Abstract
Context: Endophytic fungi are microorganisms living within the tissues of host plants, and have proven to be rich sources of biologically active secondary metabolites and therefore have attracted increasing attention in recent years.
Objective: To isolate and characterize bioactive constituents from the endophytic fungus cultures of Crocus sativus Linn. (Iridaceae).
Materials and methods: Endophytes were isolated from the corm of C. sativus. Endophytic fungus cultures were subjected to repeated column chromatography. Chemical structure was elucidated based on extensive spectroscopic methods and X-ray diffraction analysis. Several pathogenic fungi isolates and tumor cell lines were employed to evaluate the antifungal and cytotoxic activities of the isolated compound.
Results: An isolate of Penicillium vinaceum (strain no. X17) was obtained from the corm of C. sativus. Chemical investigations of the endophyte culture broth afforded an unique quinazoline alkaloid (1), identified as (-)-(1R,4R)-1,4-(2,3)-indolmethane-1-methyl-2,4-dihydro-1H-pyrazino-[2,1-b]-quinazoline-3,6-dione, which showed cytotoxic (IC50 range 40.55–76.83 μg/mL) and antifungal (MIC80 range 16–64 μg/mL) activities.
Discussion and conclusions: Endophytes in C. sativus can be a rich source of novel bioactive compounds, which prompts us to expand the medicinal resource of this valuable plant in another way. Compound 1 exhibited potential cytotoxic and antifungal activities and may be considered a lead compound for promising antifungal and anticariogenic agent.
Keywords::
Introduction
Crocus sativus Linn. (Iridaceae), commonly known as saffron, is a perennial stemless plant. Saffron stigma has become more and more popular due to its versatile biological and medicinal properties, such as anaphrodisiac, antispasmodic, expectorant, antidepressant, and stomachic activity (CitationAbdullaev, 1993; CitationRichelson, 1994; CitationWang et al., 2010). However, high demand but low yield resulted in extreme scarcity of this valuable medicinal resource.
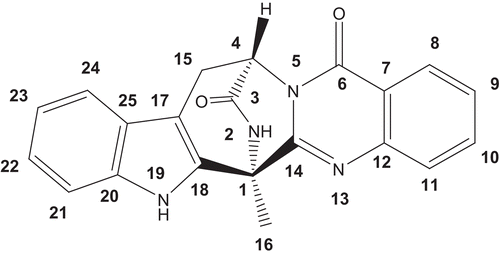
Endophytic fungi are microorganisms living within the tissues of host plants, and have proven to be rich sources of bioactive secondary metabolites, some of which are shared with the hosts (CitationYin et al., 2009; CitationKusari et al., 2009; CitationZhu et al., 2010). The present study is therefore conducted to investigate the chemical compositions of endophytic fungus isolated from C. sativus, so as to expand the medicinal resource of this valuable plant in another way. As a result, a structurally unique quinazoline alkaloid (1) was isolated from cultures of Penicillium vinaceum, an endophytic fungus in C. sativus. Herein, we describe the isolation, structural elucidation, cytotoxicities, and antifungal activity of this metabolite ().
Materials and methods
General
Optical rotations were acquired with a Perkin-Elmer 341 polarimeter. UV spectra were run on a Varian Cary Eclipse 300 spectrophotometer. IR spectra were recorded on a Bruker Vector 22 spectrometer with KBr pellets. NMR spectra were recorded on a Bruker Avance 400 NMR spectrometer with TMS as an internal standard. ESIMS were measured on an Agilent LC/MSD Trap XCT mass spectrometer, whereas HRESIMS were measured using a Q-TOF micro mass spectrometer (Waters, ). Materials for CC were silica gel (100–200 mesh; Huiyou Silical Gel Development Co. Ltd. Yantai, China), silica gel H (10–40 μm; Yantai), Sephadex LH-20 (40–70 μm; Amersham Pharmacia Biotech AB, Uppsala, Sweden), and YMC-GEL ODS-A (50 μm; YMC, Milford, MA). Preparative TLC (0.4–0.5 mm) was conducted on glass plates precoated silica gel GF254 (Yantai).
Plant and fungal materials
C. sativus were collected from the growing fields of saffron in the Changxing Island, Shanghai, China, in November 2008. Taxonomic identification was performed by Prof. Han-Chen Zheng, Department of Pharmacognosy, School of Pharmacy, Second Military Medical University. A voucher specimen (#2008-224) of the plant was deposited in the herbarium of the Department of Pharmacognosy, School of Pharmacy, Second Military Medical University. The strain, No. X17, was isolated from the corm of C. sativus. Endophytes of corm samples were isolated according to the traditional method described previously (CitationGuo et al., 2000). Endophytes obtained were maintained on potato dextrose agar (PDA) slants and subcultured every month. Slants were incubated at 25°C for 5 days and subsequently stored at 4°C. The isolate (X17) was identified as P. vinaceum from its cultural and morphological properties according to CitationRaper and Thom (1949).
Extraction and isolation
P. vinaceum was initially grown on a PDA medium in Petri dish, and then transferred into a shake flask culture by punching out 5 mm of the agar plate culture with a self-designed cutter (CitationWang et al., 2006). The shake flask culture was carried out (250 mL) containing 100 mL PDB (0.4 g potato extract, 2.0 g dextrose and 100 mL distilled water) which were autoclaved at 125°C for 20 min. Biomass was removed by filtration, and then fermentation broth (40 L) was extracted by EtOAc after incubation for 6 days at 28°C on rotary shakers at 180 rpm (CitationYou et al., 2009). Crude extract (6.20 g) was chromatographed on a silica gel column eluting with a step gradient of petroleum ether-acetone (30:1, 15:1, 10:1, 5:1, 3:1, 1:1) to give 6 fractions (Fr1-Fr6). Fr4 (268 mg) was separated over silica gel with petroleum ether-acetone (5:1) and size exclusion chromatography eluted with MeOH-H2O (80%) to afford compound 1 (6 mg).
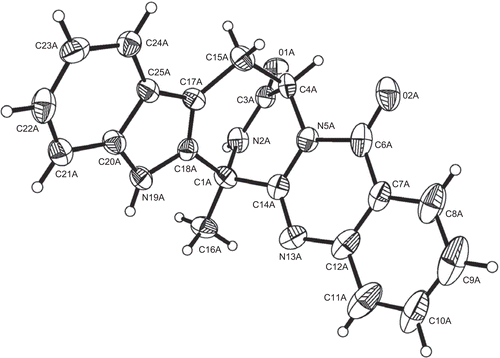
X-ray crystallographic analysis of compound 1
Upon crystallization from MeOH using the vapor diffusion method, colorless crystals were obtained for 1, a crystal was separated from the sample and mounted on a glass fiber. X-ray crystallographic analysis was carried out on a Bruker Smart Apex CCD diffractometer with graphite-monochromated Mo Kα radiation (λ = 0.71073 Å). The crystal structures were solved by direct methods, subsequent difference Fourier syntheses, and refined by full-matrix least-squares refinement on F2 by using the SHELXL-97 program. Non-hydrogen atoms were refined with anisotropic displacement parameters. Hydrogen atoms were located by geometry and rode on the related atoms during refinements with a temperature factor of 1.5 times of the latter (CitationSheldrick, 1997).
(-)-(1R,4R)-1,4-(2,3)-Indolmethane-1-methyl-2,4-dihydro-1H-pyrazino-[2,1-b]-quinazoline-3,6-dione (1): white needles; m.p. 159–160°C; [α] −136° (c 0.16, MeOH); UV λmax (MeOH) nm: 220, 268, 290; IR (KBr) vmax 3233, 3064, 2924, 2853, 1685, 1607; 1H-NMR (DMSO-d6, 400 MHz) and 13C-NMR (DMSO-d6, 100 MHz) spectra (); HRESIMS m/z 357.1355 [M + H]+ (calcd for C21H17N4O2, 357.1352).
Table 1. 1H NMR and 13C NMR data of compound 1.
Crystal data
Empirical formula: C21H16N4O2. Formula weight: 356.38. Crystal system: monoclinic. Space group: P21. Crystal size: 0.25 × 0.15 × 0.12 mm. Unit cell dimensions: a = 10.785(7) Å, b = 18.503(12) Å, c = 14.948(10) Å, α = β = γ = 90º, V = 2951(3) Å3. Index ranges: −12 ≤ h ≤ 12, −21 ≤ k ≤ 22, −17 ≤ l ≤ 15. θ Range for data collection from 1.38° to 25.20o. Z = 6. Dc = 1.203 g/cm3. F (000) = 1116. Refinement method: Full-matrix least-squares on F2. Goodness-of-fit on F2: 0.928. Final R indices [I > 2σ (I)]: R1 = 0.0584, wR2 = 0.1546. R indices (all data): R1 = 0.0950, wR2 = 0.1675. Largest differences in peak and hole: 0.210 and −0.180 e/Å−3.
Antifungal activity assay
The antifungal activities of the samples were individually tested against pathogenic fungi including Candida albicans (ATCC 76615), Cryptococcus neoformans (ATCC 32609), Trichophyton rubrum and Aspergillus fumigatus by using serial dilution method (CitationAta et al., 2009). Briefly, Sabouraud dextrose agar (SDA) slant medium was employed for pathogenic fungal growth. Dilutions of compound 1 were prepared in dimethyl sulfoxide. The tested solutions were serially diluted (2:1) in 96-well plates. Organisms at a concentration of approximately 1–5 × 103 colony forming units/mL were then added to each well. Plates were made in triplicate and incubated at 35°C for about 24 h for C. albicans, about 72 h for C. neoformans and about 168 h for T. rubrum and A. fumigatus, then their turbidity obtained by measuring optical density at 630 nm. The standard antifungal agent amphotericin B was used as a positive control and experiments were repeated at least three times. Test substance concentrations at which fungi proliferation was reduced by 80% are given as MIC80 values and the values presented are an average of triplicate ().
Table 2. Antifungal activity of compound 1 [MIC80 (μg/mL)].
Cytotoxic activity assay
The human lung tumor cell line A549, human promyelocytic leukemia cell line HL-60, human colon tumor cell line LOVO and human breast adenocarcinoma cell line MCF-7 were employed in the test. The cells viability were determined using a modified 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium (MTT) assay (CitationSharifi et al., 2005). Briefly, A549, HL-60, MKN-45 and HepG2 cells were grown in RPMI 1640 including 100 units/mL penicillin and streptomycin supplemented with 15% new-born bovine serum at 37°C in a 5% CO2 atmosphere. For experimentation, the exponentially growing cells (4–6 × 104) were used. Then, cells were incubated in the presence of 100, 75, 50, 25, 12.5 μg/mL samples in DMSO for 72 h at 37°C. After removing the sample solution and washing with phosphate-buffered saline (pH 7.4), 10 μL/well of 0.5% MTT bromide cells phosphate-buffered saline solution was added. After a further 4 h of incubation, 0.04 M HCl was added. Viable cells were determined by measuring the absorbance at 570 nm. Measurements were performed three times, and the concentration required for a 50% inhibition of viability (IC50) was determined. The values presented in are an average of triplicates.
Table 3. The in vitro cytotoxic activity of compound 1 [IC50 (μg/mL), mean ± SD, n = 3].
Results and discussion
The EtOAc extract of the cultures of P. vinaceum was successively subjected to repeated silica gel and Sephadex LH-20 column chromatography to afford a structurally unique quinazoline alkaloid (1), whose structure was determined by 1H-, 13C-, and 2D-NMR data together with X-ray crystallographic analysis.
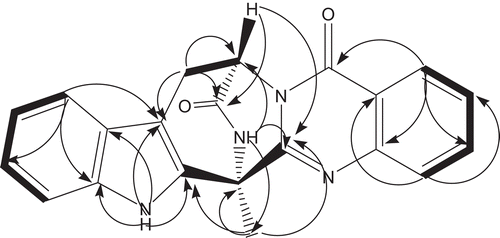
Compound 1, was obtained as colorless needles and analyzed for the molecular formula C21H16N4O2 positive HRESIMS [M + H]+ at m/z 357.1355 (calcd 357.1352), which was supported by its NMR data (). The IR spectrum of 1 indicated the existence of amide group (3233, 3064, 1685 cm−1). The 1H and 13C NMR spectra together with DEPT and 1H-13C COSY experiments indicated the presence of a methyl (δH 2.12; δC 18.3), a methylene (δH 3.42, 3.22; δC 25.6) adjacent to an olefinic group, a nitrogenated methane (δH 5.71, br s; δC 54.1), eight aromatic methines attributed to double benzene rings of 1,2-disubstitution, two carbonyl groups (δC 169.1, 159.3), and seven olefinic quaternary carbons. Two amide proton singlets at δH 11.22 and 9.58 was assigned to H-19 and H-2, respectively. Four aromatic doublets and four aromatic triplets confirmed the 1,2-disubstituted pattern of two benzene rings. The only methyl group was attached to C-1, deduced from cross-peaks of δH 2.12 (H-16) with δC 54.6 (C-1), 154.3 (C-14), and 134.0 (C-18). Analysis of the 1H–1H COSY NMR data () led to the identification of three isolated proton spin-systems corresponding to the C-4–C-15, C-8–C-11, and C-21–C-24 subunits of structure 1. All these data suggested that compound 1 contains a pyrazino-[2,1-b]-quinazoline-3,6-dione moiety and an indole ring consistent with the results of X-ray diffraction analysis (). We finally elucidated the structure of compound 1 as (-)-(1R,4R)-1,4-(2,3)-indolmethane-1-methyl-2,4-dihydro-1H-pyrazino-[2,1-b]-quinazoline-3,6-dione, a known quinazoline alkaloid synthesized as a precursor for (-)-alantrypinone (CitationHart & Magomedov, 1999). To the best of our knowledge, this is the first time that compound 1 has been found in plant endophytes, although fungi belonging to the genera Penicillium serve as a rich source of alkaloids clearly derived from amino acids (CitationHart & Magomedov, 2001). These quinazoline alkaloids have been reported to show moderate cytotoxicities in vitro (CitationTakahashi et al., 1995).
Compound 1 was tested for its antifungal against four pathogenic fungi isolates and showed good results () except for A. fumigatus (MIC80 > 128 μg/mL). It exhibited potent activity against C. neoformans with the lowest MIC80 at 16 μg/mL. Compound 1 was also active in inhibiting the growth of C. albicans and T. rubrum with MIC80 values of 32 and 64 μg/mL, respectively. Also, it was tested against several tumor cell lines. As shown in , compound 1 exhibited moderate cytotoxicities against A549, LOVO and MCF-7, with IC50 values of 76.83, 68.08 and 40.55 μg/mL, respectively, whereas no activity was observed for HL-60. Nevertheless, further studies are needed to investigate the action mechanism of this compound, which may be considered a lead compound for promising antifungal and anticariogenic agent.
Acknowledgments
The authors are grateful for the assistance of Lei Guo, Lili Xu and Hongsheng Yu of the Department of Pharmacognosy, School of Pharmacy, Second Military Medical University. Crystallographic data for the structure reported in this paper have been deposited at the Cambridge Crystallographic Data Center as supplementary publication number CCDC 793817. Copies of the data can be obtained, free of charge, on application to the Director, 12 Union Road, Cambridge CB2 1EZ, UK (Fax: +44-(0)1223–336033 or e-mail: [email protected]).
Declaration of interest
The authors report no conflicts of interest.
References
- Abdullaev FI. (1993). Biological effects of saffron. Biofactors, 4, 83–86.
- Ata A, Gale EM, Samarasekera R. (2009). Bioactive chemical constituents of Caesalpinia bonduc (Fabaceae). Phytochem Lett, 2, 106–109.
- Guo LD, Hyde KD, Liew ECY. (2000). Identification of endophytic fungi from Livistona chinensis based on morphology and rDNA sequences. New Phytol, 147, 617–630.
- Hart DJ, Magomedov NA. (2001). Synthesis of ent-alantrypinone. J Am Chem Soc, 123, 5892–5899.
- Hart DJ, Magomedov NA. (1999). Synthesis of (-)-alantrypinone. Tetrahedron Lett, 40, 5429–5432.
- Kusari S, Zühlke S, Spiteller M. (2009). An endophytic fungus from Camptotheca acuminata that produces camptothecin and analogues. J Nat Prod, 72, 2–7.
- Raper KB, Thom C. (1949). Manual of Penicillia. Williams and Wilkins Co. Balitimore, USA.
- Richelson E. (1994). Pharmacology of antidepressants-characteristic of the ideal drug. Mayo Clin Proc, 69, 1069–1081.
- Sharifi AM, Mousavi SH, Bakhshayesh M, Tehrani FK, Mahmoudian M, Oryan S. (2005). Study of correlation between lead-induced cytotoxicity and nitric oxide production in PC12 cells. Toxicol Lett, 160, 43–48.
- Sheldrick GM. (1997). SHELXL-97, program for X-ray crystal structure refinement. University of Göttingen: Göttingen.
- Takahashi C, Matsushita T, Doi M, Minoura K, Shingu T, Kumeda Y, Numata A. (1995). Fumiquinazolines A-G, novel metabolites of a fungus separated from a Pseudolabrus marine fish. J Chem Soc Perkin Trans I, 2345–2353.
- Wang Y, Han T, Zhu Y, Zheng CJ, Ming QL, Rahman K, Qin LP. (2010). Antidepressant properties of bioactive fractions from the extract of Crocus sativus L. J Nat Med, 64, 24–30.
- Wang JW, Wu JH, Huang WY, Tan RX. (2006). Laccase production by Monotospora sp., an endophytic fungus in Cynodon dactylon. Bioresour Technol, 97, 786–789.
- Yin H, Zhao Q, Sun FM, An T. (2009). Gentiopicrin-producing endophytic fungus isolated from Gentiana macrophylla. Phytomedicine, 16, 793–797.
- You F, Han T, Wu JZ, Huang BK, Qin LP. (2009). Antifungal secondary metabolites from endophytic Verticillium sp. Biochem Syst Ecol, 37, 162–165.
- Zhu D, Wang J, Zeng Q, Zhang Z, Yan R. (2010). A novel endophytic Huperzine A-producing fungus, Shiraia sp. Slf14, isolated from Huperzia serrata. J Appl Microbiol, 109, 1469–1478.