Abstract
Context: Histonedeacetylase inhibitors (HDACi) are the focus of enormous interest as a new class of anticancer drugs and discussed as novel treatment in cardiovascular diseases or memory enhancement. In the search for new active substances the structural diversity of secondary plant metabolites provides an indispensable resource. Several molecules from the plant kingdom have gained importance as anticancer drugs. Thus, a search for new therapeutic agents inhibiting histonedeacetylases (HDACs) is an important topic. To accelerate the isolation of potential candidates from plants bioassays well suited for screenings of extracts are an indispensable prerequisite.
Objective: In the presented study an enzymatic assay was modified, optimized and validated for the search for HDACi from plant origin.
Materials and methods: A fluorimetric assay was validated with respect to parameters such as temperature, incubation times, reproducibility, applicability of different enzyme sources and HDAC substrate. For the determination of the HDAC inhibitory potential of extracts the detailed study of the influence of different classes of primary and secondary metabolites probably interfering with the assay was most important.
Results: In the experimental design autofluorescent coumarins and tannins were identified to disrupt the assay. Possibilities to avoid disturbances are demonstrated and the applicability of the method in the bioactivity-guided search for new HDACi was proven on the example Leonuri herba (Leonurus cardiaca L.; Lamiaceae).
Conclusion: The optimization of the assay led to a highly efficient fluorimetric method to study plant extracts and fractions of medium/high polarity for HDAC inhibition. In the bioactivity-guided fractionation of extracts from Leonuri herba the applicability for the aimed purpose was clearly demonstrated.
Introduction
The purpose of this study was the optimization and the application of an in vitro histone deacetylase (HDAC) assay for the bioactivity-guided identification of new histone deacetylase inhibitors (HDACi) from plant origin.
Generally, HDACs comprise a family of enzymes that can deacetylate histones but also transcription factors including p53, FOXO, NF-κB and thereby regulate gene expression (CitationKim et al., 2009). In humans, eleven HDACs and seven sirtuins (SIRTs) have been identified and partly characterized until now. HDACs are divided into four classes, HDACs of classes I, II, and IV are zinc-dependent enzymes, whereas those of class III, known as SIRTs, are NAD-dependent. Class I includes HDAC1, HDAC2, HDAC3, and HDAC8, whose molecular masses range from 42 to 55 kDa and which are located in the nucleus. Class II HDACs, presented by HDAC4, HDAC5, HDAC6, HDAC7, HDAC9, and HDAC10, are larger with molecular masses from 120 to 130 kDa. These enzymes shuttle between the nucleus and the cytoplasm. Very little information is available for class IV, HDAC11 is the only representative enzyme of this group. Class III comprises the seven sirtuins SIRT1–SIRT7 which do not target histones as their primary substrate (CitationWitt et al., 2009).
The physiological role of HDACs in normal tissues and in organism development has been studied by generating knock out mice lacking one specific HDAC. Phenotypes of class I (HDAC1, 2, or 3) knock out mice resulted in defects of embryonic stem cell proliferation and multiple cardiac defects and led to an early embryonic lethality. Class II knock out mice developed defects in different tissues and organs, and the loss of HDAC7 led to embryonic death as well. Thus, HDACs of class II have been shown to be essential for the appropriate maturation of heart, smooth muscles, brain, kidney, liver, spleen, and pancreas (CitationWitt et al., 2009).
Imbalances in histone acetylation and/or deacetylation are assumed to play a crucial role in many “Western diseases” such as cancer, cardiac hypertrophy, neurodegenerative disorders, inflammation, and viral infection (CitationZhang et al., 2009).
Thus, HDACi have gained a lot of interest during the last decade. They are structurally diverse molecules which can induce growth arrest, differentiation, apoptosis, and autophagocytic cell death of cancer cells (CitationDokmanovic & Marks, 2005; CitationBolden et al., 2006; CitationRopero & Esteller, 2007; CitationFrew et al., 2009; CitationMarks & Xu, 2009; CitationOka et al., 2009; CitationSmith & Workman, 2009) and therefore have received high interest as anticancer agents (CitationFrew et al., 2009; CitationMarks & Xu, 2009). By epigenetic chromatin remodeling they influence tumor growth by repression of angiogenesis or by increasing tumor cell immunogenicity (CitationOgbomo et al., 2007). Clinical trials are studying the effects of several HDACi on different tumor types (CitationBolden et al., 2006; CitationOgbomo et al., 2007; CitationCarew et al., 2008; CitationFrew et al., 2009; CitationMarks & Xu, 2009; CitationOka et al., 2009). The contribution of HDACs to cardiovascular diseases is under investigation (CitationOlson & Schneider 2003; CitationGranger et al., 2008; CitationOka et al., 2009) and the influence of HDACs on different neural processes e.g. memory enhancement and synaptic plasticity has also been discussed (CitationGuan et al., 2009; CitationStefanko et al., 2009).
Most of the known HDACi exert a rather unselective impact on HDAC activity. Some of such pan-inhibitors, either natural or synthetic compounds, are already applied in clinical trials against cancer, but induce adverse effects such as cardiac complications. The majority of these HDACi are microbial metabolites: e.g., romidepsin or trichostatin A (TSA) are of bacterial origin, whereas depudecin, trapoxin A and B are isolated from fungi. Novel marine natural products such as largozole, apicidine, azumamides, and psammaplin A have shown HDAC inhibitory activity at nanomolar concentrations (CitationNewkirk et al., 2009).
Until now only few plant metabolites with HDAC inhibitory effects are known. Flavone and flavone derivatives from Feijoa sellowiana Bert. (Myrtaceae) inhibited HDAC1 activity (CitationBontempo et al., 2007). A sulfur compound from the roots of Pleuropterus ciliinervis Nakai (Polygonaceae) is a potent HDACi, with an IC50 value of 1.43 µM (CitationSon et al., 2007). Investigations of the rhizomes of Zingiber zerumbet L. (Zingiberaceae) provided a new insight into the anticancer efficacy of this plant. Two major sesquiterpenoids, 6-methoxy-2E,9E humuladien-8-one and zerumbone, were tested in vitro for HDAC inhibition and IC50 values of 1.25 and 8.35 µM, respectively, were determined (CitationChung et al., 2008). A recent study of the methanol extract of the stem of Microtropis japonica (Franchet & Savatier) Hall. f. (Celastraceae) has revealed the potential of triterpenoides and ursolic acid to inhibit the HDAC activity at concentrations of 20 µg/ml as shown by the increase in the acetylated state of histone 3 (CitationChen et al., 2009).
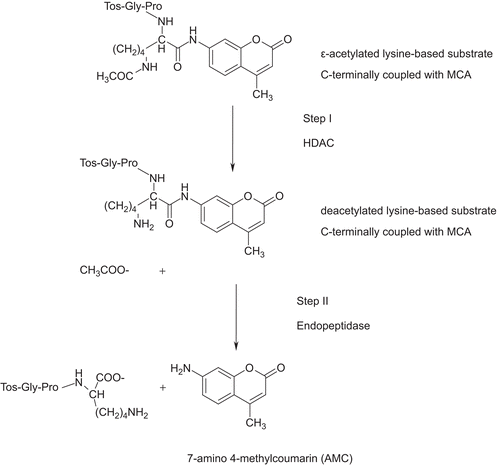
The challenge is to identify new compounds, which are effective and have particular function on “disease-related” HDACs. First examples of active plant compounds (see above) underline that in this search for selective HDAC modulators the almost infinite structural biodiversity of plant kingdom could provide new promising substances for further development. To speed-up the isolation of respective candidates in a bioactivity-guided approach, a suitable test method is indispensable. In this study a fluorimetric in vitro enzymatic assay developed earlier (CitationWegener et al., 2003) was adapted and validated for the investigation of the influence of plant compounds on the activity of class I and class II HDACs. The principle of the assay is based on the following reactions (): an ϵ-acetylated lysine-based substrate C-terminally coupled with 4-methyl-coumarin-7-amide (MCA) is deacetylated by HDAC. The deacetylated substance is recognized as substrate by an endopeptidase which releases 7-amino 4-methylcoumarin (AMC). Free AMC, in contrast to acetylated MCA, is highly fluorescent and can be monitored (CitationWegener et al., 2003; CitationMazitschek et al., 2008). To the best of our knowledge, this method has so far been employed to test pure compounds only and not yet been tested with complex mixtures nor been optimized and validated for the investigation of plant extracts. We therefore established a fast, cheap and reliable protocol of the assay that is applicable for medium to high throughput screening of natural products including complex extracts for HDAC inhibition.
Figure 1. Schematic overview over the assay reactions (CitationWegener et al., 2003). In step I the enzyme was incubated with the acetylated substrate and in step II by subsequent addition of an activator solution which contains endopeptidase and TSA the enzymatic reaction was stopped and the fluorophore was released.
Materials and methods
Cell lines, chemicals, and biochemicals
TSA was purchased from Sigma-Aldrich (Vienna, Austria). HDAC unacetylated substrate from Upstate Biotechnology (Lake Placid, NY) was used for assay calibration. All other pure compounds and reagents were obtained from Sigma-Aldrich (Vienna, Austria) or Carl Roth (Karlsruhe, Germany). The acetylated substrate was kindly provided by Ph.D. Ralph Mazitschek, Massachusetts General Hospital, Boston.
Primary rat vascular smooth muscle cells (VSMCs, kindly provided by Dr. A. Schwaiberger, University of Vienna) as well as HeLa cells (kindly provided by a.o. Prof. Dr. G. Krupitza, Medical University of Vienna) were cultured in Dulbecco’s modified Eagle medium supplemented with 10% calf serum, penicillin (1000 U/l) and streptomycin (1 mg/l) (all from LONZA, Belgium) and incubated at 37°C under an atmosphere of 5% CO2 in air.
Human umbilical vein endothelial cells (HUVECs, kindly provided by Dr. Y. Schilder, University of Vienna) were cultivated in endothelial basal medium supplemented with 10% fetal calf serum, penicillin (1000 U/l), streptomycin (1 mg/l) and additives provided by the manufacturer (LONZA, Belgium) and incubated at 37°C under an atmosphere of 5% CO2 in air.
Control HeLa nuclear extract was purchased from Upstate and used for the comparison of the activity of self-isolated nuclear extracts from VSMC, HUVECs, and HeLa cells prepared according to the protocol below.
Preparation of nuclear extracts
Cells were grown in 100 mm dishes and harvested on ice by washing them twice with phosphate-buffered saline (PBS) and scraping the cells into 1 ml of PBS. The cells were transferred to pre-cooled Eppendorf tubes and centrifuged (13 000 rpm, 10 min, 4°C), the supernatant was discarded. The pellet was incubated with 100 µl of buffer A [10 mM 4-(2-hydroxyethyl)piperazine-1-ethansulfonic acid (HEPES), 0.1 mM ethylenediaminetetraacetic acid (EDTA), 10 mM KCl, 1 mM 1,4-dithiothreitol (DTT), 0.5 mM phenylmethylsulfonylfluoride (PMSF), pH 7.5 adjusted with HCl] for 15 min at 4°C on a laboratory shaker, centrifuged (13 000 rpm, 2 min, 4°C) and the supernatant, which contained cytosolic proteins, was removed. The pellet was further incubated with buffer B [20 mM HEPES, 1 mM EDTA, 420 mM NaCl, 1 mM DTT, 0.5 mM PMSF, pH 7.0 adjusted with HCl] for 15 min at 4°C on a laboratory shaker and centrifuged (13 000 µpm, 5 min, 4°C) to precipitate DNA. The supernatant comprising the nuclear extract was used as source of HDAC proteins. Aliquots of 100 µl were stored at −80°C. The protein content was determined by the method of CitationBradford (1976).
Assay for HDAC activity
The assay was carried out in black 386-well plates. As a blank 10 µl assay buffer (25 mM Tris-HCl pH 8.0, 137 mM NaCl, 2.7 mM KCl, 1 mM MgCl2) were mixed with 50 µM assay substrate (10 µl), an ϵ-acetylated lysine-based substrate C-terminally coupled with MCA. As control (i.e., 100% HDAC activity), 5 µl assay buffer were incubated with 5 µl of the enzyme source (40 µg/ml nuclear protein) and 10 µl assay substrate. TSA (a characterized HDAC inhibitor) served as a positive inhibitory control. Here, the assay buffer (5 µl) contained 4 µM TSA, the enzyme source (5 µl) and the assay substrate (10 µl). The samples were prepared by addition of 5 µl the assay buffer, containing different concentrations of the tested compounds (200 µM–1 mM), with 5 µl the enzyme source and 10 µl the assay substrate. By subsequent addition of 10 µl activator solution (solution of endopeptidase in presence of 1 µM TSA) the deacetylation reaction was stopped and the fluorophore was selectively released from the deacetylated substrate. The fluorophore was excitated at 360 nm and its emission was measured at 465 nm in a TECAN Genios microtiter plate reader. All measurements were performed in duplicate. The activity of the enzyme was expressed in relative fluorescence units (RFUs).
RFU = [(RFU enzyme–RFU blank)/slope*reaction time]* (V enzyme+V substrate)/V enzyme]
V- volume, µl
Different natural compounds and extracts used for the study
Pure compounds and extracts were primarily solved in dimethyl sulfoxide (DMSO) and further diluted with the assay buffer (1:50) before application in the assay. The analytes were studied at different concentrations (200–1000 µM). IC50 values of some potential HDACi were determined by fitting a sigmoidal dose response curve with variable slope (GraphPad Prism).
Extraction of Leonuri herba
The herb of Leonurus cardiaca L. was purchased from Kottas-Heldenberg & Sohn Drogenhandel GmbH, Vienna, Austria (Ch. No.: KLA70811) and unambiguously identified on the basis of anatomical and morphological features by Ass. Prof. Dr. Christa Kletter, Department of Pharmacognosy, University of Vienna.
The dried plant material was milled and extracted in a solvent-series of increasing polarity with dichloromethane, ethyl acetate (EtOAc), methanol (MeOH) and water. 10 g powdered plant material was extracted with 100 ml solvent under reflux for 30 min. After filtration the extraction of the residue was repeated twice under the same conditions. The combined extracts were dried by evaporation under reduced pressure at 40°C and the yields were 190.1 mg (CH2Cl2), 98.3 mg (EtOAc), 759.4 mg (MeOH), and 767.9 mg (H2O), respectively. Dried extracts (10 mg) were dissolved in 250 µl (5 µl DMSO and 245 µl assay buffer) and diluted to final concentrations of 10 mg/ml in the assay.
For further fractionation by solid phase extraction (SPE) 400 mg of the methanol extract of Leonurus cardiaca were dissolved in 2 ml water. After conditioning of the cartridge (Varian Mega BE-C18; 5 g; 20 ml) with two volumes of methanol and two volumes of water, the aqueous extract was applied on the cartridge, followed by air purge for 10 min. The extract was eluted with five volumes each of water, 20, 40, 80, and 100% methanol. After removal of methanol from the obtained fractions by evaporation, the aqueous solutions were lyophilized. Each fraction (20 mg) was dissolved in 500 µl (10 µl DMSO and 490 ml assay buffer) and diluted to final concentrations of 1 mg/ml in the HDAC assay.
Isolation of lavandulifolioside
Due to the activity of the 40% MeOH fraction after SPE a higher amount of the drug was extracted to isolate the active compound. For this purpose, 1.0 kg of the pulverizied drug was extracted under reflux with 5.5 l methanol for 30 min. The supernatant was removed and filtered. The residue was extracted for another 30 min under reflux with 5.5 l methanol. After filtration the combined extract solutions were evaporated under reduced pressure at 40°C and yielded was 93.1 g of the methanol extract. For chlorophyll removal the dried methanol extract was dissolved in 500 ml water. The clear solution was partitioned twice with chloroform. The chloroform phases were evaporated and yielded 23.4 g. Subsequent twofold partition of the aqueous phase with ethyl acetate and evaporation resulted in a yield of 1.7 g ethylacetate fraction. The chlorophyll free aqueous extract was lyophilized (67.8 g). 60 g of this purified lyophilisate were separated by column chromatography on Sephadex LH 20 under elution with water and water-methanol mixtures of decreasing polarity. Testing of all subfractions in the HDAC assay at a concentration of 1 mg/ml showed the highest activity (77% inhibition) for subfraction SCCCF3c. 60 mg of this fraction were separated by preparative HPLC on a Nucleosil 100-7 C18 column (300 mm × 30 mm × 7 µm) and elution at a flow rate 3 ml/min starting with 40% MeOH and decreasing the solvent polarity to 95% MeOH within 30 min, resulting in the isolation of 33 mg lavandulifolioside.
Extraction of Ratanhiae radix and detannification of the extract
The root of Krameria triandra Ruiz & Pavon was obtained from Kottas-Heldenberg & Sohn Drogenhandel GmbH, Vienna, Austria (Ch. No.: KLA60374) and unambiguously identified by characteristic anatomical and morphological features by Ass. Prof. Dr. Christa Kletter, Department of Pharmacognosy, University of Vienna.
Powdered plant material (2g) was extracted with 100 ml water under reflux for 30 min. After filtration the extraction of the residue was repeated under the same conditions. The combined extract was lyophilized, yielding 299.3 mg. Dried extract (10 mg) was dissolved in 1 ml (20 µl DMSO and 980 ml assay buffer). Concentrations between 0.20 and 1 mg/ml were tested. For detannification, 50 mg lyophilized extract were dissolved in 500 µl MeOH. Partition between 2 ml water-methanol (1:9) and 2 ml hexane was performed. After separation, the apolar phase was discarded and subsequently 2 ml chloroform was added to the polar phase. The detannified chloroform phase and the aqueous phase were dried under reduced pressure at 40°C. The detannified chloroform fraction was tested at concentrations from 2 to 10 mg/ml for its effect on HDAC activity. For this purpose, 5 mg were dissolved in 125 µl solvent (5 µl DMSO and 120 µl assay buffer and further diluted). The aqueous fraction, enriched in tannins, was tested at concentrations from 0.2 to 1 mg/ml. For this purpose, 4 mg were dissolved in 1 ml solvent (20 µl DMSO and 980 µl assay buffer and further diluted).
Results and discussion
Assay optimization and validation
Various parameters had to be determined in order to make the fluorimetric assay applicable for the study of complex plant extracts and mixtures on HDAC activity.
The optimization and validation of the assay included detailed investigations of factors such as temperature, incubation times, the intra- and inter-day reproducibility, the applicability of different enzyme sources and MCA as HDAC substrate, as well as the influence of different classes of primary and secondary plant metabolites.
Linearity
A linear standard curve is required for reliable determination of the activity of the HDAC source and its modulation by the tested compounds. Optimum sensitivity for AMC fluorescence detection is obtained at 360 nm excitation and 465 nm emission. The intensity of released fluorophore was measured after incubation of the test mixture and the activator solution. A standard curve using different concentrations of unacetylated AMC (0–20 µM) displayed excellent linear correlation (r2 = 0.9975) between AMC concentration and the RFUs after correction for the appropriate blanks (data not shown).
Source of HDAC
Three different well-established cell types were used for the preparation of nuclear extracts, namely, HUVECs, HeLa cells and VSMCs. By selecting these cell types, normal human cells (HUVECs) were compared with human cancer cells (HeLa) and rodent cells (rat VSMCs).
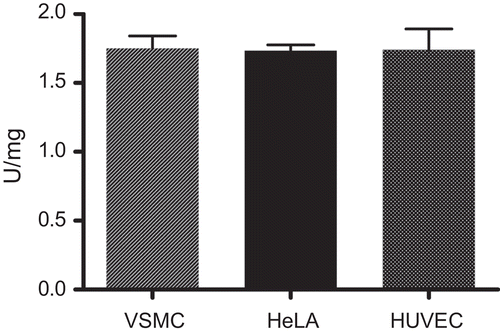
The activity of the enzyme was expressed in units, with one unit corresponding to 1 µmol deacetylated product released per min at 25°C. Based on the quantification of the protein content in correlation to the HDAC activity a specific HDAC activity of approximately 2 U/mg nuclear extract could be determined () for the three different nuclear extracts. Due to the easy cultivation of HeLa cells their nuclear extracts were used for further studies.
Figure 2. Specific activity of nuclear extracts from VSMCs, HeLa cells and HUVECs used as HDAC sources. Nuclear extracts from the three cell types were prepared and subjected to the HDAC assay. Specific activity was determined after protein determination according to CitationBradford (1976) and the assumption that 1U HDAC activity equals deacetylation of 1 µmol substrate per min at 25°C. Three independent experiments were performed for each nuclear extract.
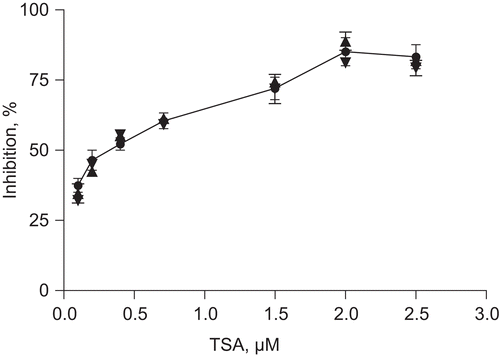
The reliability of the results obtained with the prepared HeLa nuclear extract was confirmed by comparison with published data: TSA is a very potent inhibitor of class I and class II HDACs. The IC50 values of TSA for HDAC1, 3, 4, 6, and 10 were reported to be 4.99, 5.21, 27.60, 16.40, and 24.30 nM, respectively, whereas for recombinant human HDAC8 an IC50 of 486 nM was determined (CitationStefanko et al., 2009). In our study, an IC50 value of 440.4 nM of TSA for the prepared HeLa nuclear extract, which comprises a mixture of different HDACs and additional proteins, was determined ().
Figure 3. Inhibitory activity of TSA at concentrations between 0.2 and 2.5 µM. Different concentrations of TSA were subjected to the HDAC assay with HeLa nuclear extracts at 25°C in three independent experiments. Their inhibitory potential was determined by monitoring the flourescence released during the assay (RFU, relative fluorescence units). The IC50 value was determined by fitting a sigmoidal dose response curve and was 440 nM.
To secure the optimum activity of the enzyme, the avoidance of repeated freeze-thaw cycles as well as storage of the nuclear extract at −80°C are self-evident. However, we experienced delivery of inactive nuclear extracts from few suppliers, due to the fact that obviously little attention is paid to maintain cooling during transportation.
Overall, nuclear extracts of HeLa cells (as well as from VSMCs and HUVECs) proved to be a very good and easily accessible source of HDAC for the assay.
Incubation time and temperature
Incubation time of the enzyme with substrate is one crucial parameter for the enzymatic reaction. Different time courses were compared to determine the optimum incubation time in the assay-system.
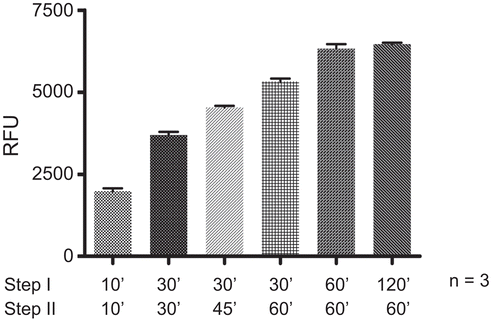
Different combinations of incubation time of the enzyme with the substrate (in step I) and further incubation of the samples with the activator solution (in step II) were monitored. The first set of experiments was performed using 10 min incubation time for both steps. In the second experiment the incubation times were increased to 30 min for both steps, the third experiment was performed 45 min for step I and 30 min for step II. In a fourth set 60 min of incubation for step I was followed by 30 min for step II. The incubation times in experiment five were 60 min for both steps; experiment six was performed with 60 min incubation time in step I and 120 min in step II (). From the experiments was deduced that obviously after 60 min of incubation for each of the two steps the reaction is complete. Thus, 60 min incubation times were used for all further experiments.
Figure 4. Monitoring different incubation times for step I and step II. The HDAC assay was performed in three independent experiments, each, with HeLa nuclear extracts at 25°C varying the incubation times for step I (enzyme + substrate) and step II (+ activator solution). The total amount of released fluorescence (RFU), a measure for the enzymatic conversion of the substrate during the assay time, was monitored.
Temperature during incubation and measurement is another factor which strongly affects enzyme activity and stability. At a temperature of 25°C, the enzyme retained its activity and displayed an adequate velocity of the reaction (data not shown). Thus, all experiments were performed at temperature 25°C.
Intra- and inter-day reproducibility
Intra- and inter-day measurements were performed to investigate the reproducibility of the assay. For assessment of intra-day reproducibility three experiments were performed at 10 and 12 am, and 4 pm (in duplicate each, ). Inter-day reproducibility included measurements on three subsequent days. The experiments were performed in duplicate at 10 am, with three different lots of the HDAC source ().
Figure 5. Intra-day reproducibility. HDAC assays (HeLa extract, 25°C, 60 min for step 1 and 2) were performed in triplicate at three time points within one day (10, 12 am and 4 pm). TSA (1 μM) was included as positive inhibitory control.
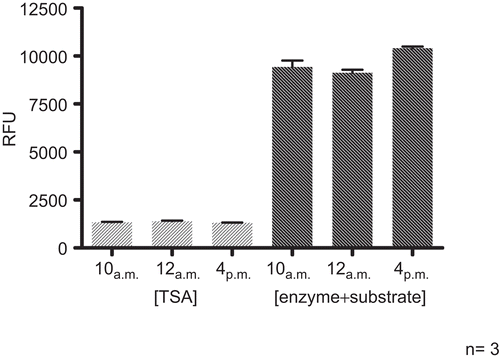
Figure 6. Inter-day reproducibility. HDAC assays (HeLa extract, 25°C, 60 min for step 1 and 2) were performed in triplicate on three subsequent days. TSA (1 μM) was included as positive inhibitory control.
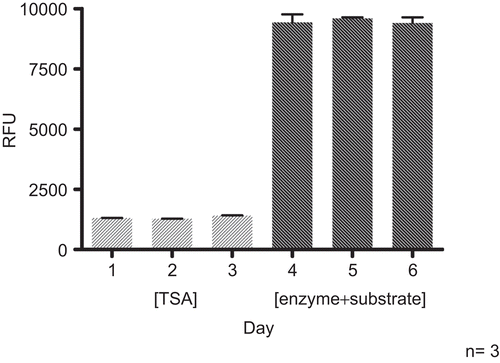
Standard deviations of ±1.82% for the positive control TSA and ±5.10% for the enzyme incubated with substrate were determined for intra-day measurements. Standard deviations of ±2.60% for TSA and ±1.08% for the enzyme incubated with the substrate, respectively, were obtained for inter-day measurements. The achieved values confirmed the reproducibility of the assay.
Solvent for the dissolution of samples
In the bioassay-guided search for HDACi extracts of different polarity have to be studied. Thus, especially the solubility of apolar fractions or extracts for application in the assay had to be checked. Only DMSO and methanol were applicable, the assay is robust against concentrations of up to 5% of these solvents (data not shown). DMSO proved to be superior for the use in the HDAC assay due to its high potency to dissolve various plant extracts with different polarity. The assay substrate, the unacetylated standard, TSA as well as all tested compounds were therefore dissolved in respective amounts of DMSO before dilution with buffer.
The activity of very apolar primary and secondary plant metabolites such as fatty acids, sterols, carotinoides or fat-soluble vitamins cannot be determined in this assay due to incomplete solubility in suitable solvents.
Autofluorescence of test substances
Autofluorescence of compounds in complex plant extracts is a parameter which can influence the precision of the measurements. Thus, the assay has to include measurement of the autofluorescence of the tested compounds and mixtures in the presence of substrate and the determined values have to be corrected accordingly.
The high autofluorescence of coumarin and its derivatives such as esculin or herniarin led to massive interferences with the fluorophore AMC even at concentrations of approximately 100 µM (data not shown), leading to false positive results for HDAC inhibition. Thus, it is strongly recommended to check extracts for dark blue fluorescent substances in suitable thin layer chromatography systems before testing, in order to avoid falsification of the results.
Test of different classes of natural compounds for HDAC modulation
To study the influence of primary and widespread secondary plant metabolites, which are common constituents in extracts, on HDAC activity, different classes of plant compounds were tested. Monosaccharides such as glucose or fructose at a concentration of 1 mM or the disaccharide saccharose (1 mM) showed no obvious interference of sugars with the assay. Chlorophylls (chlorophylla 420 µM, chlorophyllb 349 µM) as well as other plant pigments, e.g., purpurin (630 µM), did not affect the measurement either. One mM solutions of various organic acids like citric, ascorbic, gallic, salicylic, cinnamic, caffeic, ferulic, vanillic, or isovanillic acids did not interfere with the assay.
The inhibitory potential of water-soluble vitamins was studied for several representatives of the group of B vitamins at a concentration of 1 mM (thiamine, riboflavin, niacin and nicotinamide, pyridoxine and pyridoxal, folic acid, and cyanocobalamin) which did not show an inhibitory effect in the HDAC assay.
Ubiquitous flavonoids such as hyperoside, rutin and quercetin influenced the activity of the histone deacetylases only at higher micromolar concentrations. IC50 values of 603, 420, and 560 µM, respectively, were determined.
Less widespread flavonoids/phenolics such as apigenin, chrysin, saponarin, myricetin, myricitrin, taxifoline, catechin, epicatechin, biochanin A, daidzein, daidzin, formononetin, genistein, glycitein or glycitin showed no or marginal inhibitory effects at concentrations between 350 µM and 1 mM. Other phenolic compounds such as chlorogenic acid (1 mM) or verbascoside (400 µM) did not influence the assay.
For tannic acid and catechin gallate, an inhibitory influence on HDAC activity at a concentration of approximately 300 µM due to an obvious precipitation of proteins and not a real inhibition of the enzymatic activity was observed. To check the general applicability of the assay to plant extracts containing high amounts of tannins, an aqueous extract of Ratanhiae radix was prepared and tested. This herbal drug contains up to 15% tannins (CitationBlaschek et al., 2009). The aqueous extract displayed an inhibitory effect on HDAC activity with approximately 50% inhibition at a concentration of 545 µg/ml. This inhibition, however, was again due to precipitation of the enzyme. The extract after detannification according to a well-established protocol (CitationWall et al., 1996) did not show any inhibitory activity at a concentration of 10 mg/ml. These findings confirmed in practice that detannification of extracts rich in tannins before testing is indispensible in order to avoid false positive results.
Other secondary plant compounds were selected due to their structural diversity and their relationship to known HDAC inhibitors.
The IC50 values for some anthraderivatives such as aloin A (IC50 270 µM) and B (IC50 300 µM), as well as sennoside A (IC50 180 µM) and B (IC50 220 µM) were promising, although showing HDAC inhibition at micromolar concentrations only.
To the contrary, alkaloids like atropine, berberine, boldine, caffeine, cephaeline, chelidonine, quinine, emetine, eserine, papaverine, cardiac glycosides like digitoxin or gitoxin, and betaines like stachydrine or betonicine remained without effects at concentrations up to 400 µM.
All experiments with these plant compounds were performed in three independent experiments and relative standard deviations were within the range of ±10%.
Bioactivity-guided fractionation of a Leonurus cardiaca extract
Besides the study of different plant compounds, the adapted HDAC assay was further tested in a bioactivity-guided approach with extracts of the herb of Leonurus cardiaca L. (Lamiaceae). Leonuri herba (Lc) was selected as a model herbal drug because it is used in folk medicine against cardiac complaints (CitationSaukel et al., 2006; CitationHiller & Loew, 2009), which are known to be susceptible to HDAC modulation (CitationWitt et al., 2009). The preparation of extracts of different polarity, parallel testing in the optimized assay, bioguided fractionation of the most active extract and the isolation of active compounds should prove the applicability of this optimized in vitro method for the accelerated search for natural compounds with HDAC inhibitory activity.
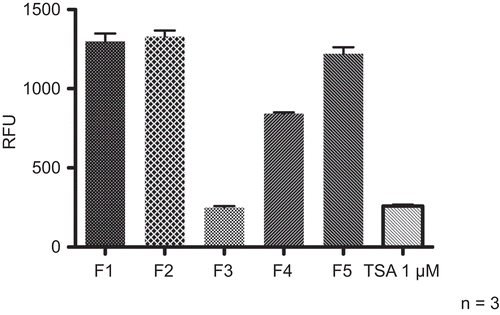
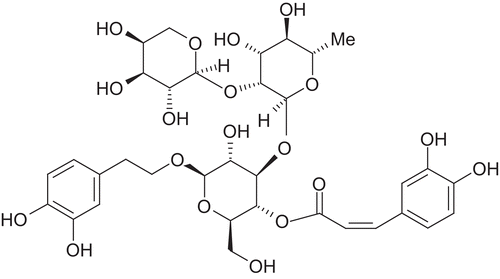
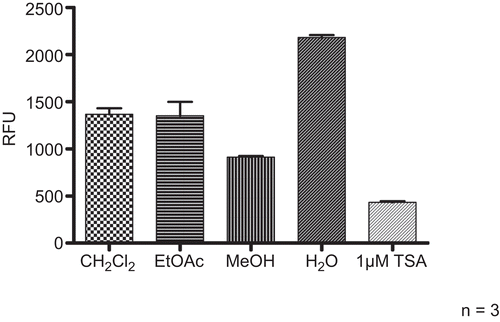
Lc extracts obtained with dichloromethane, ethyl acetate, methanol and water were tested at concentrations of 10 mg/ml. The methanol extract was most active () and further fractionation by SPE on RP-18 cartridges was performed. The fractions obtained with water, 20, 40, 80, and 100% methanol were investigated at concentrations of 1 mg/ml for their effect on HDAC activity. The 40% methanol extract showed the highest potential to inhibit HDAC activity (). HPLC fingerprinting revealed flavonoids and caffeoylquinic acid derivatives as major components. For the isolation of the active ingredients a methanol extract from the drug was prepared and further fractionated by column chromatography on Sephadex® LH 20. The most active fraction was separated by preparative HPLC in order to isolate and identify the HDAC inhibitory principle. The obtained pure compound was characterized by NMR spectroscopy and mass spectrometry and the structure unambiguously confirmed as lavandulifolioside () (CitationBasaran et al., 1988, see Appendix). The influence of lavandulifolioside was studied in the HDAC assay and an IC50 value of 220 µM was determined. Although lavandulifolioside is not a very potent HDAC inhibitor, these results confirm the applicability of the assay for successful and efficient bioactivity-guided isolations of HDAC inhibitors from plant origin.
Figure 7. Modulation of HDAC activity by different extracts from Leonuri herba. Extracts from Lc were subjected to the HDAC assay at a concentration of 10 mg/ml. TSA (1 µM) was included as positive control. Compiled results from three experiments are depicted.
Conclusions
This work presents for the first time the application of a fluorimetric in vitro assay for the search for HDAC inhibitors in complex extracts from plant origin. The method for the determination of HDAC activity is a powerful tool to accelerate the isolation and identification of potent natural product-based inhibitors of HDAC activity.
The optimized test system combines high sensitivity, speed and reproducibility and is well suited for the screening of plant extracts, fractions and natural compounds of medium to high polarity. The assay is performed in small reaction volumes which makes it a very cost effective method. A further advantage is not only the very little consumption of reagents but also of analytes. In a first pilot experiment on extracts from the herb of Leonurus cardiaca the assay successfully demonstrated its applicability for the bioactivity-guided fractionation of complex mixtures.
However, special caution has to be taken when working with extracts rich in highly autofluorescent or lipophilic substances or tannins. It was shown that coumarins and similar derivatives strongly interfere with the measurements due to autofluorescence. In the examination of extracts or fractions presumably containing such compounds a general check for blue fluorescent ingredients by chromatographic methods, e.g., TLC is highly recommended. Extracts containing tannins, as expected, lead to the precipitation of the proteins in the assay and thus to false positive results. However, after detannification according to published protocols such extracts can be tested in the assay in order to study the effect of other components on HDAC activity.
For very apolar extracts and fractions, other assays have to be used due to insufficient solubility of such mixtures in the reaction buffers.
By the use of total nuclear extracts from different cell types (HeLa, HUVECs, VSMCs) the assay is not specific to distinguish between the modulation of class I and class II HDACs or single members of the HDAC family. However, uncharacterized cofactors present in the nuclear extract also contribute to lysine hydrolysis and can be affected by the test compounds. Thus, this design of the assay mimics the natural environment of the enzyme and assures its activity and stability. To study optimally the reaction mechanisms and to determine selective HDAC inhibitors, individual recombinant purified enzymes and acetylated histones can be used instead of nuclear extract and MCA-coupled peptide as well.
To sum up, the presented results prove the applicability of the fluorimetric assay for a rapid and effective primary screening of complex extracts and fractions from plant origin for inhibitors of HDAC activity.
Acknowledgements
We are grateful to Prof. V. Dirsch, Department for Pharmacognosy, University of Vienna and Dr. D. Sorescu, M.D., Emory University Hospital Midtown, Atlanta, GA, for stimulative discussions. We thank Dr. R. Mazischek, Ph.D., Massachusetts General Hospital, Boston, MA, for provision of the HDAC substrate. We owe our thanks to Dr. V. Patel, Ph.D. and Prof. J. Clardy, Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, MA, for experimental suggestions and performed tests. We have to thank Associate Prof. Dr. H. Kaehlig, Department for Organic Chemistry, University of Vienna, for the NMR experiments and Dr. M. Zehl, Department of Pharmacognosy, University of Vienna, for the LC/MS experiments for structure elucidation of lavandulifolioside. We dedicate this work to em. O. Prof. Dr. Wolfgang Kubelka, Department of Pharmacognosy, University of Vienna, on the occasion of his 75th birthday.
Declaration of interest
The authors declare that they have no competing interests to report.
References
- Basaran AA, Calis I, Anklin C, Nishibe S, Sticher O. (1988) Lavandulifolioside. A new phenylpropanoid glycoside from Stachys lavandulifolia. Helv Chim Acta, 71, 1483–1490.
- Blaschek W, Frohne D, Loew D. (2009) Ratanhiae radix. In: Wichtl M, ed. Teedrogen und Phytopharmaka. 5th edition. Stuttgart: Wiss Verlags GmbH, 550–551.
- Bolden JE, Peart MJ, Johnstone RW. (2006). Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov, 5, 769–784.
- Bontempo P, Mita L, Miceli M, Doto A, Nebbioso A, De Bellis F, Conte M, Minichiello A, Manzo F, Carafa V, Basile A, Rigano D, Sorbo S, Castaldo Cobianchi R, Schiavone EM, Ferrara F, De Simone M, Vietri M, Cioffi M, Sica V, Bresciani F, de Lera AR, Altucci L, Molinari AM. (2007). Feijoa sellowiana derived natural flavone exerts anti-cancer action displaying HDAC inhibitory activities. Int J Biochem Cell Biol, 39, 1902–1914.
- Bradford MM. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem, 72, 248–254.
- Carew JS, Giles FJ, Nawrocki ST. (2008). Histone deacetylase inhibitors: Mechanisms of cell death and promise in combination cancer therapy. Cancer Lett, 269, 7–17.
- Chen IH, Lu MC, Du YC, Yen MH, Wu CC, Chen YH, Hung CS, Chen SL, Chang FR, Wu YC. (2009). Cytotoxic triterpenoids from the stems of Microtropis japonica. J Nat Prod, 72, 1231–1236.
- Chung IM, Kim MY, Park WH, Moon HI. (2008). Histone deacetylase inhibitors from the rhizomes of Zingiber zerumbet. Pharmazie, 63, 774–776.
- Dokmanovic M, Marks PA. (2005). Prospects: Histone deacetylase inhibitors. J Cell Biochem, 96, 293–304.
- Frew AJ, Johnstone RW, Bolden JE. (2009). Enhancing the apoptotic and therapeutic effects of HDAC inhibitors. Cancer Lett, 280, 125–133.
- Granger A, Abdullah I, Huebner F, Stout A, Wang T, Huebner T, Epstein JA, Gruber PJ. (2008). Histone deacetylase inhibition reduces myocardial ischemia-reperfusion injury in mice. Faseb J, 22, 3549–3560.
- Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, Nieland TJ, Zhou Y, Wang X, Mazitschek R, Bradner JE, DePinho RA, Jaenisch R, Tsai LH. (2009). HDAC2 negatively regulates memory formation and synaptic plasticity. Nature, 459, 55–60.
- Hiller K, Loew D. (2009) Leonuri cardiacae herba. In: Wichtl M, ed. Teedrogen und Phytopharmaka. 5th edition. Stuttgart: Wiss Verlags GmbH, 384–385.
- Kim MJ, Ahn K, Park SH, Kang HJ, Jang BG, Oh SJ, Oh SM, Jeong YJ, Heo JI, Suh JG, Lim SS, Ko YJ, Huh SO, Kim SC, Park JB, Kim J, Kim JI, Jo SA, Lee JY. (2009). SIRT1 regulates tyrosine hydroxylase expression and differentiation of neuroblastoma cells via FOXO3a. FEBS Lett, 583, 1183–1188.
- Marks PA, Xu WS. (2009). Histone deacetylase inhibitors: Potential in cancer therapy. J Cell Biochem, 107, 600–608.
- Mazitschek R, Patel V, Wirth DF, Clardy J. (2008). Development of a fluorescence polarization based assay for histone deacetylase ligand discovery. Bioorg Med Chem Lett, 18, 2809–2812.
- Newkirk TL, Bowers AA, Williams RM. (2009). Discovery, biological activity, synthesis and potential therapeutic utility of naturally occurring histone deacetylase inhibitors. Nat Prod Rep, 26, 1293–1320.
- Ogbomo H, Michaelis M, Kreuter J, Doerr HW, Cinatl J Jr. (2007). Histone deacetylase inhibitors suppress natural killer cell cytolytic activity. FEBS Lett, 581, 1317–1322.
- Oka S, Ago T, Kitazono T, Zablocki D, Sadoshima J. (2009). The role of redox modulation of class II histone deacetylases in mediating pathological cardiac hypertrophy. J Mol Med, 87, 785–791.
- Olson EN, Schneider MD. (2003). Sizing up the heart: Development redux in disease. Genes Dev, 17, 1937–1956.
- Ropero S, Esteller M. (2007). The role of histone deacetylases (HDACs) in human cancer. Mol Oncol, 1, 19–25.
- Smith KT, Workman JL. (2009). Histone deacetylase inhibitors: Anticancer compounds. Int J Biochem Cell Biol, 41, 21–25.
- Saukel J, Gerlach S, Kubelka W. (2006) Pflanzen in der österreichischen Volksmedizin. Die„VOLKSMED-Datenbank”. Sci Pharm, 74, S 36.
- Son IH, Lee SI, Yang HD, Moon HI. (2007). bis(4-Hydroxybenzyl)sulfide: A sulfur compound inhibitor of histone deacetylase isolated from root extract of Pleuropterus ciliinervis. Molecules, 12, 815–820.
- Stefanko DP, Barrett RM, Ly AR, Reolon GK, Wood MA. (2009). Modulation of long-term memory for object recognition via HDAC inhibition. Proc Natl Acad Sci USA, 106, 9447–9452.
- Wall ME, Wani MC, Brown DM, Fullas F, Olwald JB, Josephson FF, Thorton NM, Pezzuto JM, Beecher WW, Farnsworth NR, Cordell GA, Kinghorn AD. (1996). Effects of tannins on screening of plant extracts for enzyme inhibitory activity and techniques for their removal. Phytomedicine, 3, 281–285.
- Wegener D, Hildmann C, Riester D, Schwienhorst A. (2003). Improved fluorogenic histone deacetylase assay for high-throughput-screening applications. Anal Biochem, 321, 202–208.
- Witt O, Deubzer HE, Milde T, Oehme I. (2009). HDAC family: What are the cancer relevant targets? Cancer Lett, 277, 8–21.
- Zhang L, Fang H, Xu W. (2009). Strategies in developing promising histone deacetylase inhibitors. Med Research Reviews, 4, 1–16.