Abstract
Context: Cassia fistula Linn. (Caesalpiniaceae) has been used in folk medicine. Anthraquinone derivative rhein having antimicrobial properties is actively present in C. fistula fruit. Although, as yet there has been no study of its anticandidal potential.
Objective: The present study was conducted to determine the phytochemical composition of fruit pulp and seed extract and their effect on Candida albicans ATCC 10261, Candida glabrata ATCC 90030 and Candida tropicalis ATCC 750, respectively.
Materials and methods: The fruit pulp and seed extracts were tested for phytochemicals by various standard methods and rhein was identified by thin-layer chromatography. The anticandidal activity was determined by minimum inhibitory concentration (MIC), growth curve studies, cytotoxicity and ergosterol estimation assay.
Results: The fruit pulp and seed extracts showed high content of phenolic compounds. Rhein was identified in both extracts, Rf 0.38. MICs of seed extract obtained with C. albicans, C. tropicalis and C. glabrata is 350, 300 and 300 μg/ml. However, for fruit pulp extract, these values significantly reduced to 150, 250 and 100 μg/ml, respectively. Comparative MIC values for fluconazole were 16, 16 and 04 µg/ml. At MICs, pulp reduced ergosterol content in cell membrane of C. albicans, C. tropicalis and C. glabrata by 54.42, 48.78 and 68.0%, seed extract by 38.11, 47.0 and 45.0%, whereas, fluconazole showed 93.56, 89.21 and 98.0%, respectively.
Discussion and conclusion: C. fistula fruit pulp and seed extract possessed anticandidal activity. The result was significantly correlated between the MICs, cytotoxicity and ergosterol inhibition. It was concluded that the crude extract is a promising source for anticandidal compounds.
Introduction
Infectious diseases have been cured with herbal medicine throughout the history of mankind. Natural products either as pure compounds or crude extracts provide unlimited opportunities for new drugs due to the chemical diversity. The increasing failure of chemotherapeutics and antibiotic resistance exhibited by pathogenic fungal infection has led to the screening of several medicinal plants for their potential antifungal activity (CitationFicker et al., 2003; CitationKen-Ichi et al., 2008; CitationMiyasaki et al., 2010). In recent years, secondary plant metabolites have been extensively investigated as a source of medicinal agents (CitationMichal & Klaus, 2009).
Cassia fistula Linn. (Caesalpiniaceae) (Indian Laburnum or Golden shower) is frequently used as folk medicine in various disorders like hematemesis, pruritus, leucoderma, diabetes and laxatives (CitationMoshahid et al., 2009). Scientifically, the fruits parts of C. fistula have been reported as antidiabetic (CitationSingh & Bharadwaj, 1975), antitumor (CitationGupta et al., 2000), hypolipidemic (CitationGupta & Jain, 2009), sedative (CitationMazumder et al., 1998), antioxidant (CitationSiddhuraju et al., 2002), antimicrobial (CitationDhar & Qasba, 1984; CitationDuraipandiyan & Ignacimuthu, 2007), anthelmintic (CitationIrshad et al., 2010), hepatoprotective (CitationDas et al., 2008), abortifacient and purgative (CitationElujoba et al., 1999).
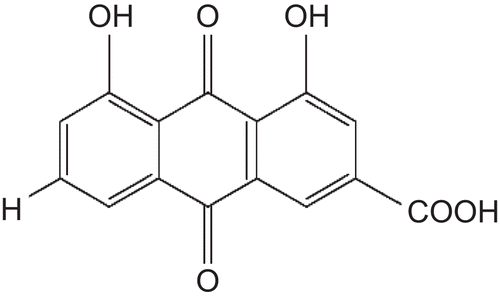
Rhein (), a major anthraquinone derivative and sennidin-like compound, identified in fruit pulp, seed, flower and leaves (CitationModi & Khorana, 1952; CitationKapadia & Khorana, 1966; CitationBahorun et al., 2005). Antibacterial and antifungal properties have been reported as (CitationDuraipandiyan & Ignacimuthu, 2007; CitationPhongpaichit et al., 2004; CitationPerumal et al., 1998).
Figure 1. Rhein (1,8-dihydroxyanthraquinone-3-carboxylic acid), an active phytochemical of Cassia fistula.
Candida is a fungus frequently found on skin and also mucosal flora. In humans with impairment of the immune system, it can lead to severe diseases from superficial mucosal to life threatening systemic disorders (CitationKaleli et al., 2007). In the last 10 years, there has been a considerable increase in mycoses and systemic infections caused by Candida albicans, particularly among immunocompromised patients (CitationEggimann et al., 2003). Studies have indicated C. albicans resistance to azoles or hepatonephrotoxic or nephrotoxic linked to polyene use, particularly amphotericin-B (CitationWhite & Goetz, 1994; CitationRex et al., 1995). Therefore, searching for the sources of new anticandidal is required from the medicinal plant derivatives owing to their versatile application.
The aim of this study was to investigate the anticandidal activity of the fruit pulp and seed extract of C. fistula against three most prevalent species causing candidiasis, C. albicans ATCC 10261, Candida glabrata ATCC 90030 and Candida tropicalis ATCC 750. Anticandidal activity is investigated by determining minimum inhibitory concentration (MIC), growth curve studies, cell cytotoxicity and membrane associated property: ergosterol extraction and estimation assay. The present study is designed to investigate new therapeutic treatments for Candida spp. infections by using plant derived principles which are low cost and readily available.
Materials and methods
Reagent and chemicals
All media constituents were obtained from HiMedia (Mumbai, India). All chemicals and solvents were of analytical grade and obtained from Merck (Mumbai, India). Fluconazole was obtained from HiMedia.
Collection and preparation of plant extract
The fresh ripe fruits of C. fistula were collected in June 2010 from the campus of Jamia Millia Islamia University, New Delhi and authenticated by Prof. M. P. Sharma. A voucher of specimen (PM 21) was stored in the laboratory for further reference. The fruit pulp and seed was separated and ground to coarse powder. The samples were defatted with hexane and then extracted with methanol in a Soxhlet apparatus for 72 h. It was filtered with Whatman filter paper and the solvent was evaporated. The crude extracts were used for the phytochemical investigation and anticandidal potential by clearance of biosafety and ethical committee of the institute.
Phytochemical analysis
The extracts were subjected to various phytochemical tests to determine the active constituents. Tests were performed for alkaloids (Mayer’s reagent and Dragendorff’s reagent test), flavanoids (Shinoda test), cardiac glycoside (Keller Killiani test), tannins (Braemer’s test), anthraquinones (Borntrager’s test), steroids and terpenoids (Salkowski test, Liebermann–Burchardt test) by standard methods (CitationHarborne, 1973; CitationTrease & Evans, 1978; CitationSofowora, 1993). The compound rhein was identified by the thin-layer chromatography on silica gel 60 F254 thin-layer chromatography aluminium sheet having mobile system of ethyl acetate: methanol: water mobile (5:1:0.5). Spot of rhein on the chromatograms was visualized by sparing with 10% methanol potassium hydroxide solution.
Growth conditions
C. albicans ATCC 10261, C. glabrata ATCC 90030 and C. tropicalis ATCC 750 used in this study were obtained from the Indian Institute of Integrative Medicine (formerly Regional Research Laboratory, Jammu). Stock cultures were maintained on slants of nutrient agar (yeast extract 1%, peptone 2%, d-glucose 2% and agar 2.5%) at 4 °C. Cells from primary culture (108 cells/ml) were inoculated in 100 ml fresh YEPD (yeast extract, peptone and dextrose) medium and grown for 8–10 h, i.e., up to mid-log phase (106 cells/ml). Microbial cultures grown at 37 °C were appropriately diluted in sterile YEPD broth to obtain the cell suspension at 106 CFU/ml.
Determination of MIC
Microtiter assay
Cells were grown for 48 h at 30 °C to obtain single colonies, which were resuspended in a 0.9% normal saline solution to give an optical density at 600 nm (OD600) of 0.1. The cells were then diluted 100-fold in yeast nitrogen base medium containing 2% glucose and the respective auxotrophic supplements. The diluted cell suspensions were added to the wells of round bottomed 96-well microtiter plates (100 µl/well) containing equal volumes of medium and different concentrations of test extracts (CitationCLSI/NCCLS, 2008). Fluconazole was included as a positive control. In addition to this, a drug free control was also included. The plates were incubated at 30 °C for 24 h. The MIC test endpoint was evaluated both visually and by observing the OD620 in a microplate reader (Bio-Rad, iMark, Hong Kong) and is defined as the lowest concentration that gave complete inhibition of growth compared to the controls.
Growth curve studies
For growth studies, 106 cells/ml (optical density A595 = 0.1) culture of Candida cells were inoculated and grown aerobically in YEPD broth for control and along with various concentrations of test extracts (1000–3500 μg/ml) in individual flasks. Growth was recorded turbidometrically at 595 nm using LaboMed spectrophotometer (LaboMed Inc., CA). The growth rate of different Candida species in the absence and presence of inhibitor was performed in triplicate for each concentration, average of which was taken into consideration.
WST1 cytotoxicity assay
The assay was monitored with a spectrophotometer using adherent or cultured cell suspension in microplate followed by incubation with 2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulphophenyl)-2H-tetrazolium, mono sodium salt (WST1). Appropriate concentrations of the test extract was added to cell culture and incubated for 24 h. WST1/CEC assay dye solution 10 µl was added to each well. Fluconazole was included as positive control. Plates were incubated for 30 min in a cell culture incubator with intermediate shaking for 1 min. The reaction was stopped by the addition of 10 µl of 1% sodium dodecyl sulfate. WST1 tetrazolium salt is reduced to formazan by cellular dehydrogenases there by generating a deep red colored formazan, measured at 450 nm and directly correlated to cell number (CitationPerez et al., 1993).
Ergosterol extraction and estimation assay
A single Candida colony from an overnight sabouraud dextrose agar (Difco, KS) plate culture was used to inoculate 50 ml of sabouraud dextrose broth (Difco) for control and for various concentrations of test extracts. Fluconazole was included as a positive control. The cultures were incubated for 16 h and harvested by centrifugation at 2700 rpm for 5 min. The net weight of the cell pellet was determined. Alcoholic potassium hydroxide solution 25% (3 ml) was added to each pellet and vortex mixed for 1 min. Cell suspensions were transferred to sterile borosilicate glass screw-cap tubes and were incubated in a 85 °C water bath for 1 h. Following incubation, tubes were allowed to cool. Sterols were then extracted by the addition of a mixture of 1 ml of sterile distilled water and 3 ml of n-heptane followed by vigorous vortex mixing for 3 min. The heptane layer was transferred to a borosilicate glass screw-cap tube and stored at −20 °C. Prior to analysis, 20 µl aliquot of sterol extract was diluted five-fold in 100% ethanol and scanned spectrophotometrically between 240 and 300 nm with a spectrophotometer (Systronics UV-Visible Spectrophotometer 117, Delhi, India). Ergosterol content was calculated as a percentage of the wet weight of the cell as described by CitationBreivik and Owades (1957).
Statistical analysis
The mean value ± standard error of mean (SEM) was determined from triplicate samples for each experimental group. Statistical significance was examined with Student’s t-test by comparing experimental groups with appropriate controls.
Results and discussion
Phytochemical analysis
The result from the present study showed that the C. fistula fruit pulp and seed extract have a significant amount of phenolic compounds like flavonoids, rhein, anthraquinone and tannins (). Thin-layer chromatography study also shows the pink color spots obtained at Rf 0.38 in the both extracts, indicating presence of rhein.
Table 1. Phytochemical analysis of Cassia fistula fruit pulp and seed extract.
Minimal inhibitory concentration (MIC)
The MICs values of fruit pulp extract against tested organisms ranged from 100 to 150 μg/ml, while that of seed extract ranged from 300 to 350 μg/ml (). The MIC results showed that the fruit pulp is more active against C. glabrata (100 μg/ml) followed by C. albicans (150 μg/ml) and C. tropicalis (250 μg/ml), although seed extract showed almost similar effects on these organisms. In comparison, the fruit pulp extract showed lowest MICs against tested organism than the seed extract. All the standard strains were fluconazole-susceptible with MIC of 16 µg/ml (C. albicans), 16 µg/ml (C. glabrata) and 4 µg/ml (C. tropicalis), respectively. The t-test significant correlation (0.0621) was observed between anticandidal activity of fruit pulp and seed extract. The anticandidal potential of crude extract of fruit pulp and seed extract is due to the presence of the phenolic compound like anthraquinone and rhein. The antimicrobial properties of these compounds are already reported (CitationAgarwal et al., 2000). Similarly, the crude extract of medicinal plants reported as antimicrobial and antifungal activity in which anthraquinone and rhein is actively present (CitationIbrahim & Osman, 1995; CitationHatano et al., 1999).
Table 2. Minimum inhibitory concentrations (MIC) (µg/ml) of fruit pulp and seed extract against different Candida species.
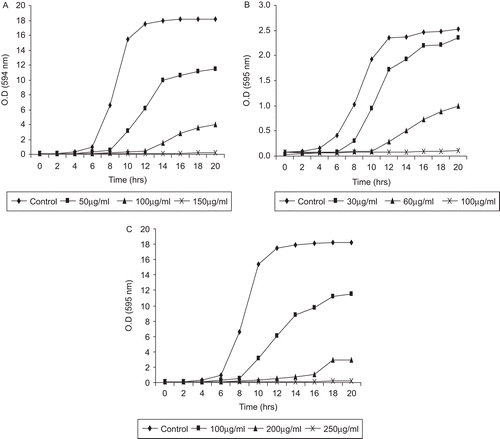
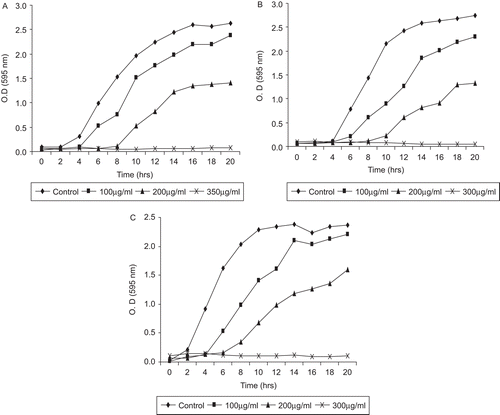
Growth studies (turbidometric measurement)
The test extracts suppressed growth and delayed exponential phases of all three tested organisms in a concentration-dependent manner ( and ). The fruit pulp and seed extract effect the growth pattern of C. albicans, C. tropicalis and C. glabrata in a similar manner. However, the control group have a normal pattern of growth with lag phase of 4–5 h, active exponential phase of 8–10 h, before attaining stationary phase. In the presence of fruit pulp and seed extract, the growth pattern decreases with increasing concentration and finally a flat line was observed at MIC values. After 24 h incubation, extract-treated Candida species were highly reduced, whereas, no such effect was seen in control groups under the microscope.
WST1 cytotoxicity assay
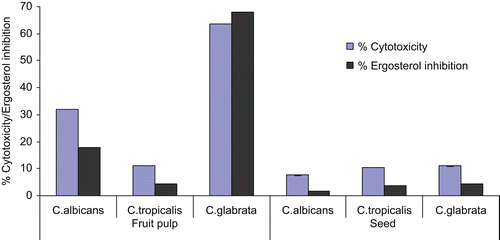
Test extracts were investigated for their anticandidal activity by WST1 cytotoxic assay. The toxicity effects on tested Candida organism increased with the increase of drug concentration. Fruit pulp extract results showed the cytotoxicity on C. tropicalis (52.31%), followed by C. albicans (56.48%) and C. glabrata (63.55%), at the minimum inhibitory concentration. Cytotoxicity of the seed extract was observed against C. albicans (39.78%) followed by C. glabrata (46.58%) and C. tropicalis (50.78%) under the same conditions (). Killing activity of fluconazole under the same experimental conditions was, however, significantly higher: 89% (C. albicans), 95% (C. glabrata) and 87% (C. tropicalis), respectively. The fruit pulp extract was seen to have greater potential compared to that of the seed extract. At MICs, fruit pulp and seed extract showed t-test significant correlation 0.099. It was concluded that crude extracts are a promising source of anticandidal compounds and the killing activity may be due to the presence of certain active compounds present in these extracts.
Table 3. Percentage cytotoxicity in the presence of different concentrations of fruit pulp and seed extract. The data represents mean ± standard error of mean.
Sterol assay
Sterol assay was performed for the mechanistic analysis of the tested drug. Most antifungal drugs target the ergosterol biosynthesis pathway and disrupt membrane fluidity, asymmetry and integrity. Ergosterol also contributes to proper functioning of membrane bound enzymes (CitationLupetti et al., 2002). Total sterol content of samples treated with varying concentrations of test extracts was studied. At MICs, fruit pulp extract showed %ergosterol inhibition in C. albicans, C. tropicalis and C. glabrata was 54.42, 48.78 and 68.0%. However, the seed extract showed 38.11, 47.0 and 45.0%, respectively (). %Ergosterol decrease in ergosterol content by fluconazole at MIC was 93.56, 89.21 and 98% with C. albicans, C. tropicalis and C. glabrata, respectively. Ergosterol inhibition was significantly correlated with the cytotoxicity. The experimental result further compared at a constant concentration 100 μg/ml, showed that fruit pulp extract had a greater effect on C. glabrata followed by C. albicans and C. tropicalis. The seed extract has less effect at the same concentration (). The finding showed that increase in concentration of extracts increased the cytotoxicity as well as %ergosterol inhibition. The decrease in ergosterol content indicates that these organisms show trailing growth in the presence of tested extracts. The correlation between the MICs, cytotoxicity and ergosterol content for all the species suggest that ergosterol biosynthesis is the primary target of the active compound of these extracts.
Table 4. Effect of fruit pulp and seed extract on %Ergosterol content in different Candida species. The data represents the mean of three experiments.
Conclusion
C. fistula displayed anticandidal activities against the tested organisms. The minimum inhibitory concentration of crude extract of fruit pulp and seed varies with the organism tested. Hence, at MICs, the cytotoxic effect and ergosterol inhibition was significantly correlated. Therefore, it was concluded that the crude extract has a promising source of anticandidal compounds. The anthraquinone and rhein was actively present in both crude extract, it may provide anticandidal potential and effects ergosterol biosynthesis of membrane.
Acknowledgements
This work was financially supported by the Life Science Research Board (LSRB-139), DRDO, Govt. of India. The authors acknowledge Prof. M. P. Sharma (Department of Botany, Jamia Hamdard, New Delhi, India) for the identification of plant materials. The assistance of Mr. Irfan Ahmad and S. Jafar Mehdi is acknowledged with thanks.
Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the article.
References
- Agarwal SK, Sudhir SS, Sushma V, Sushil K. (2000). Antifungal activity of anthraquinone derivatives from Rheum emodi. J Ethnopharmacol, 72, 43–46.
- Bahorun T, Neergheen VS, Aruoma OI. (2005). Phytochemical constituents of Cassia fistula. Afr J Biotechnol, 4, 1530–1540.
- Breivik ON, Owades JL. (1957). Spectrophotometric semi-micro determination of ergosterol in yeast. J Agri Food Chem, 5, 360–363.
- CLSI/NCCLS. (2008). Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard-3rd edition. CLSI document M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA.
- Das S, Sarma G, Barman S. (2008). Hepatoprotective activity of aqueous extract of fruit pulp of Cassia fistula (AFCF) against carbon tetrachloride (CCL4) induced liver damage in albino rats. J Clin Diag Res, 2, 1133–1138.
- Dhar DN, Qasba GN. (1984). Screening of some plant extract for antifungal activity against Venturia inaequalis. Sci Cult, 50, 209.
- Duraipandiyan V, Ignacimuthu S. (2007). Antibacterial and antifungal activity of Cassia fistula L.: An ethnomedicinal plant. J Ethnopharmacol, 112, 590–594.
- Eggimann P, Garbino J, Pittet D. (2003). Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect Dis, 3, 685–702.
- Elujoba AA, Abere AT, Adelusi SA. (1999). Laxative activities of Cassia pods sourced from Nigeria. Niger J Nat Prod Med, 3, 51–53.
- Ficker C, Smith ML, Akpagana K, Gbeassor M, Zhang J, Durst T, Assabgui R, Arnason JT. (2003). Bioassay-guided isolation and identification of antifungal compounds from ginger. Phytother Res, 17, 897–902.
- Gupta M, Mazumder UK, Rath N, Mukhopadhyay DK. (2000). Antitumor activity of methanolic extract of Cassia fistula L. seed against Ehrlich ascites carcinoma. J Ethnopharmacol, 72, 151–156.
- Gupta UC, Jain GC. (2009). Study on hypolipidemic activity of Cassia fistula legume in rats. Asian J Exp Sci, 23, 241–248.
- Harborne JB. (1973). Phytochemical Methods. Champan and Hall, London.
- Hatano T, Uebayashi H, Ito H, Shiota S, Tsuchiya T, Yoshida T. (1999). Phenolic constituents of Cassia seeds and antibacterial effect of some naphthalenes and anthraquinones on methicillin-resistant Staphylococcus aureus. Chem Pharm Bull, 47, 1121–1127.
- Ibrahim D, Osman H. (1995). Antimicrobial activity of Cassia alata from Malaysia. J Ethnopharmacol, 45, 151–156.
- Irshad M, Singh M, Rizvi MM. (2010). Assessment of anthelmintic activity of Cassia fistula L. Middle East J Sci Res, 5, 346–349.
- Kaleli I, Cevahir N, Demir M, Yildirim U, Sahin R. (2007). Anticandidal activity of Pseudomonas aeruginosa strains isolated from clinical specimens. Mycoses, 50, 74–78.
- Kapadia GJ, Khorana ML. (1966). Studies of active constituents of Cassia fistula pulp. I. Colorimetric estimation of free rhein and combined sennidin-like compounds. Lloydia, 25, 55–58.
- Ken-Ichi F, Tomoko F, Isao K. (2008). Antifungal activity of alkanols against Zygosaccharomyces bailii and their effects on fungal plasma membrane. Phytother Res, 22, 1349–1355.
- Lupetti A, Danesi R, Campa M, Del Tacca M, Kelly S. (2002). Molecular basis of resistance to azole antifungals. Trends Mol Med, 8, 76–81.
- Mazumder UK, Malaya G, Nandita R. (1998). CNS activities of Cassia fistula in mice. Phytother Res, 12, 520–522.
- Michal T, Klaus PL. (2009). Potentilla-a review of its phytochemical and pharmacological profile. J Ethnopharmacol, 122, 184–204.
- Miyasaki Y, Nichols WS, Morgan MA, Kwan JA, Van Benschoten MM, Kittell PE, Hardy WD. (2010). Screening of herbal extracts against multi-drug resistant Acinetobacter baumannii. Phytother Res, 24, 1202–1206.
- Modi MRK, Khorana ML. (1952). A study of Cassia fistula pulp. Indian J Pharm, 61–63.
- Moshahid AR, Irshad M, Gamal EH, Salaem BY. (2009). Bioefficacies of Cassia fistula: An Indian labrum. Afr J Pharm Pharmacol, 3, 287–292.
- Perez RP, Godwin AK, Handel LM, Hamilton TC. (1993). A comparison of clonogenic, microtetrazolium and sulforhodamine B assays for determination of cisplatin cytotoxicity in human ovarian carcinoma cell lines. Eur J Cancer, 29A, 395–399.
- Perumal SR, Ignacimuthu S, Sen A. (1998). Screening of 34 medicinal plants antibacterial properties. J Ethnopharmacol, 62, 173–182.
- Phongpaichit S, Pujenjob N, Rukachaisirikul V, Ongsakul M. (2004). Antifungal activity from leaf extracts of Cassia alata L., Cassia fistula L. and Cassia tora L. Songklanakarin J Sci Tech, 26, 741–748.
- Rex JH, Rinaldi MG, Pfaller MA. (1995). Resistance of Candida species to fluconazole. Antimicrob Agents Chemother, 39, 1–8.
- Siddhuraju P, Mohan PS, Becker K. (2002). Studies on the antioxidant activity of Indian laburnum (Cassia fistula L.): A preliminary assessment of crude extracts from stem bark, leaves, flowers and fruit pulp. J Agric Food Chem, 79, 61–67.
- Singh KN, Bharadwaj UR. (1975). Hypoglycaemic activity of Albizzia stipulata, Albizia moluccana and Cassia fistula leguminous seed diets on normal young rats. Indian J Pharm, 7, 47–49.
- Sofowora A. (1993). Medicinal Plants and Traditional Medicines in Africa. Chichester John Wiley & Sons, New York.
- Trease GE, Evans WC. (1978). Pharmacognosy. Bailliere Tindall Ltd, London.
- White A, Goetz MB. (1994). Azole-resistant Candida albicans: Report of two cases of resistance to fluconazole and review. Clin Infect Dis, 19, 687–692.