Abstract
Objective: The present work explored the potential hepatoprotective activity of total ethanol and successive extracts of Cyperus alternifolius L (Cyperaceae) against carbon tetrachloride (CCl4)–induced hepatotoxicity in rats and to isolate their bioactive constituents.
Methods: For isolation and identification of the compounds, column chromatography and spectroscopic analysis were used, a model of hepatotoxicity by CCl4 in rats was used to evaluate the total ethanol extract and its successive fractions.
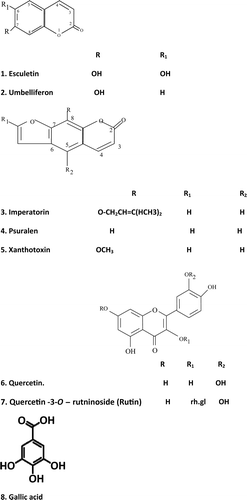
Results: Phytochemical screening of C. alternifolius revealed the presence of different phytochemical groups. The plant proved to be safe for human use because it did not induce any signs of toxicity or mortality in mice when administered orally at doses up to 5000 mg kg−1. The total alcoholic extract in doses of 100 and 200 mg kg−1 and the successive extracts (ether, chloroform and ethyl acetate) in a dose of 10 mg kg−1 exhibited a significant (p ≤ 0.05) protective effect by lowering the elevated serum levels of aspartate aminotransferase: 230.4, 218.8, 224.6, 227.4 and 231.6 U L−1, respectively, compared with 111.6 U L−1 for silymarin (25 mg kg−1). Serum levels of alanine aminotransferase were also reduced: 77.4, 72.7, 79.7, 76.0 and 79.7 U L−1 compared to 63.7 U L−1 for silymarin. Alkaline phosphatase: 164.6, 158.0, 163.6, 154.7 and 166.4 U L−1 compared to 138.2 U L−1 for silymarin. Total bilirubin: 0.50, 0.46, 0.55, 0.52 and 0.57 mg dl−1 compared to 0.42 mg dl−1 for silymarin. Cholesterol: 213.1, 200.0, 192.7, 193.6 and 197.1 mg dl−1 compared to 180.3 mg dl−1 for silymarin. Triglycerides: 237.3, 222.4, 209.5, 206.8 and 210.2 mg dl−1 compared to 196.8 mg dl−1 for silymarin. Eight phenolic compounds were isolated from C. alternifolius for the first time and identified as esculetin 1, umbelliferon 2, imperatorin 3, psoralen 4, xanthotoxin 5, quercetin 6, quercetin-3-O-rutinoside 7 and gallic acid 8.
Conclusions: The results concluded that C. alternifolius possesses significant protective effect against hepatotoxicity induced by CCl4.
Introduction
The genus Cyperus is a large genus of the sedge family, Cyperaceae. Members of Cyperaceae are monocotyledon, grass-like, flowering plants, commonly found in wet areas and known as sedges (CitationGregory & Cutler, 1960). The Cyperaceae is rich in secondary metabolites including tannins, phenolic acids, flavonoids (CitationTrease & Evans, 1976), coumarins (CitationSharma, 1993) and sesquiterpenoids (CitationMorimoto et al., 1999). The genus Cyperus comprises about 550 species which are widely distributed all over the world (CitationVare & Kukkonen, 2005). The most common species includes Cyperus alternifolius L (umbrella sedge). Phytochemical study on the aerial parts of Cyperus species led to isolation of flavonoids and coumarins (CitationSayed et al., 2008) as the most important constituents (CitationYu et al., 2007).
Liver ailments remain a serious health problem (CitationBaranisrinivasan et al., 2009). Various xenobiotics are known to cause hepatotoxicity, one among them is carbon tetrachloride (CCl4) (CitationKodavanti et al., 1989), which has proved to be highly useful as an experimental agent for the induction of acute liver injury. Conventional and synthetic drugs used in the treatment of liver diseases are sometimes inadequate and can have serious adverse effects. It is therefore necessary to search for alternatives to replace currently used drugs of doubtful efficacy and safety (CitationArhoghro et al., 2009). Natural remedies from medicinal plants are considered to be effective and safe alternative treatments for hepatotoxicity (CitationAwaad et al., 2006; CitationAwaad & Al-Jaber, 2010). The current study was undertaken to evaluate the potential protective effect of C. alternifolius on CCl4-induced hepatotoxicity in rats.
Materials and methods
Plant material
The aerial parts of C. alternifolius were collected during the flowering stage in March 2005 from Orman Garden, Egypt. The sample was kindly identified by Dr. M. Gebali, botanist, and by comparison with the published plant description (CitationEl-Gohary, 2004). A voucher specimen has been deposited in the herbarium of the Desert Research Center. Plant material was air-dried in shade, reduced to fine powder, packed in tightly closed containers and stored at room temperature for phytochemical and biological studies.
Extraction
The air-dried powder of C. alternifolius aerial parts (1 kg) was extracted by percolation in 90% ethanol (CitationAwaad et al., 2006) at room temperature for 2 days. The ethanol extract was filtered and the residues were re-percolated for four times. The total ethanol extract was concentrated under reduced pressure at a temperature not exceeding 35°C to yield a dry extract of 190 g.
The total ethanol extract (50 g) was dispersed in 1000 ml of distilled water and extracted successively with diethyl ether, chloroform, ethyl acetate and n-butanol, respectively. Each extract was dried over anhydrous sodium sulfate and concentrated under reduced pressure at a temperature not exceeding 35°C to yield 8.5, 10.6, 15.6 and 19.0 g dry extracts, respectively.
Preparation of the extracts for biological studies
The total ethanol and successive extracts of C. alternifolius and the standard silymarin were suspended in distilled water using Tween 80 (3% v/v).
Experimental animals
Both sex of adult albino mice (25–30 g body weight) and albino rats (180–200 g body weight) were used. They were housed randomly in groups in polypropylene cages, fed with standard rodent diet and water ad libitum. Animals were allowed to acclimatize to the work area environment for 2 weeks before use. Each animal was used for one experiment only. The care and handling of the animals were in accordance with the internationally accepted standard guidelines and was approved by an institutional review board.
Determination of median lethal dose (LD50)
LD50 of the total ethanol extract of C. alternifolius plant was estimated in mice by using the method of CitationLorke (1983). In a preliminary test, animals in groups of three, received one of 10, 100 or 1000 mg kg−1 of the tested extract suspended in the vehicle (3% v/v Tween 80). Animals were observed for 24 h for signs of toxicity and number of deaths. Depending on the results of the preliminary test, doses of 1250, 2500 and 5000 mg kg−1 of the tested extract were administered to fresh groups, each of six mice. Control animals were received the vehicle and kept under the same conditions. Signs of toxicity and number of deaths per dose were recorded within 24 h and the LD50 was calculated as the geometric mean of the dose that resulted in 100% mortality and that which caused no lethality at all.
Experimental induction of hepatic damage
CCl4 was dissolved in corn oil in the ratio 1:1 v/v. Liver damage was induced in rats following subcutaneous (SC) injection of CCl4 in the lower abdomen at a dose of 3 ml kg−1 (CitationThéophilea et al., 2006).
Hepatoprotective activity
Sixty adult albino rats were randomly divided into 10 groups of six animals, each. Rats of the 1st (normal control) and 2nd (CCl4-intoxicated control) groups received the vehicle in a dose of 1 ml kg−1. Animals of the 3rd group (reference) received silymarin at a dose of 25 mg kg−1. The 4th, 5th and 6th groups were treated with the total ethanol extract of C. alternifolius plant at the dose levels of 50, 100 and 200 mg kg−1, respectively. The 7th, 8th, 9th and 10th groups were administered 10 mg kg−1 of the successive extracts (ether, chloroform, ethyl acetate and n-butanol, respectively). All medications were administered orally by gastric intubation for 8 consecutive days. Two hours after the last dose, normal control rats were given a single dose of corn oil (3 ml kg−1, SC), whereas animals of the 2nd to 10th groups were received a single SC dose of CCl4 (3 ml kg−1).
Blood sampling
After 24 h of corn oil and CCl4 injections, blood samples (2 ml) were collected from all rats by puncturing their retro-orbital plexus of veins. Serum was separated by centrifugation at 2500 rpm for 15 min at 4°C and used for the biochemical assay.
Biochemical analysis
The serum activity of liver marker enzymes (AST and ALT) was assayed following the method of CitationReitman and Frankel (1957), whereas the activity of alkaline phosphatase (ALP) was estimated by the method of CitationBabson et al. (1966). The levels of total protein (CitationHenary et al., 1974) and albumin (CitationDoumas et al., 1971) were estimated in serum. The serum concentrations of total bilirubin (CitationWalter & Gerarade, 1970), cholesterol (CitationZoppi & Fenili, 1976) and triglycerides (CitationFossati & Prencipe, 1982) were assayed.
Statistical analysis
Values are expressed in mean ± SEM for six animals in each group. p value was calculated using analysis of variance followed by Dunnett’s test for multiple comparisons. Values of p < 0.05 were considered significant in all cases.
Isolation of the phenolic compounds
TLC examination of the different extractives (CitationStahl, 1969) using two different solvent systems (chloroform-methanol (a) 98:2 and ethyl acetate-methanol-water (b) 60:5:4) and spray reagents revealed that both ether and chloroform extracts are identical (same numbers and color of spots); in addition both extracts exhibit the higher activity as hepatoprotective. Therefore, both extracts were collected together (15 g), fractionated on column packed with silica gel (450 g) and eluted gradually with benzene-ethyl acetate, 120 fractions were collected (60 ml each) and reduced to 3 main fractions, these sub-fractions were reapplied on preparative thin layer chromatography using system a. For final purifications, each compound was reapplied on column packed with Sephadex LH20 and eluted with methanol. The fractions were concentrated under reduced pressure to produce compounds 1–5. Hepatoprotection was achieved in rats following medication of ethyl acetate and butanol extracts but was less significant, accordingly they were subjected to fractionation as follows.
The ethyl acetate and butanol were found to be identical adopting solvent systems (ethyl acetate-methanol-water 30:5:4) (d), visualized by UV before and after spraying with aluminum chloride, accordingly. Both of them were collected together (20 g) and fractionated on column packed with silica gel (600 g) and eluted with ethyl acetate-methanol-water (120:5:4), 100 fractions were collected (50 ml each) and reduced to 3 main fractions in different yields 3.4, 2.7 and 1.6, respectively (according to the number, color and Rf values of the spots), these sub-fractions were reapplied on preparative paper chromatography using system acetic acid-water (85:15). For final purifications, each compound was reapplied on column backed with Sephadex LH20 and eluted with methanol. The fractions were concentrated under reduced pressure to produce compounds 6-8.
Esculetin 1
Needle crystals (20 mg), Rf = 0.12 (system a), m.p. 272–274°C. UV λmax (MeOH): 226, 257, 293 and 346 nm. EI-MS m/z (% rel. int.): M+ 179 (100), 150 (20), 132 (3), 122 (22), 121 (18), 94 (20) and 69 (10). 1H NMR (DMSO-d6): δ 7.9 (1H, d, J = 9.5 Hz, H-4); δ 7.1 (1H, S, H-5); δ 6.79 (1H, S, H-8) and δ 6.2 (1H, d, J = 9.5 Hz, H-3). 13C NMR (DMSO): 161.28 (C-2′), 110.77 (C-3′), 144.60 (C-4′), 112.25 (C-5′), 148.40 (C-6′), 150.33 (C-7′), 102.53 (C-8′), 142.84 (C-9′), 110.77 (C-10′).
Umbelliferone 2
White crystals (25 mg), Rf = 0.75 (system a), m.p. 272–274°C. UV λmax (MeOH): (nm), 244, 257, 320 and 324. EI-MS m/z (% rel. int.): 162 (M+) (100), 134 (15), 106 (10), 78 (9), 77 (10) and 51 (5). 1H NMR (DMSO-d6): δ 7.79 (1H, d, J = 9.5 Hz, H-4); δ 7.42 (1H, d, J = 8.4 Hz, H-5); δ 6.77 (1H, dd, J = 8.4, J = 2.2 Hz, H-6); δ 6.71 (1H, d, J = 2.2 Hz, H-8) and δ 6.1 (1H, d, J = 9.5 Hz, H-3). 13C NMR (DMSO): 161.101 (C-2′), 121.40 (C-3′), 145.10 (C-4′), 129.78 (C-5′), 113.42 (C-6′), 162.11 (C-7′), 103.10 (C-8′), 146.5 (C-9′), 112.34 (C-10′).
Imperatorin 3
Obtained as needle crystal from methanol (30 mg). Rf = 0.65 (system a), m.p. 102–103°C. λmax (EtOH) 302, 265 and 250 nm. EI-MS m/z (% rel. int.): 270 (100), 201 (50), 215 (10), 255 (30) and 174 (80). 1H NMR (CDCl3): δ 6.35 (1H, d, J = 9.5 Hz, H-3); δ 7.73 (1H, d, J = 10 Hz, H-4); δ 7.29 (1H, S, H-5); δ 6.79 (1H, d, J = 2.1 Hz, H-4′); δ 7.69 (1H, d, J = 2.6 Hz, H-5′); δ 5.08 (2H, d, OCH3, H-8) and δ 1.68 (6H, S, 2CH3). 13C NMR (DMSO): 161.46 (C-2), 113.58 (C-3), 145.43 (C-4), 113.80 (C-5), 126.41 (C-6), 148.73 (C-7), 131.06 (C-8), 143.75 (C-9), 116.62 (C-10), 147.15 (C-2′), 106.59 (C-3′), 69.51 (C-1′′), 119.53 (C-2′′), 139.70 (C-3′′), 16.67 (C-4′′), 24.53 (C-5′′).
Xanthotoxin 4
Obtained as needle crystals from methanol (50 mg). Rf = 0.54 (system a), m.p. 144–146°C. λmax (EtOH) 202, 186, 249 and 300 nm. MS showed M+ at m/e 216 (100), and fragment ions at m/e 202 (20), 186 (18), 185 (23), 173 (21), 145 (16) and 71 (12). IR (KBr) Vmax cm−1: 1736, 1628, 1579, 1510, 1214 and 1008. 1H NMR (CDCl3): δ 6.33 (1H, d, J = 9.5 Hz, H-3); δ 7.8 (1H, d, J = 9.5 Hz, H-4); δ 7.35 (1H, S, H-5), δ 7.1 (1H, d, J = 2.5 Hz, H-4′); 7.62 (1H, d, J = 2.5 Hz, H-5′).1H NMR (CDCl3): 161.36 (C-2), 113.59 (C-3), 142.80 (C-4), 116.65 (C-5), 126.66 (C-6), 147.73 (C-7), 60.41 (C-8), 147.17 (C-2′), 106.60 (C-3′), 145.30 (C-4′), 113.70 (C-5′).
Psoralen 5
Obtained as white needle crystals, soluble in methanol, m.p. 102–103°C, Rf value: 0.46 (system a). UV showing λmax at 250, 265 and 302 nm. EI-MS showed m/z: 200 (100%), 270 (70%), 202 (50%), 253 (25%) and 238 (35%). IR (KBr) Vmax cm−1: 1721, 1628, 1579, 1510, 1214 and 1008. 1H NMR (CDCl3): δδ 6.36 (1H, d, J = 9.6 Hz, H-3), δ 8.00 (1H, d, J = 9.6 Hz, H-4), δ 7.54 (1H, s, H-5), δ 7.86 (1H, d, J = 2.3 Hz, H-2′), δ 6.92 (1H, d, J = 2.3 Hz,H-3′), δ 4.95 (1H, d, J = 7.2 Hz, H-1′′), δ 5.53 (1H, t, J = 7.2 Hz, H-2′′), δ 1.68 (3H, S, H-4′′), δ 1.64 (3H, S, H-5′′). 13C NMR (methanol-d3): 161.46 (C-2), 113.58 (C-3), 145.43 (C-4), 113.80 (C-5), 126.41 (C-6), 148.73 (C-7), 131.06 (C-8), 143.75 (C-9), 116.62 (C-10), 147.15 (C-2′), 106.59 (C-3′), 69.51 (C-1′′), 119.53 (C-2′′), 139.70 (C-3′′), 16.67 (C-4′′), 24.53 (C-5′′).
Quercetin 6
Yellow needle crystals (20 mg) Rf = 0.7 (system b), m.p. 313–315°C. UV λmax (MeOH): (nm) 255, 269 (sh), 370; (AlCl3): 270, 290 (sh), 455; (AlCl3/HCl): 270, 357, 426; (NaOAc): 274 (Dec.), 325; (NaOAc/H3BO3): 261, 385; (NaOMe): 246, 330, 398. 1H NMR (DMSO-d6): δ 8.16 (1H, d, J = 2 Hz, H-6); δ 7.67 (1H, d, J = 2 Hz, H2′); δ 7.6 (1H, dd, J6′,2′ = 8 Hz, H-6′); δ 6.80 (1H, d, J = 8 Hz, H-5′) and δ 6.3 (1H, d, J = 2 Hz, H-8).
Quercetin-3-O-rutinoside 7
Yellow crystals (600 mg) Rf = 0.5 (system b), m.p. 190°C. UV λmax (MeOH): (nm) 256, 265 (sh), 290, 355; (AlCl3): 274, 302 (sh), 330 (sh), 432; (AlCl3/HCl): 270, 298, 359, 399; (NaOAc): 272, 324, 398; (NaOAc/H3BO3): 263, 292 (sh), 368; (NaOMe): 272, 310, 410. 1H NMR (DMSO-d6): δ 8.10 (1H, d, J = 2 Hz, H2′); δ 7.86 (1H, d, J = 8 Hz, H-6′); δ 6.89 (1H, d, J = 8 Hz, H-5′); δ 6.65 (1H, d, J = 2 Hz, H-8); δ 6.5 (1H, d, J = 2 Hz H-6); δ5.13 (1H, d, J = 7.50 Hz H1′′); δ 4.55 (1H, d, J = 1.3 Hz, H1′′′); δ 3.82 (1H, dd, J = 10 Hz, J = 2 Hz H-6′′); δ 3.65 (1H, dd, J = 3.5, H2′′′); δ 3.47–3.87 (6H, m, sugar protons) and δ 1.23 (3H, d, J = 6 CH3).
Gallic acid 8
Obtained as white crystals, soluble in methanol. Rf value = 0.74 (system b). m.p. 252°C. 1H NMR (methanol-D3): δ (ppm) 7.0 (2H, s). 13C NMR (methanol-d3): δ.120.7 C-1, 108.9 C-2, 145.5 C-3, 138.2 C-4, 145.0 C-5, 108.9 C-6, 169.1 C-7 (carbonyl).
Results and discussion
Isolation of phenolic compounds
Eight phenolic compounds were isolated from C. alternifolius for the first time () and identified as esculetin, umbelliferon, imperatorin, psoralen, xanthotoxin, quercetin, rutin and gallic acid using different spectroscopic analysis such as 1H NMR, 13C NMR DEPT, COSY, HMBC, HMQC and mass spectrometry.
Compounds 1–5 were isolated from ether and chloroform extracts. They were identified as esculetin, umbelliferon, imperatorin, psoralen and xanthotoxin by comparing their TLC chromatograms, UV spectrum in methanol and with different shift reagents, EI-MS, 1H NMR and 13C NMR spectra with authentic samples and published data (CitationMurray et al., 1982). Compound 6 migrated with quercetin. Its spectral data were in agreement with those published for this compound (CitationMabry et al., 1970).
Acid hydrolysis (CitationHarborne et al., 1975) of compound 7 revealed the sugar rhamnose glucose which identified by PC and TLC (systems e and f) and an aglycone which was found to be identical with compound 6 when compared with its TLC and UV shift reagents. There are substituted at position 3 as indicated by their UV spectra upon addition of diagnostic shift reagent. Accordingly, based on the results obtained and some of the published data (CitationGeissman, 1962; CitationMabry et al., 1970; CitationHarborne et al., 1975), this compound was identified as quercetin-3-O-rutinoside (rutin).
Compound 8 was isolated from ethyl acetate and butanol extracts. It was obtained as a white crystal after crystallization from methanol and identified as gallic acid.
Determination of median lethal dose (LD50)
The total ethanol extract of C. alternifolius did not produce any behavioral changes and mortality in mice in doses up to 5000 mg kg−1. Accordingly, it suggested that oral LD50 of the total extract was higher than 5000 mg kg−1. Therefore, the tested plant can be categorized as highly safe because substances possessing LD50 higher than 50 mg kg−1 are non-toxic (CitationBuck et al., 1976).
Hepatoprotective activity
Hepatotoxicity induced by CCl4 is the most commonly used model system for the screening of hepatoprotective activity of plant extracts. Acute exposure to CCl4 produces rapid cellular injury due to its reductive dehalogenation in the endoplasmic reticulum of hepatocytes to generate an unstable highly reactive complex CCl3• or trichloroperoxyl radical (CCl3O3). These radicals attack microsomal lipids and proteins causing lipid peroxidation. Products of lipid peroxidation may cause damage to the biological membranes leading to serious cellular injury and leakage of serum marker enzymes like AST, ALT and ALP (CitationCotran et al., 1994) and finally cell death (CitationWei, 1998). The prevention of this phenomenon can be considered as hepatoprotective activity (CitationPoon et al., 2003).
Estimations of serum transaminases and ALP are the most sensitive tests for diagnosis of liver diseases (CitationMahendale et al., 1985). In this study, SC injection of CCl4 to rats leads to a marked elevation in the levels of serum AST, ALT and ALP after 24 h of exposure compared to normal control rats, indicating acute hepatocellular damage (). This might be due to the release of these enzymes from the cytoplasm, into the blood circulation rapidly after rupture of the plasma membrane and cellular damage (CitationSallie et al., 1991). Pretreatment with the total ethanol extract of C. alternifolius plant (100 and 200 mg kg−1) and the ethereal, chloroform and ethyl acetate successive extracts (10 mg kg−1) significantly reversed the levels of hepatic enzymes when compared to CCl4-treated animals (). In the group where silymarin was given, the levels of hepatic enzymes were significantly lower (p < 0.05) than in the CCl4-treated group. The protective effect of plant extracts against CCl4 may be attributed to the presence of flavonoids (CitationGilani & Janbaz, 1995) or coumarins (CitationPark et al., 2004) among the plant constituents.
Table 1. Effect of the total ethanolic and successive extracts of C. alternifolius on the activity of AST, ALT and ALP enzymes and levels of total proteins and albumin in serum of rats with CCl4-induced hepatotoxicity (n = 6).
The administration of CCl4 alone adversely interferes with protein metabolism probably by inhibiting the synthesis of proteins in the liver such as albumin. In the present study, the levels of total protein (5.10 ± 0.15 g dl−1) and albumin (3.38 ± 0.10 g dl−1) were significantly (p < 0.05) reduced after intoxication with CCl4 in comparison with normal control rats (6.16 ± 0.25 and 4.02 ± 0.16 g dl−1, respectively). The lowered levels of total protein and albumin in the serum reveal the intensity of hepatopathy (CitationAniya et al., 2005). Moreover, defects in protein metabolism, evidenced by changes in total protein and/or albumin level, are used to indicate the severity of the hepatic diseases (CitationHenry, 1984). Pretreatment with the total ethanol extract of C. alternifolius plant (100 and 200 mg kg−1) and the ethereal, chloroform and ethyl acetate successive extracts (10 mg kg−1) to CCl4-intoxicated rats showed a significant reversal of these parameters toward the normal (). The effects of these extracts were comparable to that of standard silymarin activity. This assures the hepatoprotective activity of these extracts against damage by CCl4.
Further, animals receiving CCl4 had higher serum levels of bilirubin, cholesterol and triglyceride, as compared to the intact rats (). The rise in the level of serum bilirubin is the most sensitive parameter that confirms the intensity of jaundice (CitationDrotman & Lawhorn, 1978). The ability of the total ethanol extract (100 and 200 mg kg−1) and the ethereal and chloroform successive extracts (10 mg kg−1) to reduce the level of total bilirubin in the serum of intoxicated rats () suggests their potential in clearing bilirubin from the serum when its level is elevated.
Table 2. Effect of the total ethanolic and successive extracts of C. alternifolius L plant on levels of total bilirubin, cholesterol and triglycerides in serum of rats with CCl4-induced hepatotoxicity (n = 6).
Administration of the total ethanol extract (50 mg kg−1) and butanol successive extract (10 mg kg−1) once daily for 8 days before CCl4, did not elicit any significant effect on the altered serum levels of ALT, ALP, total protein, albumin, total bilirubin, cholesterol and triglycerides, when compared with CCl4-intoxicated group.
Conclusions
In the present study, the total ethanol extracts (100 and 200 mg kg−1) showed significant hepatoprotective activity against acute hepatotoxicity induced by CCl4 in rats, which was comparable with the standard drug silymarin. The bioactive principles of the ether and chloroform successive extracts that have been identified could be responsible for the observed hepatoprotective effect. Further studies with the individual isolated compounds are underway which will enable us to understand the exact mechanism of hepatoprotective activity of C. alternifolius.
Acknowledgment
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for the work through the research group project NO RGP-VPP-060.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aniya Y, Koyama T, Miyagi C, Miyahira M, Inomata C, Kinoshita S, Ichiba T. (2005). Free radical scavenging and hepatoprotective actions of the medicinal herb, Crassocephalum crepidioides from the Okinawa Islands. Biol Pharm Bull, 28, 19–23.
- Arhoghro EM, Ekpo KE, Anosike EO, Ibeh GO. (2009). Effect of aqueous extract of bitter leaf (Vernonia Amygdalina Del) on carbon tetrachloride (CCl4) induced liver damage in albino Wistar rats. Eur J Sci Res, 26, 122–130.
- Awaad AS, Al-Jaber NA. (2010). Antioxidant natural plant. RPMP, 27, 1–35.
- Awaad AS, Maitland DJ, Soliman GA. (2006). Hepatoprotective activity of Schouwia thebica Webb. Bioorg Med Chem Lett, 16, 4624–4628.
- Babson L, Greeley S, Coleman C, Philips G. (1966). Serum alkaline phosphatase determination. Clin Chem, 12, 482–490.
- Baranisrinivasan P, Elumalai EK, Sivakumar C, Viviyan Therasa S, David E. (2009). Hepatoprotective effect of Enicostemma littorale Blume and Eclipta alba during ethanol induced oxidative stress in albino rats. Int J Pharmacol, 5, 268–272.
- Buck WB, Osweiter GD, Van Gelder AG. (1976). Clinical and diagnostic veterinary toxicology, 2nd edn. St. Louis, Iowa: Kendall/Hunt Publishing Co., 5211.
- Cotran R, Kumar V, Robbins S. (1994). Cell injury and cellular diseases, 5th edn. Bengaluru, India: Prism Book. Pub. Ltd., 379–430.
- Doumas BT, Watson WA, Biggs HG. (1971). Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta, 31, 87–96.
- Drotman RB, Lawhorn GT. (1978). Serum enzymes as indicators of chemically induced liver damage. Drug Chem Toxicol, 1, 163–171.
- El-Gohary HM. (2004). Study of essential oils of the tubers of Cyperus rotundus L. and Cyperus alopecuroides Rottb. Bull Fac Pharm Cairo Univ, 42, 157–164.
- Fossati P, Prencipe L. (1982). Triglyceride estimations. Clin Chem, 28, 2077–2080.
- Geissman TA. (1962). Chemistry of flavonoid compounds. New York: The McMillan Company Ltd.
- Gilani AH, Janbaz KH. (1995). Preventive and curative effects of Berberis aristata fruit extract on paracetamol and CCl4-induced hepatotoxicity. Gen Pharmacol, 26, 627–631.
- Gregory M, Cutler DF. (1960). Anatomy of the monocotyledons. Vol. 2. USA: Oxford University Press, 308.
- Harborne TB, Mabry TJ, Mabry H. (1975). The Flavonoids. London: Chapman and Hall, 219.
- Henary RJ, Cannon DC, Winkleman JW. (1974). Clinical chemistry principles and techniques, 2nd edn. New York: Harper and Row.
- Henry JB. (1984). Clinical diagnosis and management by laboratory methods. 17th edn. London: W.B. Saunders.
- Kodavanti PR, Joshi UM, Young RA, Meydrech EF, Mehendale HM. (1989). Protection of hepatotoxic and lethal effects of CCl4 by partial hepatectomy. Toxicol Pathol, 17, 494–505.
- Lorke D. (1983). A new approach to practical acute toxicity testing. Arch Toxicol, 54, 275–287.
- Mabry TJ, Markham KR, Thomas MB. (1970). The systematic identification of flavonoids. Berlin: Springer-Verlag.
- Mahendale H, Gupta P, Shalunka D. (1985). Hepatic Toxicity. Vol. 1. New Delhi, India: Metropolitan Books, 225.
- Morimoto M, Fujii Y, Komai K. (1999). Antifeedants in Cyperaceae: Coumarin and quinones from Cyperus spp. Phytochemistry, 51, 605–608.
- Murray RD, Mendez J, Brown SA. (1982). The natural coumarins, occurrence, chemistry, and biochemistry. Chichester: John Wiley and Sons Ltd., 509.
- Park EJ, Oh H, Kang TH, Sohn DH, Kim YC. (2004). An isocoumarin with hepatoprotective activity in HepG2 and primary hepatocytes from Agrimonia pilosa. Arch Pharm Res, 27, 944–946.
- Poon MK, Chiu PY, Mak DH, Ko KM. (2003). Metformin protects against carbon tetrachloride hepatotoxicity in mice. J Pharmacol Sci, 93, 501–504.
- Reitman S, Frankel S. (1957). A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol, 28, 56–63.
- Sallie R, Tredger JM, Williams R. (1991). Drugs and the liver. Part 1: Testing liver function. Biopharm Drug Dispos, 12, 251–259.
- Sayed HM, Mohamed MH, Farag SF, Mohamed GA, Omobuwajo OR, Proksch P. (2008). Fructose-amino acid conjugate and other constituents from Cyperus rotundus L. Nat Prod Res, 22, 1487–1497.
- Sharma OP. (1993). Plant Taxonomy. New York: Tata McGraw-Hill, pp. 452.
- Stahl E. (1969). Thin Layer chromatography. A Laboratory Handbook, 2nd edn. Berlin, Heidelberg, New York: Springer-Verlag, 880.
- Théophilea D, Tsala D, Dzeufiet D, Désiréa P, Njifutie N. (2006). Effects of alfia multiflora stapf on lipid peroxidation and antioxidant enzyme status in carbon tetrachloride-treated rats. Pharmacology Online, 2, 76–89.
- Trease GE, Evans WC. (1976). A Text Book of Pharmacognosy, 12th edn. London: Bailliere Tindall, 83.
- Vare H, Kukkonen I. (2005). Seven new species of Cyperus (Cyperaceae) section Arenarii and one new combination and typification. Ann Bot Fennici, 42, 473–483.
- Walter M, Gerarade H. (1970). An ultramicromethod for the determination of conjugated and total bilirubin in serum or plasma. Microchem J, 15, 231–243.
- Wei YH. (1998). Oxidative stress and mitochondrial DNA mutations in human aging. Proc Soc Exp Biol Med, 217, 53–63.
- Yu HH, Lee DH, Seo SJ, You YO. (2007). Anticariogenic properties of the extract of Cyperus rotundus. Am J Chin Med, 35, 497–505.
- Zoppi F, Fenili D. (1976). Letter: Enzymatic determination of total serum cholesterol with the Vickers D-300 analyzer. Clin Chem, 22, 690–691.