptisine, epiberberine and jatrorrhizine from Coptidis Rhizoma in rat plasma. Inter J Mass Spectro 268:30–37.
- Yu Y, Nie Y, Sun YX, Lu HF, Zhang HL, Kong FQ. (2004).Microcalorimetric investigation of artemisia capillaris thumb for bacterial activity. J Qufu Norm Univ 1:72–74.
- Zheng D, Liu Y, Zhang Y, Chen XJ, Shen YF. (2006).Microcalorimetric investigation of the toxic action of Cr(VI) on themetabolism of Tetrahymena thermophila BF5 during growth. Environ Toxicol Pharm 22:121–127.
- Zhu JC, Liu Y, Wong WK, Zhou B, Yin J. (2006).Investigation of antibacterial activity of two kinds of novel schiff bases on Escherichia coli by microcalorimetry. Chin J Chem 24:1295–1300.