Abstract
Context: Broccoli [Brassica oleracea L. var. italica Plenck. (Brassicaceae)] contains substantial quantities of bioactive compounds, which are good free radical scavengers and thus might have strong antitumor properties. Enhancing production of plant secondary metabolites could be obtained with phytohormones that have significant effects on the metabolism of secondary metabolites. In that manner, in vitro culture presents good model for manipulation with plant tissues in order to affect secondary metabolite production and thus enhance bioactive properties of plants.
Objective: Estimation of the antioxidative and antitumor properties of broccoli cultivated in different in vitro conditions.
Materials and methods: In vitro germinated and cultivated broccoli seedlings, as well as spontaneously developed calli, were subjected to Soxhlet extraction. Antioxidative activity of the herbal extracts was determined using 1,1-diphenyl-2-picrylhydrazyl (DPPH•) radical method. Antitumor properties of the extracts were determined using crown-gall tumor inhibition (potato disc) assay.
Results: Three, 10, 20, and 30 days old broccoli seedlings, cultivated in vitro on three different Murashige–Skoog media, two types of callus, and seedlings from sterile filter paper were used for extraction. In total, 15 aqueous extracts were tested for antioxidative and antitumor potential. Three day-old seedlings showed the highest antioxidative activity. Eleven out of 15 aqueous extracts demonstrated above 50% of crown-gall tumor inhibition in comparison with the control. Tumor inhibition was in association with types and concentrations of phytohormones presented in growing media.
Discussion and conclusions: It is demonstrated that phytohormones in plant-growing media could affect the bioactive properties of broccoli either through increasing or decreasing their antioxidative and antitumor potential.
Introduction
Oxidative stress, induced by free oxygen radicals, is a frequent cause of carcinogenesis. There are few mechanisms of protection against oxidative stress and one of those involves inactivation by antioxidants. All the aerobic organisms, including human beings, have antioxidant defense; however, these mechanisms are highly dependent on dietary intake. Numerous aromatic and medicinal plants, spices, and vegetables contain chemical compounds exhibiting antioxidant properties. Plants are a good source of many antioxidants such are ascorbate, carotenoids, tocopherols, and phenolics. These antioxidants scavenge free radicals, inhibit the oxidation of lipid, or act as preventive antioxidants, which slow the rate of oxidation by several actions but do not convert free radicals (CitationOu et al., 2002; CitationOthman et al., 2007; CitationEl-Qudah, 2008; CitationSemalty et al., 2009).
Broccoli [Brassica oleracea L. var. italica Plenck. (Brassicaceae)] contains substantial quantities of bioactive compounds, such as vitamin C, β-carotene, phenolic compounds, and glucosinolates (CitationVallejo et al., 2003; CitationHeimler et al., 2006; CitationJagdish et al., 2006; CitationMoreno et al., 2006), which are good free radical scavengers (CitationSørensen et al., 2001; CitationGülcin et al., 2004; CitationEberhardt et al., 2005) and thus might have strong antitumor properties (CitationVerhoeven et al., 1997; CitationBrandi et al., 2005). Furthermore, 3 day old broccoli seedlings contain 10 to 100 times more glucosinolate than mature plants. Consuming small quantities of broccoli sprouts has the same protective effect as consuming large amounts of mature plants (CitationFahey et al., 1997).
Plant growth regulators presented in the media has significant effects on the metabolism of secondary metabolites. The type of growth regulators, their quality, as well as quantity has the major role in the production capability of a given in vitro culture. Phytohormones have an effect on cells multiplication and division, which is also in a function of increasing production of secondary metabolites. Effects of some phytohormones on secondary metabolite production has been already studied and confirmed in different medicinal plants (CitationNair et al., 1992; CitationBais et al., 2001; CitationWeathers et al., 2005; CitationKhan et al., 2008; CitationShilpashree & Ravishankar, 2009).
Crown gall is a neoplastic disease that affects many plant species and it is caused by Agrobacterium tumefaciens (CitationLippincott & Lippincott, 1975; CitationGalsky & Wilsey, 1980; CitationKahl & Schell, 1982). A. tumefaciens contain Ti-plasmid, which is responsible for rapid multiplication of plant cells without inducing cell apoptosis, which finally results in formation of tumors similar (histologically and in nucleic acid contents) to human and animal cancers (CitationMcLaughlin, 1991; CitationAgrios, 1997). Potato disc assay is a simple and inexpensive antitumor pre-screening assay that can be used as an alternative to extensive animal testing in the search for new anticancer drugs. In this bioassay on the surface of potato discs, A. tumefaciens tumors are formed, and the treatment with different compounds could inhibit tumor formations. Namely, plant extract inhibition of tumor formation on potato might have a high predictability of showing antiproliferative activity against the P388 (3PS) leukemia in mice (CitationGalsky & Wilsey, 1980; CitationFerrigni et al., 1982).
Materials and methods
Plant materials
Seeds of broccoli were used as a starting material for establishing in vitro tissue culture. Sterilized seeds were germinated on three different types of a Murashige–Skoog (MS) medium (CitationMurashige & Skoog, 1962). The first one (WH) was basal MS medium. Second (H) was MS medium supplemented with 0.1 mg/L 6-benzylaminopurine (BAP), and 0.1 mg/L indole butyric acid (IBA). The third one (3H) was MS medium with 0.5 mg/L BAP, 0.2 mg/L IBA, and 0.1 gibberellic acid (GA3). Three, 10, 20, and 30 day old seedlings, cultivated on MS media, were used for extraction. Additionally, spontaneously formed calli on H and 3H media were also subjected to extraction. Three day old seedlings germinated on sterile filter paper (FP-3) was used as a positive probe since it is reported as having strong antitumor properties (CitationFahey et al., 1997; CitationNestle, 1997, Citation1998; CitationRosselot et al., 2005).
Extract preparation
Plant materials (2 g) were subjected separately to Soxhlet extraction with 96% ethanol. Each extract was filtered and concentrated in rotary evaporator at ~40°C. Water extracts were obtained after exhaustive ethanol extraction. Finally, extracts were sterilized filtrating throughout syringe filters with 0.20 μm pores and stored at +4°C.
1,1-Diphenyl-2-picrylhydrazyl radical-scavenging activity
The free radical-scavenging activity of broccoli extracts was determined spectrophotometrically, using a Perkin-Elmer Lambda 25 UV/VIS spectrophotometer, by slightly modified method of CitationBrand-Williams and Cuvelier (1995) as described below. Extract solutions were diluted to three different concentrations (1:1, 1:3, 1:5). Aliquots of each extract solution (100 µL) were mixed with 3 mL of 0.001% 1,1-diphenyl-2-picrylhydrazyl (DPPH•) dissolved in absolute ethanol. The reaction of scavenging DPPH• was carried out at room temperature in dark conditions for 30 min. Absolute ethanol was used to zero the spectrophotometer. The blank was freshly prepared DPPH• solution, whereas thymol was used as a positive probe. Reduction in absorbance was recorded at 520 nm (CitationMolyneux, 2003). The radical-scavenging activity of the tested samples, expressed as percentage inhibition of DPPH, was calculated according to CitationYen and Duh (1994):
where A0 is absorbance of the control at t = 0 min and At absorbance of the antioxidant at t = 15 and 30 min.
Bacterial culture preparation
For induction of crown galls on potato [Solanum tuberosum L. (Solanaceae)] disc surface, A. tumefaciens strain 2215 (shooty type) was used (CitationJelenić et al., 2000). Agrobacterium strains were cultured on Luria-Bertani (LB) agar medium, prepared according to CitationDevi et al. (2007). Single colony was transferred into 5 mL LB liquid medium and incubated at 28°C for 24 h. Optical density was adjusted spectrophotometrically at OD600 = 0.2 (CitationArican et al., 2000).
Antitumor potato disc assay
For testing antitumor properties of broccoli extracts, the potato disc bioassay was used. Cylindrical explants of potato tuber were sterilized and cut into 12 mm × 3 mm discs with a sterilized cork borer (CitationMcLaughlin et al., 1998). According to the slightly modified procedure (CitationArican et al., 2000), potato discs were transferred to MS basal media and inoculated with bacterial suspension (OD600 = 0.2). Inoculated discs were left in the dark condition at 28°C for 48 h. Sterile solution of cefotaxime (500 mg/L) was used for removing bacteria from discs. Dried potato discs were then transferred to basal MS medium supplemented with 250 mg/L cefotaxime (CitationTsong-Ann et al., 2001). After 48 h, discs were transferred into the fresh MS medium, containing antibiotic, and on the disc surface, 50 µL of each broccoli extracts was added. As a negative control, sterile water was used. Twenty discs per treatment were used and three independent experiments were performed. Potato discs supplemented with extracts were incubated at 25–28°C for 4 weeks. Thereafter, tumors were observed on potato discs. Percent of tumor inhibition was calculated using formula (CitationMcLaughlin, 1991):
Statistical analysis
The experimental results were represented as the mean value of the three replicates with standard deviation (SD). One-way ANOVA was used for testing differences between broccoli extracts cultivated at different media. Spearman–Pearson correlation test was applied for measuring correlation between antioxidative and antitumor activity of broccoli extracts. One sample t-test was used to test difference between means of tumor number on control discs and discs with extracts.
Differences were considered significant at P < 0.05. For all mentioned analysis, WINKS 4.5 Professional software was used.
Results
Plants cultivated at different MS media showed a good rate of germination and seeds germinated into plantlets. Seedling, growth, shoot induction, and rhizogenesis were also successful. Callus was developed spontaneously, directly on the hypocotyls of the seedlings cultivated on both MS media supplemented with phytohormones (H, 3H). Calli were also subjected to extraction. The highest yield (17.12%) was found for the samples of 3 days old broccoli sprouts from 3H medium and the lowest for sprouts cultivated for 20 days at a 3H medium (3.62%).
DPPH radical-scavenging activity
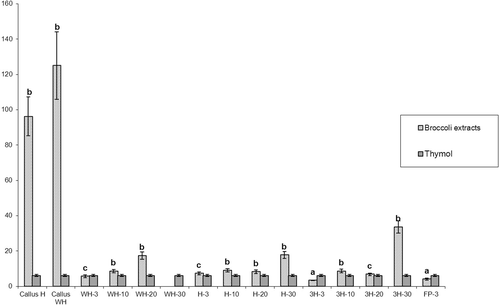
Extract of 3 day old seedlings from 3H medium demonstrated the highest antioxidative potential (3.48 mg/mL). Generally, 3 day old plants notably reduced the concentration of DPPH free radical, and their potential was stronger than the positive probe used (). Extracts of 10 and 20 day old seedlings also had strong antioxidative activity and the IC50 values varied from 6.86 mg/mL for 3H-20 to 17.47 mg/mL for WH-20. In our study, the IC50 values of callus extracts were high, indicating low antioxidant potential. Also, extract of broccoli seedlings cultivated on basal MS medium for 30 days (WH-30) showed pro-oxidative activity. Statistical analysis showed no differences in antioxidative activity between extracts of broccoli cultivated for different periods of time (f = 2.77; P = 0.11), nor between extracts of broccoli cultivated on different MS media (f = 0.58; P = 0.58).
Figure 1. Antioxidative activities of broccoli extracts compared with positive probe (thymol). All results are presented as mean values of three independent experiments ± SD. Higher IC50 values indicate lower antioxidative activity. Letters (a, b, and c) presents statistical significance of antioxidative activity in comparison with positive probe where a is significantly higher antioxidative activity then of positive probe (P < 0.05); b is significantly lower antioxidative activity than of positive probe (P < 0.05); and c is not statistically significant different between positive probe and broccoli extract (P < 0.05).
Antitumor potato disc assay
Antitumor potential of broccoli extracts is shown in . Eleven out of the 15 aqueous extracts demonstrated above 50% of tumor inhibition in comparison with the control. All tested extracts of broccoli reduce significantly the number of tumors per disc (t = 9.177; P < 0.0001) comparing with control. The aqueous extract of 3 days old broccoli seedling from sterile filter paper (FP-3) had the highest percentage of tumor inhibition (86.96%). Also, seedlings cultivated on 3H medium had very strong inhibitory activity (above 75%). The lowest percent of tumor inhibition was observed on potato discs treated with extracts of broccoli cultivated for 20 days on H medium. Also, the extract of 3 day old broccoli cultivated on H medium showed low antitumor capacity even if it has strong antioxidative activity ().
Figure 2. Antitumor activity of broccoli extracts presented as an average ± SD of tumors number recorded after treatment with extracts in comparison with control treatment. Also, results are presented as a percent of tumor inhibition induced in treatment with broccoli extracts.
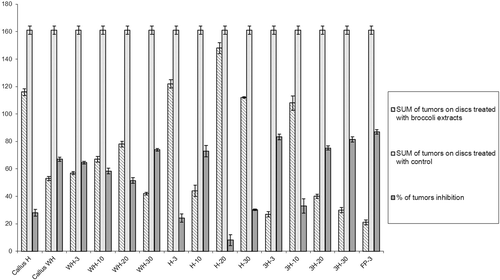
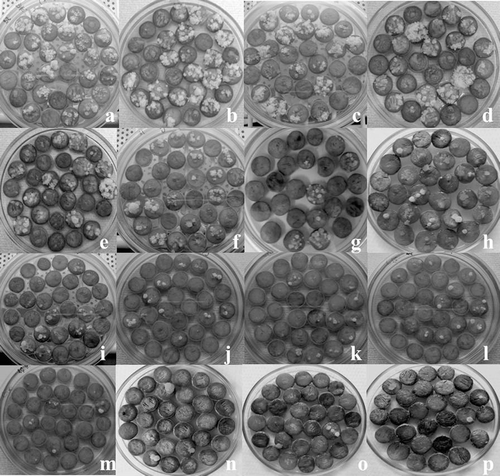
Figure 3. Antiproliferative properties of different broccoli extracts. The pictures of potato explants with tumors are ordered in the manner of decreasing numbers of tumors recorded after treatments with: (A) control, (B) H-20, (C) H-3, (D) callus H, (E) H-30, (F) 3H-10, (G) WH-20, (H) WH-10, (I) WH-3, (J) callus 3H, (K) WH-30, (L) H-10, (M) 3H-20, (N) 3H-30, (O) 3H-3, and (P) FP-3.
Statistically significant differences (f = 4.52; P < 0.05) were observed between tumor inhibitions on potato discs treated with extracts of broccoli from different types of medium. Additionally, Newman–Keuls multiple comparison tests showed that tumor inhibition with all extracts of broccoli cultivated on H medium are statistically lower then tumor inhibition with extracts of broccoli cultivated on other two types of medium. In our study, the statistical analysis showed no positive correlation between antioxidative and antitumor properties of broccoli extracts (r = 0.59; P > 0.05).
Discussion
Although almost all living organisms have antioxidative mechanisms for preventing and repairing oxidative damage, often these endogenous mechanisms are not sufficient to inhibit oxidative stress. Plants are a rich source of exogenous antioxidants and are frequently used for preventing many degenerative and age-related disorders (CitationTesoriere et al., 2007). Prospective health-promoting effects of plant secondary metabolites have encouraged research about their potential to prevent or treat cancers, circulatory diseases, and viral or bacterial infections (CitationHarinder et al., 2007). Certain studies indicate that frequent intake of vegetables from Brassicaceae family (broccoli, cauliflower, cabbage, and beet) reduces risk of cancers occurrence in all stages of carcinogenesis (CitationProchaska et al., 1992; CitationGuo et al., 2001; CitationChun et al., 2004; CitationChang & Lin, 2005). Among all edible Brassicaceae varieties, broccoli has the strongest antiradical activity measured by the DPPH radical assay (CitationHeimler et al., 2006). Extracts of broccoli have strong antioxidant and antitumor properties (CitationSørensen et al., 2001; CitationChun et al., 2004; CitationEberhardt et al., 2005). Moreover, 3 day old broccoli sprouts are commercially available in the form of pharmaceutical preparations.
Although antioxidative and antitumor activities of broccoli are well-studied, it was interesting to see if treatments with different phytohormones could improve bioactive properties of broccoli seedlings. A few studies showed that treatment with auxins could increase a concentration of glucosinolates, compounds that occur in broccoli and have antitumor properties (CitationPasquali et al., 1992; CitationZhang et al., 2005).
In current study, extracts of 3 day old broccoli had the highest antioxidant activity measured by DPPH free radical-scavenging method. CitationPiao et al. (2005) has isolated two active compounds (1,2-disinapoylgentiobiose and 1-sinapoyl-2-feruloylgentiobiose) from broccoli extracts and measured antioxidative properties of these two compounds by the DPPH free radical-scavenging method. IC50 values of mentioned compounds were 5.18 and 7.52 mg/mL, respectively. Three day old broccoli seedlings cultivated on media supplemented with different combinations of phytohormones had the same or slightly better antioxidative potential. Plant sprouts are a rich source of many biologically active compounds, so strong antioxidant activity was expected. Extracts of broccoli cultivated on basal MS medium for 30 days have shown pro-oxidative properties. At very high concentrations, antioxidants may have a pro-oxidative activity and could induce oxidative stress (CitationLing et al., 2010). Maybe the pro-oxidative activity of extracts of plantlets cultivated for 30 days on WH medium could be contributed to pro-oxidative properties of some unknown component(s) (CitationHu et al., 2000) that has been accumulated at higher concentrations in plantlets during longer cultivation.
Extracts of broccoli in general showed good antitumor properties. Treatment with broccoli extracts had inhibited growth of potato tumors in high percent. Moreover, 3 day old broccoli sprouts from filter paper and from 3H medium inhibited over 80% of tumors. CitationRosselot et al. (2005) examined antitumor potential of 3 day old broccoli sprouts on three cell lines of urinary bladder cancer cells. In their study, broccoli sprouts reduced growth of cancer cells in all tested cell lines.
In the current study, 3 day old sprouts cultivated on H medium did not significantly affect the growth of tumors, indicating that plant growth conditions strongly influenced their antitumor activity. Extracts of broccoli sprouts from H medium showed statistically lower antitumor properties comparing with extracts from the other two types of medium. It could be attributed to a specific combination and concentration of phytohormones used in culture media, which finally results in reduction of secondary metabolite production.
Many investigations have been conducted to establish and to enhance plant bioactive compounds production. The quality and quantity of phytohormones added in the media seems to have a significant effect on the production of secondary metabolites (CitationKhan et al., 2008). The production of secondary metabolites in plant cell culture is in a function of both cell multiplication and division. Consequently, growth regulators have a major role in the production of a given culture (CitationKurz & Constabel, 1979; CitationStaba, 1980; CitationDörnenburg & Knorr, 1995). Effects of the type of auxin and cytokinine on secondary metabolite synthesis have been studied in different medicinal plants. It was found that secondary metabolite production in tissue culture was increased with indole acetic acid (IAA) and naphthalene acetic acid (NAA) (CitationFuruya et al., 1971; CitationNair et al., 1992) or with 2,4-D (CitationIkeda et al., 1976). Also, BAP improved the production of plant secondary metabolites in shoot culture (CitationGadzovska et al., 2005; CitationDanova et al., 2009). In our study, higher concentrations of both BAP and IBA promoted antitumor properties of broccoli in shoot and callus culture.
Even if some biotic and abiotic factors can regulate production, the role of phytohormone supplementation in the culture medium needs further investigations. Culture condition optimization could allow significant change in the level of bioactive metabolites in plant species.
Interestingly, WH-30 extract that had pro-oxidant properties showed strong antitumor activity and inhibited over 70% of potato tumors. Statistical analysis showed no correlation between antioxidative and antitumor activity of broccoli extracts. That may be ascribed to an involvement of different compounds in these two types of activities.
Further research should emphasize the identification of active compounds that are responsible for antioxidative and antitumor properties. Furthermore, understanding the mechanisms of free radicals scavenging and tumor proliferation inhibition would be of great importance for improving phytotherapy in the prevention and treatment of certain types of cancer.
Conclusions
Extract of broccoli cultivated in in vitro conditions have shown good antioxidative and antitumor properties. Strong antioxidative and antitumor properties were mainly found for extracts of 3 day old broccoli plantlets. Anyhow, in a recent study, 3 day old sprouts from H medium had low antitumor potential indicating that combinations and concentrations of phytohormones are not suitable for desirable results. On the other hand, combination and concentrations of phytohormones used in 3H medium resulted in broccoli seedlings with strong antitumor properties. Our results suggest that plant growth regulators play a major role in secondary metabolite production and could be used to enhance bioactive properties of some medicinal plants that are already used in phytotherapy. However, a lot of biotic and abiotic factors are involved in the regulation of secondary metabolite pathway and further studies should reveal their role in antioxidative and antitumor activity of broccoli.
Declaration of interest
The authors report no conflicts of interest.
References
- Agrios GN. (1997). Plant Diseases Caused by Prokaryotes: Bacteria and Mollicutes. San Diego, CA: Plant Pathology Academic Press.
- Arican E, Bajrović K, Gozukirmizi N. (2000). Inhibition grown-gall of tumors on the potato discs with plant extracts. BIOS, 5, 45–49.
- Bais HP, Sudha G, George J, Ravishankar GA. (2001). Influence of exogenous hormones on growth and secondary metabolite production in hairy root cultures of Cichorium intybus L. cv. Lucknow Local. In Vitro Cell Dev Biol Plant, 37, 293–299.
- Brand-Williams W, Cuvelier ME. (1995). Use of a free radical method to evaluate the antioxidant activity. Lebensmittel-Wissenshaft und Technologie, 28, 25–30.
- Brandi G, Schiavano GF, Zaffaroni N, De Marco C, Paiardini M, Cervasi B, Magnani M. (2005). Mechanisms of action and antiproliferative properties of Brassica oleracea juice in human breast cancer cell lines. J Nutr, 135, 1503–1509.
- Chang CY, Lin CH. (2005). Textural change and antioxidant properties of broccoli under different cooking treatments. Food Chem, 90, 9–15.
- Chun OK, Smith N, Sakagawa A, Lee CY. (2004). Antioxidant properties of raw and processed cabbages. Int J Food Sci Nutr, 55, 191–199.
- Danova K, Bertoli A, Pistelli L, Dimitrov D, Pistelli L. (2009). In vitro culture of Balkan endemic and rare Pulsatilla species for conservational purposes and secondary metabolites production. Botanica Serbica, 33, 157–162.
- Devi PU, Murugan S, Suja S, Selvi S, Chinnaswamy P, Vijayananda E. (2007). Antibacterial, in vitro lipid peroxidation and phytochemical observation on Achyranthes bidentata Blume. Pakistan J Nutr, 6, 447–451.
- Dörnenburg H, Knorr D. (1995). Strategies for the improvement of secondary metabolite production in plant cell cultures. Enzyme Microb Technol, 17, 674–684.
- Eberhardt MV, Kobira K, Keck AS, Juvik JA, Jeffery EH. (2005). Correlation analyses of phytochemical composition, chemical, and cellular measures of antioxidant activity of broccoli (Brassica oleracea L. var. italica). J Agric Food Chem, 53, 7421–7431.
- El-Qudah JM. (2008). Dietary intake of selected common vegetable foods and their total carotenoid determination. Am J Agric Biol Sci, 3, 729–733.
- Fahey JW, Zhang Y, Talalay P. (1997). Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci USA, 94, 10367–10372.
- Ferrigni NR, Putnam JE, Anderson B, Jacobsen LB, Nichols DE, Moore DS, McLaughlin JL, Powell RG, Smith CR Jr. (1982). Modification and evaluation of the potato disc assay and antitumor screening of Euphorbiaceae seeds. J Nat Prod, 45, 679–686.
- Furuya I, Kojima H, Syono K. (1971). Regulation of nicotine biosynthesis by auxins in tobacco callus tissue. Phytochemistry, 10, 1529–1532.
- Gadzovska S, Maury S, Ounnar S, Righezza M, Kascakova S, Refregiers M, Spasenoski M, Joseph C, Hagège D. (2005). Identification and quantification of hypericin and pseudohypericin in different Hypericum perforatum L. in vitro cultures. Plant Physiol Biochem, 43, 591–601.
- Galsky AG, Wilsey JP. (1980). Crown gall tumor disc bioassay: A possible aid in the detection of compounds with antitumor activity. Plant Physiol, 65, 184–185.
- Gülcin I, Sat IG, Bezdemir S, Küfrevioglu ÖI. (2004). Evaluation of the in vitro antioxidant properties of broccoli extracts (Brassica oleracea L.). Ital J Food Sci, 16, 17–30.
- Guo JT, Lee HL, Chiang SH, Lin FI, Chang, CY. (2001). Antioxidant properties of the extracts from different parts of broccoli in Taiwan. J Food Drug Anal, 9, 96–101.
- Harinder PS, Makkar P, Siddhuraju P, Becker K. (2007). Plant secondary metabolites. In: Walker J, ed. Methods in Molecular Biology. Totowa, NJ: Humana Press, pp. 1–113.
- Heimler D, Vignolini P, Dini MG, Vincieri FF, Romani A. (2006). Antiradical activity and polyphenol composition of local Brassicaceae edible varieties. Food Chem, 99, 464–469.
- Hu C, Zhang Y, Kitts DD. (2000). Evaluation of antioxidant and prooxidant activities of bamboo Phyllostachys nigra var. henonis leaf extract in vitro. J Agric Food Chem, 48, 3170–3176.
- Ikeda T, Matsumoto T, Noguchi M. (1976). Formation of ubiquinone by tobacco plant cells in suspension culture. Phytochemistry, 15, 954–959.
- Jagdish S, Rai M, Upadhyay AK, Bahadur A, Chaurasia SNS, Singh KP. (2006). Antioxidant phytochemicals in broccoli (Brassica oleracea L. var. italica Plenck) cultivars. J Food Sci Technol, 43, 391–393.
- Jelenić S, Mitrikeski PT, Papeš D, Jelaska S. (2000). Agrobacterium-mediated transformation of broad bean Vicia faba L. Food Technol Biotechnol, 38, 167–172.
- Kahl G, Schell JS (1982). Molecular Biology of Plant Tumors. New York: Academic Press.
- Khan T, Krupadanam D, Anwar SY. (2008). The role of phytohormone on the production of berberine in the calli cultures of an endangered medicinal plant, turmeric (Coscinium fenestratum L.). Afr J Biotechnol, 7, 3244–3246.
- Kurz WG, Constabel F. (1979). Plant cell cultures, a potential source of pharmaceuticals. Adv Appl Microbiol, 25, 209–240.
- Ling LT, Palanisamy UD, Cheng HM. (2010). Prooxidant/antioxidant ratio (ProAntidex) as a better index of net free radical scavenging potential. Molecules, 15, 7884–7892.
- Lippincott JA, Lippincott BB. (1975). The genus Agrobacterium and plant tumorigenesis. Annu Rev Microbiol, 29, 377–405.
- McLaughlin JL. (1991). Crown gall tumors on potato discs and brine shrimp lethality: Two simple bioassay for higher plant screening and fractionation. In: Hostettman K, ed. Methods in Plant Biochemistry. Academic Press: London, vol. 9, pp. 383–409.
- McLaughlin JL, Rogers LR, Anderson JE. (1998). The potato discs bioassay modified for plant extracts and compound. Drug Information J, 32, 513–524.
- Molyneux J. (2003). The use of free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. J Sci Technol, 26, 211–219.
- Moreno DA, Carvajal M, López-Berenguer C, García-Viguera C. (2006). Chemical and biological characterisation of nutraceutical compounds of broccoli. J Pharm Biomed Anal, 41, 1508–1522.
- Murashige T, Skoog FA. (1962). A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plantarum, 15, 473–497.
- Nair AJ, Sudhakaran PR, Madhusudana JR, Ramakrishna SV. (1992). Berberine synthesis by callus and cell suspension cultures of Coscinium fenestratum. Plant Cell Tissue Organ Cult, 29, 7–10.
- Nestle M. (1997). Broccoli sprouts as inducers of carcinogen-detoxifying enzyme systems: Clinical, dietary, and policy implications. Proc Natl Acad Sci USA, 94, 11149–11151.
- Nestle M. (1998). Broccoli sprouts in cancer prevention. Nutr Rev, 56, 127–130.
- Othman A, Ismail A, Abdul GN, Adenan I. (2007). Antioxidant capacity and phenolic content of cocoa beans. Food Chem, 100, 1523–1530.
- Ou B, Huang D, Hampsch-Woodill M, Flanagan JA, Deemer EK. (2002). Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: A comparative study. J Agric Food Chem, 50, 3122–3128.
- Pasquali G, Goddijn OJ, de Waal A, Verpoorte R, Schilperoort RA, Hoge JH, Memelink J. (1992). Coordinated regulation of two indole alkaloid biosynthetic genes from Catharanthus roseus by auxin and elicitors. Plant Mol Biol, 18, 1121–1131.
- Piao XL, Kim HY, Yokozawa T, Lee YA, Piao XS, Cho EJ. (2005). Protective effects of broccoli (Brassica oleracea) and its active components against radical-induced oxidative damage. J Nutr Sci Vitaminol, 51, 142–147.
- Prochaska HJ, Santamaria AB, Talalay P. (1992). Rapid detection of inducers of enzymes that protect against carcinogens. Proc Natl Acad Sci USA, 89, 2394–2398.
- Rosselot RA, Clinton SK, Schwartz SJ, Tian Q. (2005). Broccoli sprout extracts inhibit bladder cancer cell proliferation. IFT Annual Meeting, July 15–20. New Orleans, Louisiana, Session 54G, Nutraceutical & Functional Foods: General II, 54G-5.
- Semalty M, Semalty A, Joshi GP, Rawat MSM. (2009). Comparison of in vitro antioxidant activity of Trigonella foenum-graecum and T. corniculata seed. Res J Phytochem, 1–5.
- Shilpashree HP, Ravishankar R. (2009). In vitro plant regeneration and accumulation of flavonoids in Hypericum mysorense. Int J Integr Biol, 8, 43–49.
- Sørensen M, Jensen BR, Poulsen HE, Deng X, Tygstrup N, Dalhoff K, Loft S. (2001). Effects of a Brussels sprouts extract on oxidative DNA damage and metabolising enzymes in rat liver. Food Chem Toxicol, 39, 533–540.
- Staba EJ (1980). Plant Tissue Culture as a Source of Biochemicals. Boca Raton, FL: CRC Press.
- Tesoriere L, Butera D, Gentile C, Livrea MA. (2007). Bioactive components of caper (Capparis spinosa L.) from Sicily and antioxidant effects in a red meat simulated gastric digestion. J Agric Food Chem, 55, 8465–8471.
- Tsong-Ann Y, Shyi-Dong Y, Jiu-Sherng Y. (2001). Effect of carbenicillin and cefotaxime on callus growth and somatic embryogenesis from adventitious roots of papaya. Bot Bull Acad Sin, 42, 281–286.
- Vallejo F, García-Viguera C, Tomás-Barberán FA. (2003). Changes in broccoli (Brassica oleracea L. var. italica) health-promoting compounds with inflorescence development. J Agric Food Chem, 51, 3776–3782.
- Verhoeven DT, Verhagen H, Goldbohm RA, van den Brandt PA, van Poppel G. (1997). A review of mechanisms underlying anticarcinogenicity by Brassica vegetables. Chem Biol Interact, 103, 79–129.
- Weathers, PJ., Bunk, G, McCoy, MC. (2005). The effect of phytohormones on growth and artemisinin production in Artemisia annua hairy roots. In Vitro Cell Dev Biol Plant, 41, 47–53.
- Yen GC, Duh PD. (1994). Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. J Agric Food Chem, 42, 629–632.
- Zhang Y, Li J, Tang L. (2005). Cancer-preventive isothiocyanates: Dichotomous modulators of oxidative stress. Free Radic Biol Med, 38, 70–77.