Abstract
Context: Nephrotoxicity induced by several synthetic drugs is a major problem of modern age. Medicinal plants and phytomedicine are the prime choice of research as they possess better activity and lesser side effects.
Objective: To investigate the protective effect of Cardiospermum halicacabum Linn. (Sapindaceae), methanol and petroleum ether extracts against acetaminophen-induced nephrotoxicity in rats.
Materials and methods: Nephrotoxicity was induced by the administration of acetaminophen suspension (750 mg/kg, p.o.) after the pretreatment with methanol extract (MECF) and petroleum ether extract (PEECF) of Cardiospermum halicacabum for 7 days. Forty-eight h after the acetaminophen administration estimations of serum alkaline phosphate, creatinine, blood urea nitrogen, uric acid, total proteins, cholesterol, albumin level and histological analysis of kidney injuries were determined.
Results: In nephrotoxic animals, a significant (P < 0.01) elevation of serum alkaline phosphate, creatinine, blood urea nitrogen, uric acid, cholesterol and depletion of total proteins and albumin were observed. Pretreatment with MECF and PEECF (400 mg/kg) significantly (P < 0.01, P < 0.05) decreased serum alkaline phosphate, creatinine, blood urea nitrogen, uric acid, cholesterol level and causes elevation of total protein and albumin level, though MECF produces better effect than PEECF in rats. Histopathological studies also confirm the protective effect of extracts. The protective effect of Cardiospermum halicacabum was associated with restoration of serum alkaline phosphate, creatinine, blood urea nitrogen, uric acid, cholesterol, total protein and albumin level.
Discussion and conclusions: Methanol and petroleum ether extracts of Cardiospermum halicacabum had a significant nephroprotective activity against acetaminophen-induced nephrotoxicity in rats.
Introduction
Acetaminophen (N-acetyl-p-aminophenol; APAP), popularly known as paracetamol, is a most common and widely used as analgesic and antipyretic drug that is safe at therapeutic dosages for a wide range of treatments (CitationYapar et al., 2007). Acute overdose of acetaminophen is known to cause hepatic and renal damage in both human and experimental animals (CitationGhosh & Sil, 2007). Nephrotoxicity is less common compared to hepatotoxicity in acetaminophen overdose, but renal damage and acute renal failure can occur even in the absence of liver injury and can even lead to death in humans and experimental animals (CitationCekmen et al., 2009; CitationPalani et al., 2009). Hepatic-derived acetaminophen metabolites, particularly glutathione conjugates, play important role in nephrotoxicity. Acetaminophen induces renal injury by formation of reactive intermediate metabolite N-acetyl-p-benzoquinone imine (NAPQI), which at therapeutic doses is removed by conjugation with glutathione sulhydrile. NAPQI is capable to initiate lipid peroxidation after binded to cellular protein and leads renal injury (CitationHart et al., 1994; CitationLi et al., 2003).
Cardiospermum halicacabum Linn. (Sapindaceae) is a deciduous, branching, herbaceous climber widely distributed in topical and subtropical region. It is found throughout the plains of India; different parts of the plant are employed in traditional management of diverse veterinary and human diseases (CitationJoshi et al., 1992). In Indian traditional medicine, the plant is highly valued for its diaphoretic, rheumatism, diuretic, emetic, emmenagogue, laxative, refrigerant, stomachic and sudorific properties (CitationNadkarni, 1976; CitationChopra, 1980; CitationJoshi et al., 1992). Leaves of Cardiospermum halicacabum are used as a poultice on swelling and juice of the leaves is useful in treatment of earache and itchy skin (CitationMalaviya et al., 2009). Stalks and leaves are useful in the treatment of dysentery, diarrhea and headache (CitationRao et al., 2006). Reports suggest that the plant is also used in lumbago, nervous diseases, and as a demulcent in orchitis and in dropsy. The plant is found beneficial in the treatment of skeletal fractures in Sri Lanka (CitationVeeramani et al., 2008).
Phytochemical investigation of the plant revealed the presence of flavone, aglycones, triterpinoids, glycosides, carbohydrates, fatty acids and volatile ester in the different extracts of the plant (CitationHopkins et al., 1968; CitationFerrara et al., 1996; CitationSrinivas et al., 1998; CitationRao et al., 2006). In addition, pharmacological evaluation of Cardiospermum halicacabum extracts showed that the plant possesses antimalarial (CitationWaako et al., 2005), antifilarial (CitationKhunkitti et al., 2000), antiparasitic (CitationBoonmars et al., 2005), antipyretic (CitationAsha & Pushpangadan, 1999), anti-inflammatory (CitationSadique et al., 1987), and antianxiety (CitationMalaviya et al., 2009) activities. Also, the plant’s antiulcer activity (CitationSheeba & Asha, 2006), and antihyperglycemic activity against streptozotocin-induced diabetes in rats as well as its antidiarrheal potentials in mice (CitationRao et al., 2006) are well documented in literature.
Cardiospermum halicacabum is a plant of interest in traditional systems. However, no scientific studies on nephroprotective effects of Cardiospermum halicacabum are reported and its chemical constituents may be useful for the treatment of nephrotoxicity. Therefore, the present study was undertaken to study the nephroprotective effect of methanol and petroleum ether extracts of the whole plant against acetaminophen induced nephrotoxicity.
Materials and methods
Chemicals
Acetaminophen and solvents like petroleum ether and methanol were procured from Sigma-Aldrich. The kits for the measurement of serum alkaline phosphate, creatinine, blood urea nitrogen, uric acid, total proteins, cholesterol and albumin were purchased from Span Diagnostics Limited, India. All other chemicals and reagents used were of analytical grade.
Plant materials
Cardiospermum halicacabum, locally known as ‘Buddakakara’, was collected from Gundumala, Anantapur district of Andhra Pradesh, India in the first week of January 2009. The plant was identified by Professor K. Madhava Chetty, Department of Botany, Sri Venkateswara University, Tirupati, Andhra Pradesh and deposited in voucher record 1321.
The whole plant was collected and air-dried at room temperature (28 ± 2°C) protected from direct sunlight and heat for 2 weeks, until completely dried. The dried plant material was pulverized by a mechanical grinder, passed through a 40 mesh to get fine powder. The powdered part was kept in nylon bags in a deep freezer (Remi) at −20°C until the time of use to avoid any hidden infestation.
Preparation of plant extract
The Cardiospermum halicacabum, the whole plant’s powder (200 g) was extracted exhaustively with methanol and petroleum ether separately (1800 mL for each solvent) in a Soxhlet apparatus for 18 h. After extraction, the solvent was filtered and the filtrate was distilled at 60°C under reduced pressure and about 1100 mL and 1050 mL of methanol and petroleum ether were distilled off respectively. The solvent was further evaporated to afford methanol (13.8 g) and petroleum ether (16.2 g) extracts. The extracts were of semisolid, sticky nature and dark green in color. The yields of methanol and petroleum ether extracts were found to be 6.9% and 8.1%, respectively. The obtained extracts were kept in a capped container and stored in the refrigerator. Different concentrations of the extracts were reconstituted from this stock.
Experimental animals
Healthy Wistar rats between 2 and 3 months of age, and weighing 150–200 g were purchased from Raghavendra Enterprises, Bangalore, India and used for the study. The animals were acclimatized for at least 7 days in an institutional animal room where they housed in standard polypropylene cage and maintained them under standard laboratory conditions of temperature (20 ± 2°C), relative humidity (50 ± 15%), 12 h light/dark cycle, diet and water ad libitum. The Institutional Animal Ethics Committee (Registration No: 1305/ac/09/CPCSEA) approved the study.
Preparation of the test samples
Methanol extract of Cardiospermum halicacabum (MECH) and petroleum ether extract of Cardiospermum halicacabum (PEECH) were suspended in 2% acacia in distilled water prior to oral administration to the experimental animals (5 mL/kg). Rutin (20 mg/kg) was used as the reference drug. Animals in the saline control group and vehicle control group received only normal saline and 2% acacia (5 mL/kg), respectively.
Experimental design
Experiment is carried out as per the procedure described by CitationPalani et al. (2010). Wistar rats were divided into six groups of six rats in each group. Group I treated with vehicle (2% gum acacia) and was kept as normal (control group). Groups II and III animals served as saline control and vehicle control respectively, were treated orally with 5 mL/kg/day of normal saline or 2% gum acacia, respectively. Group IV animals were treated with rutin (20 mg/kg) and were kept as standard. Groups V and VI were treated with methanol (MECH) and petroleum ether extracts Cardiospermum halicacabum (PEECH) at a dose of 400 mg/kg body weight respectively.
All treatments were continued for 7 days. Following the termination of treatment on the 7th day, rats of Group II-VI were fasted overnight for 14 h and acetaminophen suspension was given by oral route in a dose of 750 mg/kg body weight. Exactly 48 h after the acetaminophen administration, animals were anaesthetized with ether and sacrificed. Blood samples and pieces of kidney from each group were collected for biochemical and histopathological examination.
Biochemical analysis
Blood samples were collected by the retro-orbital method using heparin coated capillaries. Serum was separated by centrifugation for 5 min at 1000 × g and stored at −20°C until analysis. Serum samples were used to determine alkaline phosphate, creatinine, blood urea nitrogen, uric acid, total proteins, cholesterol, and albumin level. All estimations were carried out using a diagnostic kit (Span Diagnostics Ltd., India) as per the method described by the manufacturer.
Histopathological examination
After the animals were sacrificed, for qualitative analysis of kidney histology, sections of kidneys from each group were fixed immediately in 10% neutral formalin for a period of at least 24 h, then dehydrated in graded (50–100%) alcohol and embedded in paraffin. Cross-sections of the kidney tissue (5–6 μm thick) were prepared and stained with haematoxylin-eosin dye. The sections were evaluated by microscopical examination.
Preliminary phytochemical study
Methanol and petroleum ether extracts of Cardiospermum halicacabum were analyzed for their chemical constituents. Preliminary qualitative phytochemical screening will give idea about the chemical constituents present in the extract and will help for further investigation. Phytochemical screening was done as explained in literature (CitationFarnsworth, 1966; CitationIkhiri et al., 1992; CitationSilva et al., 1993; CitationHarborne, 1998; CitationHoughton & Raman, 1998).
Preliminary phytochemical properties of the extracts were studied using the following reagents and methods: alkaloids with Mayer’s, Hager’s, Wagner’s and Dragendorff’s reagents; flavanoids with the use of sodium acetate, ferric chloride, amyl alcohol and metalic magnesium plus HCl; tannins with 1% gelatin and ferric chloride reagent; saponins with the ability to produce suds and by hemolysis method; triterpnes and sterols with Salkowski’s and Leibermann Burchard test; protein with Millon’s, Biuret, and xanthoprotein test; carbohydrate with Molish’s, Fehling’s and Benedict’s reagent; glycosides with Keller Killiani test, Baljet’s test, bromine water test; gum was tested using Molish’s reagent and Ruthenium red reagent; fixed oils and fats with spot test.
Statistical analysis
Values are presented as mean ± standard deviation (SD). Statistical differences between the treatments and the controls were tested by one-way analysis of variance (ANOVA) followed by the Tukey’s test. A difference in the mean values of P < 0.05 was considered to be statistically significant.
Results
Serum analysis for alkaline phosphate, creatinine, blood urea nitrogen, uric acid, total proteins, cholesterol and albumin were analyzed after acetaminophen-induced toxicity and the protective potential of Cardiospermum halicacabum extracts in rats. The histopathology study for kidney was also carried out to assess the protective effect of Cardiospermum halicacabum.
Effect of pretreatment of MECH and PEECH on serum biochemical parameters
shows the effect of 400 mg/kg/day of oral administration of Cardiospermum halicacabum methanol and petroleum ether extracts on serum alkaline phosphate, creatinine, blood urea nitrogen, and uric acid level in acetaminophen nephrotoxic rats treated for 7 days. Alkaline phosphate, creatinine, blood urea nitrogen, and uric acid levels significantly increased in saline control (normal saline + APAP) and vehicle control (2% gum acacia + APAP) groups when compared to the control group, but there was no significant difference found between the saline control and vehicle control groups suggesting that the vehicle group (2% gum acacia) does not possess any effect itself. Therefore, all the values of drug treated groups were compared with the vehicle control group.
Table 1. Effect of Cardiospermum halicacabum extract pretreatment on the biochemical activities in paracetamol-induced nephrototoxicity.
Oral administration of MECH (400 mg/kg) and PEECH significantly (P < 0.01) reduced the serum alkaline phosphatase level to 310.21 ± 9.78 and 410.81 ± 8.60 U/L, respectively, compared to the vehicle control group (797.43 ± 5.81 U/L). The standard drug rutin (20 mg/kg) significantly (P < 0.01) reduced the alkaline phosphatase level to 294.30 ± 7.65 U/L.
Administration of acetaminophen increased the level of creatinine, blood urea nitrogen, and uric acid from 2.89 ± 0.30, 18.21 ± 0.32, 5.42 ± 0.50 mg/dL in the control to 16.51 ± 1.68, 32.54 ± 1.42, 13.50 ± 1.05 mg/dL, respectively, in the vehicle control group. Pre-treatments with MECH, PEECH and rutin significantly reduced the creatinine to 3.87 ± 0.23, 8.61 ± 0.81 and 3.96 ± 0.26 mg/dL; blood urea nitrogen to 21.01 ± 0.95, 29.42 ± 0.73 and 14.75 ± 1.2 mg/dL; uric acid to 5.23 ± 0.61, 9.16 ± 0.44, and 4.91 ± 0.78 mg/dL, respectively.
shows the effect of MECH and PEECH on serum total proteins, cholesterol and albumin levels after a single 400 mg/kg/day of oral administration in acetaminophen nephrotoxic rats treated for 7 days.
Table 2. Effect of MECH and PEECH pretreatment on total protein, cholesterol and alumin in paracetamol-induced nephrototoxicity.
Serum levels of total proteins and albumin were significantly decreased and cholesterol levels were significantly increased by paracetamol administration as compared to the control group. However, elevation in serum cholesterol was significantly (P < 0.01, P < 0.05) decreased by MECH and PEECH pretreatments and the decline of total proteins and albumin level was significantly (P < 0.01, P < 0.05,) increased by MECH and PEECH pretreatments. Between the two extracts, the methanol extract of Cardiospermum halicacabum was found to produce a better effect than petroleum ether extract. Pre-treatment with MECH increases the total protein and albumin level to 6.60 ± 0.54 and 2.74 ± 0.20 g/dL, respectively, and decreased the level of cholesterol to 202.74 ± 4.60 mg/dL whereas total proteins, albumin and cholesterol levels in the vehicle control group were found to be 4.68 ± 0.25 g/dL, 1.73 ± 0.23 g/dL and 251.05 ± 2.63 mg/dL, respectively.
Histopathological observations
To further confirm the preventive effect of Cardiospermum halicacabum methanol and petroleum ether extracts against the acetaminophen-induced renal injury, we next histopathologically examined kidney tissue after MECH and PEECH pretreatment.
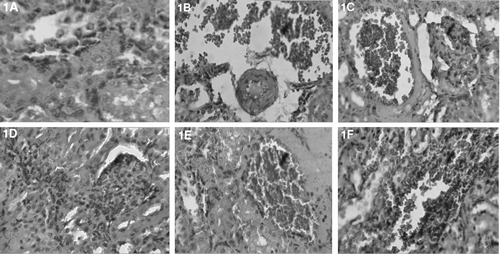
Morphological changes including marked tubular necrosis, swelling and flattening of proximal tubular cells with brush border loss and infiltration of RBCs were clearly observed in the acetaminophen-treated rats ( and ). Rats treated with rutin + APAP and MECH + APAP showed the presence of minimal degree of infiltration of RBCs and mild tubular degeneration ( and ). Rats treated with PEECH + APAP showed a moderate degree of infiltration of RBCs and flattening of proximal tubular cell (). This observation clearly showed a better morphology of kidney tissue in extract treated groups compared with the acetaminophen treated groups.
Figure 1. Photomicrographs of kidney sections of rat stained with haematoxylin and eosin (×100). (A) Hemotoxylin and eosin-stained sections of normal rat kidney. (B) Kidney section of 2% gum acacia + APAP treated rats showing focal areas of tubular necrosis, swelling, infiltration of RBCs and tubular brush border loss. (C) Kidney section of normal saline + APAP-treated rats showing marked tubular necrosis, swelling and flattening of proximal tubular cells with brush border loss and infiltration of RBCs. (D) Kidney section of rutin (20 mg/kg) + APAP-treated rats showing almost normal mucosa with very less degree of RBCs infiltration and flattening of proximal tubular cells with brush border loss. (E) Section of MECH (400 mg) + APAP-treated rat kidney showing minimal degree of inflammatory cells with RBCs infiltration and there is a decreased flattening of proximal tubular cells with brush border loss. (F) Kidney section of PEECH (400 mg) + APAP-treated rat showing moderate degree of RBCs infiltration and flattening of proximal tubular cells with brush border loss.
Preliminary phytochemical analysis
Our qualitative preliminary phytochemical tests showed that sterol, saponins, carbohydrate, flavonoids and tannin were present in the methanol extract of Cardiospermum halicacabum, while glycosides, alkaloid, protein, fixed oil, fat, gum, mucilage and triterpenes were absent; and petroleum ether extract of the plant were found to contain sterol, carbohydrate, flavonoids and triterpenes.
Discussion
The results of present study demonstrate that methanol and petroleum ether extracts of Cardiospermum halicacabum is effective in protecting against the nephrotoxic effects of acetaminophen in rats. This is evident biochemically by a mitigation of acetaminophen elevated on serum alkaline phosphate, creatinine, blood urea nitrogen, uric acid, cholesterol level and decreased total proteins, albumin level. The protective effect of the extract was also confirmed pathologically by amelioration of acetaminophen-induced renal lesions, proximal tubular cell necrosis, including infiltration of RBCs and congestion.
The kidney is one of the complex organs of the body consisting of well-defined components that function in an extremely coordinated fashion. A number of drugs, chemicals, and heavy metals have been shown to have toxic effects on kidney by altering its structure and function (CitationPriyamvada et al., 2010). Acetaminophen is an effective, well-tolerated, household, widely used general medicine used as an analgesic and antipyretic alternative to aspirin (CitationAdeneye & Benebo, 2008). Nephrotoxicity and hepatotoxicity are the potential well-known complications of paracetamol, initiated by the biotransformation is the first step paracetamol (CitationLi et al., 2003). Paracetamol is metabolized through glucuronidation and sulfation reactions primarily occurring in the liver which result in the water-soluble metabolites that are excreted via the kidney (CitationCekmen et al., 2009). Nephrotoxicity due to paracetamol results from the toxic effects of its highly reactive intermediate metabolite NAPQI. The proteins (specifically selenium-binding protein and glutamine synthetase) in the S3 segment of the proximal tubule are arylates by NAPQI, initiating cell death of renal tubular cells (CitationTarloff & Kinter, 1997; CitationAdeneye & Benebo, 2008). Glutathione depletion and the consequent lipid peroxidation also are considered as important factors of nephrotoxicity that causes due to paracetamol overdose. The selective renal accumulation of paracetamol is thought to result in a chain of biochemical reactions in animals and humans which culminate in acute or chronic nephropathies (CitationAdeneye et al., 2008). Marked elevations in blood urea nitrogen, serum creatinine and acute tubular necrosis are often associated with drug-induced nephrotoxicities. Therefore, the estimation of biochemical parameters such as blood urea, serum creatinine, creatinine clearance, and enzyme urea is used to investigate drug-induced nephrotoxicity in animals and humans (CitationAdeneye & Benebo, 2008).
The kidney is vulnerable to damage because of larger perfusion and the amplified concentration of excreted compounds that generate in renal tubular cell. Urea is the foremost nitrogen-containing metabolic product of protein metabolism and uric acid is the main product of purine nucleotides, adenosine and guanosine (CitationRenugadevi & Prabu, 2009). Blood urea nitrogen is derived from the diet or tissue sources and found in the liver protein that is normally excreted in the urine. In renal disorder, the rate of serum urea production exceeds the rate of renal clearance and as a result urea accumulates in serum. High protein diet, increased catabolism due to starvation, tissue damage, sepsis or steroid treatment and absorption of amino acids and peptides from digested blood after hemorrhage into the gastrointestinal lumen or soft tissue also causes uremia (CitationAdeneye et al., 2008; CitationPalani et al., 2009, Citation2010). Serum levels of urea and creatinine are considered as indicators of renal function. Creatinine is mostly derived from endogenous sources by tissue creatinine breakdown. Increased urea level in blood/serum is known to be correlated with an increased protein catabolism and increased synthesis of arginase in mammals causes the conversion of ammonia to urea (CitationRenugadevi & Prabu, 2009). Acetaminophen administration to control rats produced a typical pattern of nephrotoxicity which was manifested by marked increase in serum alkaline phosphate, creatinine, blood urea nitrogen, uric acid and cholesterol accompanied by significant decrease in total proteins and albumin. This indicates that acetaminophen at a dose of 750 mg/kg body weight is capable of causing significant nephrotoxicity. The data obtained from the present work clearly show that the increased levels of kidney function markers in serum (viz. blood urea nitrogen, creatinine, uric acid, cholesterol and serum alkaline phosphate) after acetaminophen administration, which reflect its interaction with cell membrane, leading to altered cell membrane permeability and loss of functional integrity in the kidney. In contrast, MECF and PEECF treated rats showed significant reduction in these markers, thus showing its ability to protect against acetaminophen-induced kidney damage. Total protein levels including albumin levels depressed in hepatotoxic conditions due to faulty protein biosynthesis in liver (CitationKumar et al., 2009). The acetaminophen-induced nephrotoxic condition in rats also causes a similar situation, and that the treatment with extracts causes increased level of total proteins and albumin to return to normal, justifying its nephroprotective activity.
The preliminary phytochemical studies showed the presence of different phytochemicals such as flavanoids, saponins, tannins, triterpenoids, and alkaloids which may be responsible for its protective activity. Several research studies proved the nephroprotective effect of saponins, triterpenoids and alkaloids (CitationSon et al., 2007; CitationOlagunju et al., 2009; CitationBalakumar et al., 2010). Preliminary studies are useful to get a preliminary idea about the characteristics of chemical constituents present in the extract. Therefore further studies are needed to discover bioactive phytochemicals responsible for its nephroprotective activity and to determine the exact mechanistic pathways of Cardiospermum halicacabum that can be used in protecting persons exposed to acetaminophen.
Conclusions
In conclusion, acetanimophen-treatment resulted in impairments of renal function markers, and histopathological changes in the kidneys of rats. Pre-treatment with Cardiospermum halicacabum extracts led to a significant decrease in all of these parameters in acetaminophen-treated rats.
These beneficial effects of Cardiospermum halicacabum may be attributed to the amelioration of the renal function markers. Our findings suggest that Cardiospermum halicacabum might be a potential agent against acetaminophen-induced nephrotoxicity.
Acknowledgments
The authors wish to thank the Principal and the Management of Creative Education Society’s College of Pharmacy for providing facilities and support.
Declaration of interest
The authors declare that they have no conflicts of interest to disclose. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- Adeneye AA, Benebo AS. (2008). Protective effect of the aqueous leaf and seed extract of Phyllanthus amarus on gentamicin and acetaminophen-induced nephrotoxic rats. J Ethnopharmacol, 118, 318–323.
- Adeneye AA, Olagunju JA, Benebo AS, Elias SO, Adisa AO, Idowu BO, Oyedeji MO, Isioye EO, Braimoh OB, Oladejo OO, Alana EO. (2008). Nephroprotective effects of the aqueous root extract of Harungana madagascariensis (L.) in acute and repeated dose acetaminophen renal injured rats. Int J Appl Res Nat Prod, 1, 6–14.
- Asha VV, Pushpangadan P. (1999). Antipyretic activity of Cardiospermum halicacabum. Indian j Exp Biol, 37, 411–414.
- Balakumar P, Rohilla A, Thangathirupathi A. (2010). Gentamicin-induced nephrotoxicity: Do we have a promising therapeutic approach to blunt it? Pharmacol Res, 62, 179–186.
- Boonmars T, Khunkitti W, Sithithaworn P, Fujimaki Y. (2005). In vitro antiparasitic activity of extracts of Cardiospermum halicacabum against third-stage larvae of Strongyloides stercoralis. Parasitol Res, 97, 417–419.
- Cekmen M, Ilbey YO, Ozbek E, Simsek A, Somay A, Ersoz C. (2009). Curcumin prevents oxidative renal damage induced by acetaminophen in rats. Food Chem Toxicol, 47, 1480–1484.
- Chopra RN.(1980). Glossary of Indian Medicinal Plants. New Delhi, India: CSRI Publication.
- Farnsworth RN. (1966). Review on biological and phytochemical screening of plants. J Pharm Sci, 55, 225–276.
- Ferrara I, Schettino O, Motesano D. (1996). Triterpenoids from Cardiospermum halicacabum L. Phytother Res, 10(Suppl. 1), S192–S194.
- Ghosh A, Sil PC. (2007). Anti-oxidative effect of a protein from Cajanus indicus L against acetaminophen-induced hepato-nephro toxicity. J Biochem Mol Biol, 40, 1039–1049.
- Harborne JB. (1998). Phytochemical Methods. A Guide to Modern Techniques of Plant Analysis, 3rd edn. London: Chapman and Hall.
- Hart SG, Beierschmitt WP, Wyand DS, Khairallah EA, Cohen SD. (1994). Acetaminophen nephrotoxicity in CD-1 mice. I. Evidence of a role for in situ activation in selective covalent binding and toxicity. Toxicol Appl Pharmacol, 126, 267–275.
- Hopkins CY, Ewing DF, Chiosholm MJ. (1968). A short chain ester from seed oil of Cardiospermum halicacabum Linn. Phytochemistry, 7, 619–624.
- Houghton PJ, Raman A. (1998). Laboratory Handbook for Fractionation of Natural Extracts, 3rd edn. London: Chapman and Hall.
- Ikhiri K, Boureima D, Dan-Kouloudo D. (1992). Chemical screening of medicinal plants used in traditional pharmacopoeia of Niger. Int J Pharmacog, 30, 251–262.
- Joshi SK, Sharma BD, Bhatia CR, Singh RV, Thakur RS. (1992). The Wealth of India. Raw Materials series, vol 3. New Delhi, India: CSIR Publication.
- Khunkitti W, Fujimaki Y, Aoki Y. (2000). In vitro antifilarial activity of extracts of the medicinal plant Cardiospermum halicacabum against Brugia pahangi. J Helminthol, 74, 241–246.
- Kumar SS, Kumar BR, Mohan GK. (2009). Hepatoprotective effect of Trichosanthes cucumerina var cucumerina L. on carbon tetrachloride induced liver damage in rats. J Ethnopharmacol, 123, 347–350.
- Li C, Liu J, Saavedra JE, Keefer LK, Waalkes MP. (2003). The nitric oxide donor, V-PYRRO/NO, protects against acetaminophen-induced nephrotoxicity in mice. Toxicology, 189, 173–180.
- Malaviya S, Nandakumar K, Vaghasiya JD, Bhalodiya YS, Jivani NP, Sheth N, Manek RA, Chauhan SP. (2009). Anxiolytic activity of root extracts of Cardiospermum halicacabum in mice. Int J Pharmacol, 7, 1–6.
- Nadkarni KM.(1976). Indian Meteria Medica. Bombay, India: Popular Book Depot.
- Olagunju JA, Adeneye AA, Fagbohunka BS, Bisuga NA, Ketiku AO, Benebo AS, Olufowobi OM, Adeoye AG, Alimi MA, Adeleke AG. (2009). Nephroprotective activities of the aqueous seed extract of Carica papaya Linn. in carbon tetrachloride induced renal injured Wistar rats: A dose- and time-dependent study. Biol Med, 1, 11–19.
- Palani S, Raja S, Kumar RP, Jayakumar S, Kumar BS. (2009). Therapeutic efficacy of Pimpinella tirupatiensis (Apiaceae) on acetaminophen induced nephrotoxicity and oxidative stress in male albino rats. Int J Pharm Tech Res, 1, 925–934.
- Palani S, Raja S, Kumar RP, Parameswaran P, Kumar BS. (2010). Therapeutic efficacy of Acorus calamus on acetaminophen induced nephrotoxicity and oxidative stress in male albino rats. Acta Pharma Sci, 52, 89–100.
- Priyamvada S, Khan SA, Khan MW, Khan S, Farooq N, Khan F, Yusufi AN. (2010). Studies on the protective effect of dietary fish oil on uranyl-nitrate-induced nephrotoxicity and oxidative damage in rat kidney. Prostaglandins Leukot Essent Fatty Acids, 82, 35–44.
- Rao NV, Prakash KC, Kumar SM. (2006). Pharmacological investigation of Cardiospermum halicacabum (Linn) in different animal models of diarrhoea. Indian J Pharmacol, 38, 346–349.
- Renugadevi J, Prabu SM. (2009). Naringenin protects against cadmium-induced oxidative renal dysfunction in rats. Toxicology, 256, 128–134.
- Sadique J, Chandra T, Thenmozhi V, Elango V. (1987). Biochemical modes of action of Cassia occidentalis and Cardiospermum halicacabum in inflammation. J Ethnopharmacol, 19, 201–212.
- Sheeba MS, Asha VV. (2006). Effect of Cardiospermum halicacabum on ethanol-induced gastric ulcers in rats. J Ethnopharmacol, 106, 105–110.
- Silva LG, Lee IS, Kinghorn DA. (1993). Special problems with the extraction of plants. In: Cannell JPR, ed. Methods in Biotechnology Natural Product Isolation. New Jersey: Humana Press Inc., 329–363.
- Son IH, Park YH, Lee SI, Yang HD, Moon HI. (2007). Neuroprotective activity of triterpenoid saponins from Platycodi radix against glutamate-induced toxicity in primary cultured rat cortical cells. Molecules, 12, 1147–1152.
- Srinivas K, Choudhary KA, Rao SS, Sathyanarayana T, Rao RVK. (1998). Phytochemical investigation of Cardiospermum halicacabum Linn. Indian J Nat Prod, 14, 24–7.
- Tarloff JB, Kinter LB. (1997). In vivo methodologies used to assess renal function In: Sipes, IG, McQueen, CA, Gandolfi, AJ. eds, Comprehensive Toxicology, vol 7. Oxford: Elsevier Publishing Co, 99–120.
- Veeramani C, Pushpavalli G, Pugalendi KV. (2008). Antihyperglycaemic effect of Cardiospermum halicacabum Linn. leaf extract on STZ-induced diabetic rats. J Appl Biomed, 6, 19–26.
- Waako PJ, Gumede B, Smith P, Folb PI. (2005). The in vitro and in vivo antimalarial activity of Cardiospermum halicacabum L. and Momordica foetida Schumch. et Thonn. J Ethnopharmacol, 99, 137–143.
- Yapar K, Kart A, Karapehlivan M, Atakisi O, Tunca R, Erginsoy S, Citil M. (2007). Hepatoprotective effect of l-carnitine against acute acetaminophen toxicity in mice. Exp Toxicol Pathol, 59, 121–128.