Abstract
Context: Fructus Corni is derived from the dry ripe sarcocarp of Cornus officinalis Sieb. et Zucc. (Cornaceae). It has attracted increasingly much attention as one of the most popular and valuable herbal medicine in clinic. This paper applied a rapid and validated method to the intrinsic quality control of Fructus Corni.
Materials and methods: The components of crude Fructus Corni were investigated by means of solid-phase extraction (SPE) and LTQ-linear ion trap mass spectrometry (MS) technique in the negative ion mode.
Results: The 29 detected compounds were identified by comparing the retention time and mass spectrometry data and retrieving the reference literatures.
Discussion and conclusion: It was concluded that a rapid and validated method was successfully applied based on SPE-LC-DAD–LTQ-linear-MSn which showed high sensitivity and resolution that was more suitable for identifying main components in Traditional Chinese medicines (TCMs) and their prescriptions, which would be helpful to their quality control.
Introduction
Traditional Chinese medicines (TCMs) have been used by TCM practitioners for thousands of years and have been attracting ever-increasing attention for their therapeutic effects to western medicines with few side effects (CitationTilburt & Kaptchuk, 2008; CitationLee, 2000). Although many TCMs have been proven effective by modern pharmacological studies and clinical trials, their bioactive components and the remedial mechanism are still not well understood. So far it is widely accepted that TCMs are mostly used in combination, and the composite formulae will produce a synergistic effect or antagonistic action (CitationBaker et al., 2007; CitationCao et al., 2009; CitationLiang et al., 2004). So, the clarification of bioactive ingredients of TCMs needs an integrative method that can make it possible to perform bioactive assay, chemical isolation, and identification of captured compounds almost simultaneously (CitationLiu & Liang, 2008; CitationBent & Ko, 2004; Zhang et al., 2004).
Fructus Corni is derived from the dry ripe sarcocarp of Cornus officinalis Sieb. et Zucc (Cornaceae). The crude Fructus Corni is used clinically for nourishing liver and kidney (CitationWang et al., 2010; CitationCao et al., 2009). It has been increasingly paid much attention as one of the most popular and valuable herbal medicine in the clinical world and can be used for medicine, hygienic food, and cosmetic purposes, due to its biological and pharmacological activities such as anti-inflammation, anti-virus, and anti-oxidation (CitationDu et al., 2008; CitationChen et al., 2008). In the past few years, reports on the detection and identification of active ingredients in crude Fructus Corni have been steadily increasing, especially for the recently accelerated development of various hyphenated and hybrid mass spectrometry (MS) techniques such as HPLC and LC–MS (Ding et al.; CitationLiu et al., 2008; CitationWang et al., 2010; CitationZhao & Sun, 2008). Although those analytical methods were efficient for identification of one or two types of components in Fructus Corni, they cannot be extended to other kinds of components. In this article, SPE and LC–LTQ-linear ion trap mass spectrometry were first utilized to corroborate the main constituents in the crude Fructus Corni; then, the character spectra were established. A novel method was developed to study the potential active components in TCM.
Materials and methods
Materials and standards
The crude Fructus Corni was acquired from Henan suppliers. LC grade acetonitrile was purchased from E. Merck (Darmstadt, Germany). Deionized aqueous was purified by Milli-Q system (Millipore, Bedford, MA, USA). Solvents (Millipore Corp., Bedford, MA) of LC grade were used for all preparations. All other chemicals were of analytical grade and commercially available.
HPLC analysis
Analyses were performed using an Agilent 1200 HPLC system (Agilent Technologies, Palo Alto, CA, USA) with a diode array detector. The monitoring wavelengths were set at 218, 240, and 284 nm. An Agilent Zorbax Extend C18 column (250 mm × 4.6 mm, 5 μm) was used with a flow rate of 1.0 mL/min. The mobile phase consisted of 98% aqueous formic acid (0.1%, v/v) (A) and 2% acetonitrile (B) using a gradient elution of 2% B at 0–10 min, 2–5% B at 10–15 min, 5–15% B at 15–45 min, 15–25% B at 45–55 min, 25–90% B at 55–70 min, 90% B at 80 min. All samples were centrifuged at 15000 × g for 10 min. The column temperature was maintained at 30°C, and 10 μL aliquots of the supernatants were injected into the LC–LTQ-linear ion trap mass spectrometry system for analysis.
Mass spectrometry
All mass spectra were determined on an LTQ XL linear ion trap instrument (Thermo Fisher Scientific, Bremen, Germany) equipped with an electrospray ion source, which is capable of analyzing ions up to m/z 2000. The scan scope was chosen from m/z 50 to 1000. By means of the comparison of LC–LTQ-linear ion trap mass spectra in positive ion mode and negative ion mode, the latter was chosen. The spray voltage was set to −3.0 kV. The capillary voltage was fixed at 4.0 V and the temperature at 270°C. The ion gauge pressure was 4.0 × 10−3 Pa. Nitrogen was used as a sheath gas (∼6.9 × 10−3 Pa) and the flow rate was 40 arbitrary units. Helium was used as the buffer gas. Data acquisition and processing was performed using Xcalibur software (version 2.0.7, Thermo Fisher Scientific, Inc.) and Metworks (version 1.1.0).
Preparation of sample solutions
The coarse powder of the crude Fructus Corni (about 1 g) was weighed in a round bottomed flask and it was soaked in 10 mL of distilled water for 1 h, and then extracted for 2 h. The extract was then concentrated, transferred to a 5-mL volumetric flask and diluted to the mark with distilled water. The mixed solution was applied to a pre-activated OASIS HLB SPE extraction C18 column (30 μm, 60 mg, Waters Corporation, USA). The column was washed with 1 mL of water, 1 mL of 2% methanol, and 2 mL of 100% methanol. The 100% methanol elutes were collected and dried under nitrogen gas at 45°C. The residues were re-dissolved in 300 μL of methanol and filtered through a 0.20-μm filter, and the filtrate was used as LC sample.
Results and discussion
Optimization of chromatographic conditions
Different mobile phase compositions were tested: water−methanol; water−acetonitrile; aqueous formic acid (0.1%, v/v)−acetonitrile. As a result, the combination of aqueous formic acid (0.1%, v/v)−acetonitrile for the mobile phase gave the best separation. Furthermore, other chromatographic variables were also optimized, including the analytical column (Hanbon Hedera ODS-2, Hanbon Lichrospher C18, and Agilent-Zorbax Extend C18), the column temperature (20°C, 25°C, and 30°C), and the flow rate (0.8 and 1.0 mL/min). The optimal separation was achieved on an Agilent Zorbax Extend C18 column (250 mm × 4.6 mm, 5 μL) at a column temperature of 30°C with a flow rate of 1.0 mL/min.
Optimization of Ms conditions
In order to optimize the mass spectrometric detection, electrospray ionization (ESI) spectra were acquired both in the positive ion (PI) and negative ion (NI) mode in the range of m/z 50–1000. ESI tests were performed by direct infusion of a 10.5 mg/L solution of morroniside and sweroside. In the negative ion mode, there was adduct formation for both compounds, while the positive ion mode gave low signal for both compounds and adduct formation for morroniside was observed. Flow-injection tests (10 μL) for ESI of the same solutions of daidzein and genistein showed a good signal for both compounds without adduct formation. As a result, all further investigations were performed in the ESI mode. In order to find the best settings, the capillary temperature was optimized by varying the temperature from 165 to 300°C, the optimum temperature was found to be 270°C. The tests were initially performed both in the PI and NI mode, which showed that NI provided better signals. Therefore, all further optimizations were done for this mode only. The optimized conditions obtained for NI-ESI were: a capillary voltage of 4.0 V, ion gauge pressure of 4.0 × 10−3 Pa and nitrogen was used as a sheath gas (6.9 × 10−3 Pa) and the flow rate was 40 arbitrary units.
LC−LTQ-linear-MSn analysis of crude Fructus Corni
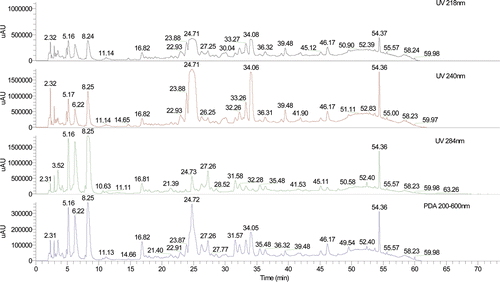
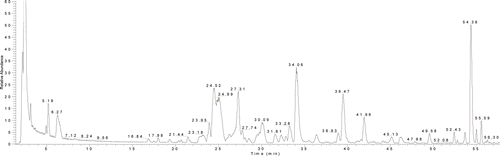
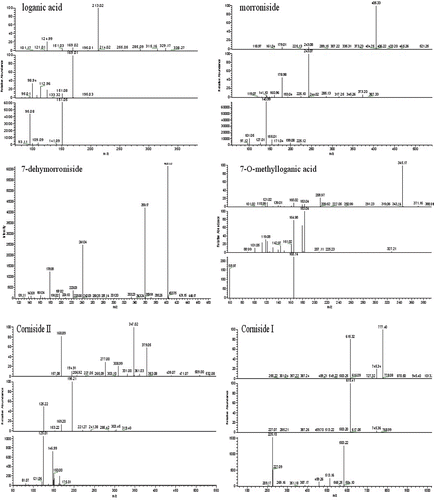
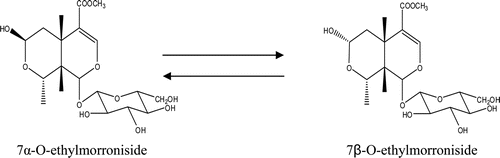
The chromatogram of the sample is shown in and the negative ion mode is shown in . The constituents in crude Fructus Corni were well separated by using the LC-DAD–LTQ-linear-MSn method with 29 compounds and detected; the structures of the identified compounds are show in For most of the constituents, [M − H]−, [M + HCOOH]− were observed. The results provided reliable information for confirming molecular weights of components from the crude Fructus Corni extract according to m/z data separately. Compounds 9, 10, 11, 12, 17, 18, 26, and 27 were attributed to loganic acid, morroniside, 7-dehymorroniside, 7-O-methylloganic acid, sweroside, loganin, cornuside I, and cornuside II, respectively, by comparing the retention time and mass data (CitationZhou et al., 2008; CitationWang et al., 2008; CitationZhang et al., 2009). They are classified as iridoid glycosides and the accurate ion trap mass spectra of these eight compounds were shown in . Other compounds were identified from several aspects, utilizing electrospray ionization in combination with the sequential tandem mass spectrometry, comparing with the literature data (CitationZhou et al., 2008; CitationWang et al., 2008; CitationZhang et al., 2009; CitationYang et al., 2005). For example, a series of fragment ions of compound 2 were given in negative ion ESI model, including 133 at [M − H]−, 115 at [M − H2O − H]− and 71 at [M − H2O − CO2 − H]−. Its molecular weight could be confirmed 134 and the considerable structural being deduced by specific fragment ions. Accordingly, the literature data compound 2 was deduced as malic acid. Some peaks, such as peak 15 and peak 16 had same m/z value of quasi-molecular ion in the MS spectra and a similar fragment ion in MS–MS spectra, respectively, but the retention times are different, and the structural identification of these compounds are in progress. The equilibrium sketch map of epimers of 7-O-ethylmorroniside in solution is shown in .
Conclusion
To the best of our knowledge, we report for the first time an easy, fast, and effective SPE- LC-DAD–LTQ-linear-MSn method for the characterization of main compounds in crude Fructus Corni. The SPE extraction constitutes about 90% of sample preparation and therefore a single SPE extraction would be a significantly improve in sample throughput. In this work, the presently developed method and strategy were successfully applied into the rapid detection and identification of the complicated components contained in crude Fructus Corni extraction. More than 29 compounds were readily detected and structurally characterized from crude Fructus Corni. The presented method might be universally applicable for identifying the complicated components from most TCMs and their prescriptions. SPE- LC-DAD–LTQ-linear-MSn method will also provided a type of validated rapid and higher throughput methodology for intrinsic quality control of TCMs.
Table 1. MS data of (-)ESI–MS spectra, and the identification of the constituents of crude Fructus Corni.
Acknowledgments
The authors are grateful to the financial support of the National Natural Science Foundation of China (No. 30873438) and Science and technology of Chinese Medicines of Zhejiang province (No. 2009CB008).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Baker DD, Chu M, Oza U, Rajgarhia V. (2007). The value of natural products to future pharmaceutical discovery. Nat Prod Rep, 24, 1225–1244.
- Bent S, Ko R. (2004). Commonly used herbal medicines in the United States: a review. Am J Med, 116, 478–485.
- Cao G, Zhang Y, Cong XD, Cai H, Cai BC. (2009). Advances in research on polysaccharides from Fructus Corni. Asian Tradit Herbal Med, 4, 205–209.
- Cao G, Zhang Y, Cong XD, Cai H, Cai BC. (2009). Research progress on the chemical constituents and pharmacological activities of Fructus corni. J Chin Pharm Sci, 18, 208–213.
- Chen CC, Hsu CY, Chen CY, Liu HK. (2008). Fructus corni suppresses hepatic gluconeogenesis related gene transcription, enhances glucose responsiveness of pancreatic beta-cells, and prevents toxin induced beta-cell death. J Ethnopharmacol, 117, 483–490.
- Ding X, Wang MY, Yao YX, Li GY, Cai BC. (2010). Protective effect of 5-hydroxymethylfurfural derived from processed Fructus corni on human hepatocyte LO2 injured by hydrogen peroxide and its mechanism. J Ethnopharmacol, 128, 373–376.
- Du W, Cai H, Wang M, Ding X, Yang H, Cai B. (2008). Simultaneous determination of six active components in crude and processed Fructus corni by high performance liquid chromatography. J Pharm Biomed Anal, 48, 194–197.
- Lee KH. (2000). Research and future trends in the pharmaceutical development of medicinal herbs from Chinese medicine. Public Health Nutr, 3, 515–522.
- Liang YZ, Xie P, Chan K. (2004). Quality control of herbal medicines. J Chromatogr b Analyt Technol Biomed Life Sci, 812, 53–70.
- Liu HR, Tang XY, Dai DZ, Dai Y. (2008). Ethanol extracts of Rehmannia complex (Di Huang) containing no Corni fructus improve early diabetic nephropathy by combining suppression on the ET-ROS axis with modulate hypoglycemic effect in rats. J Ethnopharmacol, 118, 466–472.
- Liu S, Yi LZ, Liang YZ. (2008). Traditional Chinese medicine and separation science. J Sep Sci, 31, 2113–2137.
- Tilburt JC, Kaptchuk TJ. (2008). Herbal medicine research and global health: an ethical analysis. Bull World Health Organ, 86, 594–599.
- Wang MY, Zhao FM, Peng HY, Lou CH, Li Y, Ding X, Yu XY, Yang GM, Xu DQ, Jiang LH, Zhang X, Ye LH, Cai BC. (2010). Investigation on the morphological protective effect of 5-hydroxymethylfurfural extracted from wine-processed Fructus corni on human L02 hepatocytes. J Ethnopharmacol, 130, 424–428.
- Wang X, Sun W, Sun H, Lv H, Wu Z, Wang P, Liu L, Cao H. (2008). Analysis of the constituents in the rat plasma after oral administration of Yin Chen Hao Tang by UPLC/Q-TOF-MS/MS. J Pharm Biomed Anal, 46, 477–490.
- Yang J, Chen SQ, Ji CR, Liu YZ. (2005). Chemical constituents from the Fruit of Cornus officinalis. Tradit Herbal Drug, 36, 1780–1784.
- Zhang L, Liu W, Zhang R, Wang Z, Shen Z, Chen X, Bi K. (2008). Pharmacokinetic study of matrine, oxymatrine and oxysophocarpine in rat plasma after oral administration of Sophora flavescens Ait. extract by liquid chromatography tandem mass spectrometry. J Pharm Biomed Anal, 47, 892–898.
- Zhang YE, Liu EH, Li HJ, Li P. (2009). Chemical constituents from the fruit of Cornus officinalis. Chin J Nat Med, 7, 365–367.
- Zhao XF, Sun MQ. (2008). Rapid identification of compounds in Fructus Corni by liquid chromatography/tandem mass spectrometry. Chin Tradit Herbal Drug, 39, 180–183.
- Zhou LL, Wu GG, Liu ZQ, Liu SY. (2008). Studies on the components of crude and processed Fructus Corni by ESI-MSn. Chem Res Chin Univ, 24, 270–274.