Abstract
Context: Prevalence of diabetes mellitus type 2 (DM-II) is increasing in Japan. Brown alga Ecklonia kurome Okamura (Laminariaceae) (kurome in Japanese) is rich in phlorotannins, a kind of polyphenol. Phlorotannins have been reported to possess various bioactivities; however, few studies have reported its effect on DM-II.
Objective: The present study was conducted to investigate the antidiabetic effect of polyphenols from E. kurome (KPP) on KK-Ay mice, the animal model for human DM-II.
Materials and methods: Inhibitory activities of KPP against α-amylase and α-glucosidase in vitro, and effects on oral carbohydrate tolerance test in vivo were investigated. KK-Ay mice were fed with 0.1% KPP containing water for 5 weeks. A glucose tolerance test was conducted at week 4 of the 5-week period. At the end of experiment, blood biochemical parameters, including blood glucose, insulin, glycoalbumin, and fructosamine were determined. Furthermore, the kidneys and pancreatic islets were histologically examined.
Results: KPP showed inhibitory activities on carbohydrate-hydrolyzing enzymes and decreased postprandial blood glucose levels. The body weight gain and blood glucose levels in the KPP group were lower than the control group during the experimental period. KPP improved glucose tolerance and decreased the fasting blood glucose and insulin levels, fructosamine and glycoalbumin levels compared with the control group. Furthermore, KPP contracted the pancreatic islet size and decreased renal mesangial matrix in KK-Ay mice.
Discussion and conclusion: These results suggest that KPP is effective against DM-II and might provide a source of therapeutic agents for DM-II.
Introduction
The prevalence of diabetes, especially type 2 diabetes, is increasing in Japan. In Japan, it is estimated that there are 8.9 million diabetes patients, and an additional 13.2 million individuals with impaired glucose tolerance (Kubo et al., 2010). Diabetes mellitus (DM) is one of the most serious diseases worldwide, and the number of adults with diabetes is expected to rise to at least 300 million by the year 2025 (CitationKing et al., 1998). DM is a group of metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both. Chronic hyperglycemia of diabetes is associated with long-term damage, dysfunction, and failure of various organs, especially the eyes, kidneys, nerves, heart, and blood vessels (The Expert Committee, 1997). It is clear that decreasing high blood glucose levels and correcting blood glucose levels in diabetes is most important to prevent or at least delay DM development.
DM is a group of chronic metabolic disorders; thus, patients need to take medicine over long periods of time safely and effectively. Herbal medicines are frequently considered to be less toxic with fewer side effects than synthetic medicines (CitationYao et al., 2008; CitationChen et al., 2009). We have studied the research for naturally occurring substances to prevent diabetes and diabetic complications; thus far, we have focused on edible seaweeds in Japan. In a previous study, we reported that various kinds of seaweed had beneficial effects on DM, especially Eisenia bicyclis (Kjellman) Setchell (Laminariaceae) (CitationOkada et al., 2004) and Ecklonia cava Kjellman (Laminariaceae) (CitationNakamura et al., 1997).
In the present study, we investigate the antidiabetic effect of E. kurome Okamura (Laminariaceae), which belongs to the same family as E. bicyclis and E. cava. E. kurome is an edible brown alga (kurome in Japanese) that is widely distributed on the south coast of Japan along the Pacific, Seto Inland Sea, Kyushu Island and the midland coast of the Sea of Japan. In those some local fishing village, eating E. kurome is part of their traditional food culture. E. kurome contains abundant phlorotannin derivatives, such as eckol, dieckol, 8,8′-bieckol, that have been previously reported (CitationFukuyama et al., 1983, 1985, 1989, 1990). However, there has been little study on DM in terms of phlorotannins, especially DM-II.
It is well known that over 90% of prevalence is DM-II and KK-Ay mouse is considered suitable as a polygenic model for human DM-II, developed marked adiposity and diabetic symptoms, for example, impaired tolerance to glucose, hyperglycemia, hyperinsulinemia and nephropathy (CitationIwatsuka et al., 1970; CitationTakada et al., 1996; CitationIto et al., 2006). There were numerous studies used KK-Ay mouse as a model for investing the antidiabetic effects on insulin resistance and diabetic nephropathy for human DM-II (CitationOdaka et al., 1992; CitationMatsuzaki et al., 1997).
Thus, we carried out this study on the antidiabetic effects of polyphenols from E. kurome (KPP) in KK-Ay mice.
Materials and methods
Chemicals
Folin-Ciocalteu phenol reagent, phloroglucinol, Glucose CII Test Wako assay kit and Amylase Test Wako, acarbose and voglibose were purchased from Wako Pure Chemical Industries, Ltd. (Tokyo, Japan). Porcine pancreatic α-amylase was purchased from Merck Ltd. (Tokyo, Japan). Intestinal acetone powder from rat was obtained from Sigma-Aldrich Japan (Tokyo, Japan). All chemicals used were of biological grade.
Plant materials
Cultivated E. kurome was collected from Kumamoto Prefecture, Japan. Air-dried E. kurome powder was extracted with 70% (v/v) ethanol for 24 h at room temperature and then filtered. Extract was evaporated and adsorbed onto Diaion HP-20. Subsequently, the concentrated extract was washed with purified water, and then the extract was dissolved with 60% (v/v) ethanol to give a crude polyphenol-rich fraction. The fraction was evaporated and freeze-dried, and then obtained as an E. kurome polyphenol (KPP)-rich powder (yield, 6.52%). Percentage of polyphenolic compounds in KPP was over 70% (w/w), calculated as a phloroglucinol by the Folin-Ciocalteu method.
Determination of total phenolic contents in KPP
Determination of total phenolic contents was confirmed by the Folin-Ciocalteu method (CitationSingleton & Rossi, 1965) using phloroglucinol as a standard phenolic compound. Briefly, Folin-Ciocalteu phenol reagent (3 mL) was added to the standard compound or KPP solution (3 mL), vigorously shaken, and then reacted for 3 min at room temperature. After reaction, 10% sodium carbonate solution (3 mL) was added and then the solution was left for 1 h. After 1 h, the mixed solution was centrifuged (1500 3 g, 4°C, 10 min). The supernatant was measured for absorption at 700 nm. The polyphenol content in the KPP was determined in milligrams of phloroglucinol equivalent per gram of dry powder.
Measurement of α-glucosidase inhibitory activity in vitro
Inhibition assay for α-glucosidase was performed according to Inoue’s method (CitationInoue et al., 1994) with the following minor modification. The crude enzyme solution was prepared with intestinal acetone powder from rat. One gram of rat intestinal acetone powder was suspended in 10 mL of 0.9% saline. The suspension was sonicated in an ice bath and centrifuged (1500 3 g, 4°C, 10 min). The supernatant was used as the crude enzyme solution in this experiment.
Substrate and sample were dissolved with 0.1 M maleic acid buffer (pH 6.0). Two percent (w/v) sucrose or maltose solution (200 µL), each concentration of sample solution (50 µL), distilled water (200 µL), and enzyme solution (50 µL) were mixed and incubated at 37°C for 1 h. Then, the samples were put in boiling water for 5 min for inactivation. The released glucose level was measured by Glucose CII Test Wako at 540 nm. Acarbose and voglibose were used as positive controls in this assay.
Measurement of α-amylase inhibitory activity in vitro
α-Amylase activity was determined according to Inoue’s method (CitationInoue et al., 1994) with the following minor modification using a commercial assay kit, Amylase Test Wako. Porcine pancreatic α-amylase was prepared at 375 U/mL with 0.25 M sodium phosphate buffer (pH 7.0). Sample was also dissolved in 0.25 M sodium phosphate buffer (pH 7.0) to prepare each concentration. Sample (20 µL) and substrate solution (40 mg/dL, 90 µL) from the assay kit were mixed and preincubated at 37°C for 5 min. Subsequently, 1000-fold dilution of enzyme solution (90 µL) was added, and the reaction mixture was incubated at 37°C for 5 min. The remaining starch was determined colorimetrically using Amylase Test Wako at 660 nm. Acarbose and voglibose were used as positive controls in this assay.
Animals and diets
Male 6-week-old ICR mice were purchased from Japan SLC Inc. (Shizuoka, Japan). Mice were housed individually in stainless wire-netting plastic cages in a room maintained at 23 ± 0.5°C with 50 ± 2.5% humidity and 12 h light/dark cycles (08:00–20:00). ICR mice were acclimated to a CRF-1 diet in 1 week with free access to diet and water. After acclimation, mice were subjected to an oral carbohydrate tolerance test.
Male 6- to 7-week-old KK-Ay mice were purchased from CLEA Japan. Inc. (Tokyo, Japan) and were also subjected to the same conditions as ICR mice; acclimated to CRF-1 diet in 3 weeks with free access to diet and water during the experimental period.
All animals were handled in accordance with the guidelines for the care and use of laboratory animals at Nagase Research and Development Center.
Oral carbohydrate tolerance test in ICR mice
After 1 week of acclimation, ICR mice were divided into eight groups of mice and fasted for 16 h. The sucrose tolerance test was used in four groups (n = 6) of mice. KPP was orally administered (control: saline) at doses of 100 or 500 mg/kg body. Then, sucrose (2 g/kg body) was orally administered 15 min after KPP administration. Voglibose was used as a positive control at a dose of 0.1 mg/kg body. Blood from the tail vein was collected 5 min before KPP administration and at 15, 30, 60, and 120 min after KPP administration. Blood glucose was then determined using a Medisafe Reader GR-101 (Terumo Co., Tokyo, Japan).
The starch tolerance test also used the same animal design as the sucrose tolerance test. Starch was orally administered at a dose of 2 g/kg body to each group (n = 8). KPP was orally administered (control: saline) at doses of 50 or 100 mg/kg body. Acarbose was used as a positive control at a dose of 10 mg/kg body.
Effect on spontaneous DM-II model of KK-Ay mice
After 3 weeks of acclimation, the mice weighed 33.1–39.6 g, and were assigned to two groups of seven animals. The mice were given free access to diet and tap water containing 0.1% KPP (control: tap water) during the 5-week experimental period. The tap water was sterilized by UV 253.7 nm and exchanged everyday. Diet and water consumption were measured twice a week and body weight and blood glucose level were measured once a week during 4 weeks of the 5-week experimental period, respectively. Blood was collected from tail vein and blood glucose was measured using a Medisafe Reader GR-101.
At the end of 4 weeks, the oral glucose tolerance test (OGTT) were conducted. KK-Ay mice were fasted for 16 h and all mice were administered the same 60 mg regardless of differences in weight. Then, blood was collected from the tail vein at 0, 30, 60, 120 min after glucose administration. When the OGTT was finished, mice were returned to their cages and the experiment continued.
At the end of the 5 weeks, mice were fasted for 16 h and blood was collected. Blood was centrifuged (1500 3 g, 4°C, 15 min) to obtain serum. Blood glucose, insulin, fructosamine and glycoalbumin of serum samples were analyzed by HITACHI 7180 auto-analyzer (Tokyo, Japan) in Nagahama Life Science Laboratory, Oriental Yeast Co., Ltd.
Histological examination
Samples of kidney and pancreas were fixed in 10% neutral-buffered formalin, embedded in paraffin, and sectioned.
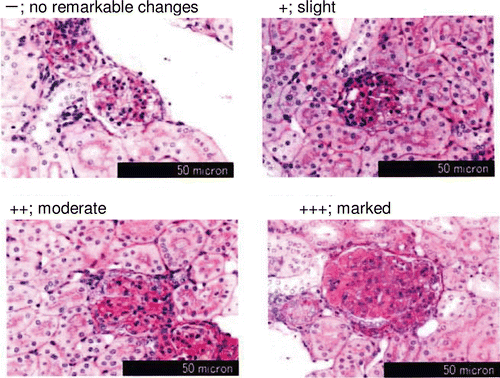
Kidney was stained with Periodic-Acid Schiff, and 100 glomeruli per mouse were randomly selected and observed microscopically (3 micron) to evaluate the progress of diabetic nephropathy. Every kidney section was evaluated with grades of no remarkable changes (−), slight changes (+), moderate changes (++), and marked changes (+++) ().
Pancreas was stained with hematoxylin/eosin (H.E.). Twenty pancreatic islets per mouse were randomly selected and the size of the pancreatic islets was examined microscopically. Photos of the islets were taken by digital camera (PDMCII digital microscope-camera, Polaroid Japan, Tokyo, Japan) to analyze the area of the pancreatic islet by image analysis system (Win ROOF Ver. 3.6, Mitani Corporation, Fukui, Japan). Furthermore, to confirm the vindication for 20 selections of pancreatic islets, the 10 largest and 3 largest of the pancreatic islets were selected from among the aforementioned 20 pancreatic islets to compare the mean area (3 1000 µm2).
All of the histological data were evaluated by Kamakura Techno-Science, Torey Industries, Inc.
Statistical analysis
All data are expressed as mean ± S.E. Comparisons of data were analyzed by Student’s t-test. Values were considered significantly different at p values <0.05.
Results
Determination of total phenolic compounds in KPP
The Folin-Ciocalteu reagent is a solution of complex polymeric ions formed from the combination of phosphomolybdic and phosphotungstic acids. The reagent oxidizes phenolates (ionized phenolics) present in the sample and reduces the acids to form a blue complex (CitationSingleton & Rossi, 1965). The total polyphenol content of KPP was determined to be over 70%, calculated as a phloroglucinol in this study.
Inhibitory activities against α−glucosidase and α-amylase in vitro
Inhibitory activities of KPP are shown in . The concentrations which gave 50% inhibition (IC50) of sucrase, maltase and α-amylase were 761, 1882 and 16 µg/mL, respectively. KPP showed selective potent inhibitory activity on α-amylase, resulting in an IC50 value that was higher than with sucrase and maltase. In contrast, acarbose and voglibose showed potent inhibitory activities on sucrase and maltase. Meanwhile, eckol, one of the main components of KPP, showed low inhibitory activity on α-amylase and high inhibitory activity on sucrase compared with KPP.
Table 1. Inhibitory effects on carbohydrate hydrolyzing enzyme.
Oral carbohydrate tolerance test in ICR mice
The change in plasma glucose level with orally administrated sucrose is shown in . The blood glucose levels of three groups quickly increased to a maximum level in 30 min, with the exception of the high-dose KPP group. The blood glucose level of the high-dose KPP group was significantly lower at 15, 30, and 60 min when compared with the control group, indicating a delay in glucose absorption.
Figure 1. Effects of KPP on carbohydrate tolerance test in normal ICR mice. Mice were administered with 2 g/kg sucrose (n = 6) or starch (n = 8) (control: saline). Sample was administered 5 min before carbohydrate administration. Blood glucose was measured 10 min before carbohydrate administration and at 15, 30, 60, and 120 min after carbohydrate administration. All values represent the mean± S.E. * p < 0.05, ** p < 0.01, *** p < 0.001 (Student’s t-test).
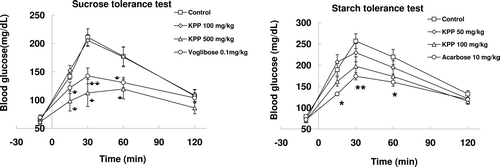
There were no significant differences in KPP groups compared with the control group in the oral starch tolerance test. However, the blood glucose level was decreased in a dose-dependent manner in KPP groups. ()
Effect on spontaneous DM-II model of KK-Ay mice
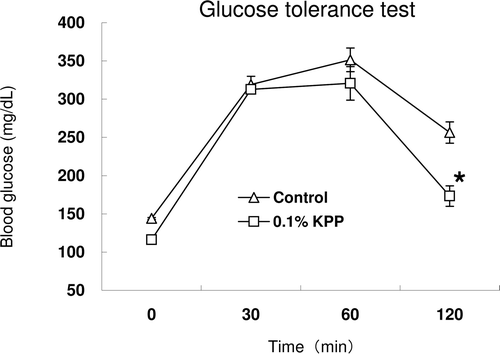
There were no significant differences between the two groups in terms of diet (Control: 6.50 ± 0.26 g, KPP: 6.24 ± 0.16 g) and water (Control: 17.76 ± 0.37 mL, KPP: 15.19 ± 0.21 mL) intakes, body weight gain, liver and kidney weight (data not shown) of KK-Ay mice in this experiment. The average of KPP intake per mouse was 15.19 ± 0.21 mg/day calculated from water intake. The change of body weight and blood glucose level over the period of 4 weeks is shown in . During the initial stages of the experiment, there was no difference in the blood glucose among the two groups. Increasingly, blood glucose levels increased in the two groups, especially in the diabetes control group. Blood glucose levels of the KPP group also increased but those increases were clearly suppressed, and significant differences were found when compared with the diabetes control group at 1 and 4 weeks. On the other hand, in the glucose tolerance test, the blood glucose level was significantly decreased 2 h after glucose administration when compared with the control group ().
Figure 2. Effects of KPP intake on body weight and blood glucose time courses in KK-Ay mice. Animals were fed with 0.1 % KPP water (control: tap water). Body weight and blood glucose was measured once a week. Blood was collected from tail vein and blood glucose was measured by blood glucose meter. Each value represents the mean ± S.E. (n = 7), *p < 0.05 (Student’s t-test).
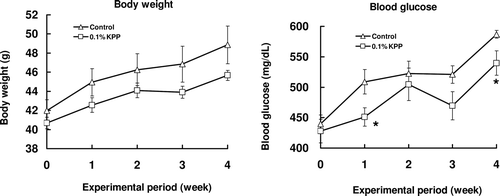
Figure 3. Oral glucose tolerance test in KK-Ay mice. Sixteen-hour fasted mice were orally administered 60 mg glucose regardless of difference in weight at 4 weeks. Blood was collected from tail vein at 0, 30, 60,120 min after glucose administration and blood glucose was measured by blood glucose meter. Each value represents the mean ± S.E. (n = 7), * p < 0.05 (Student’s t-test).
At 5 weeks, the end of the experiment, blood glucose and insulin, fructosamine and glycoalbumin levels were measured. There was no significant difference in the level of blood glucose; however, blood insulin level was significantly different compared with the control group. Subsequently, there was also a significant difference in the level of fructosamine, and the glycoalbumin level was decreased when compared with the control group ().
Table 2. Effects of KPP intake on blood biochemical parameters in KK-Ay mice.
Histological examination
Kidney and pancreas showed remarkable abnormalities with advanced diabetic state in KK-Ay mice. There were observed increases of mesangial matrix in glomeruli in both groups. To illustrate the degree of increased mesangial matrix (slight-marked) of 100 glomeruli, the KPP group had significantly decreased mesangial matrix compared with the control group (, ).
Table 3. Effects of KPP intake on renal mesangial matrix and pancreatic islet histological change in KK-Ay mice.
Figure 4. Effect of KPP intake on increase of kidney mesangial matrix in KK-Ay mice. Kidney was stained with Periodic-Acid Schiff, and 100 glomeruli per mouse were picked up for microscopic evalutaion with grades of no remarkable changes (−), slight changes (+), moderate changes (++), and marked changes (+++).
When examining the area of pancreatic islets, the 20 islet areas of the KPP group tended to be less than the area of pancreatic islets in the control group. The same results were observed in the 10 and 3 largest islets among the 20 pancreatic islets.
Discussion
In the present study, we investigated the antidiabetic effect of polyphenols from E. kurome (KPP) in vitro and in vivo. The results suggest that intake of KPP for 5 weeks exerts an antidiabetic effect in KK-Ay mice by decreasing blood glucose levels and resolving insulin resistance.
Seaweed is a common marine alga and popular seafood, e.g. tangle, wakame, and laver in Japan. E. kurome is known as seafood in some fishing villages in Japan and is rich in phlorotannins (CitationShibata et al., 2004). Those phlorotannins have been reported to possess various bioactivities, especially an antioxidant effect (CitationNakamura et al., 1996; CitationShibata et al., 2006; CitationAhn et al., 2007), but little is known with regard to DM, especially DM-II.
In DM, one therapeutic approach is to decrease postprandial hyperglycemia by reducing absorption of glucose by inhibition of carbohydrate-hydrolyzing enzymes, such as α-amylase and α-glucosidase (CitationMcCue et al., 2005; CitationWang et al., 2010). Postprandial hyperglycemia is considered as a risk factor of cardiovascular disease in DM. New DPP-4 inhibitors and a GLP-1 mimetic primarily target postprandial hyperglycemia (CitationCeriello, 2003; CitationAryangat & Gerich, 2010). Numerous studies indicate that polyphenols, such as tea polyphenols, have inhibitory activities like those of carbohydrate hydrolyzing enzymes (CitationLee et al., 2010; CitationLi et al., 2010). In the present study, KPP showed inhibitory activity on α-glucosidase, especially the marked inhibition of α-amylase (). Numerous studies confirm that α-amylase and α-glycosidase inhibitors, such as polyphenols, are known to delay the digestive absorption of carbohydrate in the gut, decrease the postprandial increase in plasma glucose, and result in lower blood glucose level (Tsujima & Takaku, 2008; CitationLee et al., 2010). Therefore, it is expected that KPP may have a hypoglycemic effect from postprandial elevation of blood glucose levels.
Starch and sucrose tolerance tests showed that KPP decreased postprandial hyperglycemia in a dose-dependent manner in both tests (). A larger dose of KPP is necessary in the sucrose tolerance test than the starch tolerance test, and these results are consistent with in vitro data (). It was shown that KPP mainly inhibited α-amylase in intestinal glucose absorption and that it may have a hypoglycemic effect on DM.
Based on these results, we investigated the antidiabetic effect of KPP on KK-Ay mice. KK-Ay mice are one of the animal models for DM-II with hyperinsulinemia (CitationIto et al., 2006; CitationTomino, 2009). KPP decreased the blood glucose level during the experimental period (). In addition, KPP decreased blood fructosamine and glycoalbumin levels (). It has been known that blood fructosamine and glycoalbumin levels represent the history of blood glucose status during the previous 1–2 weeks (CitationHosoda et al., 2003; CitationInaba et al., 2007; CitationTakahashi et al., 2007). This indicates that the mean blood glucose level in the KPP-fed KK-Ay mice was lower than the control group during the experimental period. Furthermore, the oral glucose tolerance test at the point of 4 weeks showed that the blood glucose level was significantly decreased at 2 h after administration of glucose in the KPP group (). At the end of the experiment, blood parameters showed that the blood glucose level was decreased in the KPP group and also that the blood insulin level was significantly decreased (). Under histological examination, the KPP group tended to exhibit suppressed islet hypertrophy (). This finding indicates that KPP consumption can ameliorate glucose tolerance and insulin resistance in KK-Ay mice.
It is well known that KK-Ay mice are considered to be a suitable animal model for human DM-II nephropathy and that mesangial matrices are closely related to hyperglycemia (CitationIwatsuka et al., 1970; CitationOkazaki et al., 2002). At the end of the experiment, KPP ameliorated some of these renal lesions. The glomerular mesangial matrices were significantly decreased compared with the control group (, ). Those findings indicate that KPP may have potential to prevent diabetic nephropathy in KK-Ay mice.
Regarding the inhibitory activity of carbohydrate-hydrolyzing enzymes, one of the main components of eckol showed low inhibitory activity on α-amylase and high inhibitory activity on sucrase when compared with KPP. This illustrates that other phlorotannins will have more potent inhibitory activity than KPP. In fact, dieckol, a dimer of eckol, showed potent inhibitory activities on α-glucosidase and α-amylase (CitationLee et al., 2009, 2010). In the present study, two mechanisms of antihyperglycemia by KPP were considered: one resulting in decreased carbohydrate absorption by KPP and these compounds, and another from the amelioration of hyperinsulinemia. Further investigation is necessary to find more evidence on the amelioration of hyperinsulinemia.
Conclusion
From our finding in addition to little toxicity of phlorotannins and those main components (CitationNagayama et al., 2002; CitationJung et al., 2009; CitationKim et al., 2009; CitationShim et al., 2009; CitationLee et al., 2010), we suggest that E. kurome polyphenol (KPP) consumption might be useful to prevent DM-II and diabetes-related complications.
Declaration of interest
The authors report no conflict of interest. The authors are responsible for the content and writing of the paper.
References
- Ahn G-N, Kim K-N, Cha S-H, Song C-B, Lee J, Heo M-S, Yeo I-K, Lee N-H, Jee Y-H, Kim J-S. (2007). Antioxidant activities of phlorotannins purified from Ecklonia cava on free radicalscavenging using ESR and H2O2-mediated DNA-damage. Eur Food Res and Technol, 226, 71–79.
- Aryangat AV, Gerich JE. (2010). Type 2 diabetes: Postprandial hyperglycemia and increased cardiovascular risk. Vasc Health Risk Manag, 6, 145–155.
- Ceriello A. (2003). The possible role of postprandial hyperglycaemia in the pathogenesis of diabetic complications. Diabetologia, 46 Suppl 1, M9–16.
- Chen J, Ma M, Lu Y, Wang L, Wu C, Duan H. (2009). Rhaponticin from rhubarb rhizomes alleviates liver steatosis and improves blood glucose and lipid profiles in KK/Ay diabetic mice. Planta Med, 75, 472–477.
- Fukuyama Y, Kodama M, Miura I, Kinzyo Z, Mori H, Nakayama Y, Takahashi M. (1989). Anti-plasmin inhibitor. V. Structures of novel dimeric eckols isolated from the brown alga Ecklonia kurome Okamura. Chem Pharm Bull, 37, 2438–2440.
- Fukuyama Y, Kodama M, Miura I, Kinzyo Z, Mori H, Nakayama Y, Takahashi M. (1990). Anti-plasmin inhibitor. VI. Structure of phlorofucofuroeckol A, a novel phlorotannin with both dibenzo-1,4-dioxin and dibenzofuran elements, from Ecklonia kurome Okamura. Chem Pharm Bull, 38, 133–135.
- Fukuyama Y, Miura I, Kinzyo Z, Mori H, Kido M, Nakayama Y, Takahashi M, Ochi M. (1985). Eckols, novel phlorotannins with a dibenzo-p-dioxin skeleton possessing inhibitory effects on α2-macroglobulin from the brown alga Ecklonia kurome Okamura. Chem Lett, 739–742.
- Fukuyama Y, Miura I, Kinzyo Z, Nakayama Y, Takahashi M, Kido M.(1983). Dibenzo-p-dioxin derivatives as inhibitors of antiplasmin agents. Tennen Yuki Kagobutsu Toronkai Koen Yoshishu, 26, 126–133.
- Hosoda K, Wang MF, Liao ML, Chuang CK, Iha M, Clevidence B, Yamamoto S. (2003). Antihyperglycemic effect of oolong tea in type 2 diabetes. Diabetes Care, 26, 1714–1718.
- Inaba M, Okuno S, Kumeda Y, Yamada S, Imanishi Y, Tabata T, Okamura M, Okada S, Yamakawa T, Ishimura E, Nishizawa Y; Osaka CKD Expert Research Group. (2007). Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: Effect of anemia and erythropoietin injection. J Am Soc Nephrol, 18, 896–903.
- Inoue Y, Hosomi N, Tuzita T, Okuda H. (1994). Action of a-amylase inhibitor form leaf extracts. New Food Industry, 36, 1–7.
- Ito T, Tanimoto M, Yamada K, Kaneko S, Matsumoto M, Obayashi K, Hagiwara S, Murakoshi M, Aoki T, Wakabayashi M, Gohda T, Funabiki K, Maeda K, Horikoshi S, Tomino Y. (2006). Glomerular changes in the KK-Ay/Ta mouse: A possible model for human type 2 diabetic nephropathy. Nephrology (Carlton), 11, 29–35.
- Iwatsuka H, Shino A, Suzuoki Z. (1970). General survey of diabetic features of yellow KK mice. Endocrinol Jpn, 17, 23–35.
- Jung WK, Heo SJ, Jeon YJ, Lee CM, Park YM, Byun HG, Choi YH, Park SG, Choi IW. (2009). Inhibitory effects and molecular mechanism of dieckol isolated from marine brown alga on COX-2 and iNOS in microglial cells. j Agric Food Chem, 57, 4439–4446.
- Kim AR, Shin TS, Lee MS, Park JY, Park KE, Yoon NY, Kim JS, Choi JS, Jang BC, Byun DS, Park NK, Kim HR. (2009). Isolation and identification of phlorotannins from Ecklonia stolonifera with antioxidant and anti-inflammatory properties. J Agric Food Chem, 57, 3483–3489.
- King H, Aubert RE, Herman WH. (1998). Global burden of diabetes, 1995-2025: Prevalence, numerical estimates, and projections. Diabetes Care, 21, 1414–1431.
- Kubo T, Fujino Y, Murata A, Ichimiya Y, Kuwabara K, Fujimori K, Horiguchi H, Matsuda S. (2011). Prevalence of type 2 diabetes among acute inpatients and its impact on length of hospital stay in Japan. Intern Med, 50, 405–411.
- Lee SH, Han JS, Heo SJ, Hwang JY, Jeon YJ. (2010). Protective effects of dieckol isolated from Ecklonia cava against high glucose-induced oxidative stress in human umbilical vein endothelial cells. Toxicol in Vitro, 24, 375–381.
- Lee S-H, Li Y, Karadeniz F, Kim M-M, Kim S-K. (2009). α-Glucosidase and α-amylase inhibitory activities of phloroglucocinol derivatives from edible marine brown alga, Ecklonia cava. J Sci Food Agr, 89, 1552–1558.
- Lee SH, Park MH, Heo SJ, Kang SM, Ko SC, Han JS, Jeon YJ. (2010). Dieckol isolated from Ecklonia cava inhibits α-glucosidase and α-amylase in vitro and alleviates postprandial hyperglycemia in streptozotocin-induced diabetic mice. Food Chem Toxicol, 48, 2633–2637.
- Lee W-K, Lin L-W, Ying Y-L, Stefan K, Huang D-J. (2010). Evaluation of different teas against starch digestibility by mammalian glycosidases. J Agr Food Chem, 58, 148–154.
- Li DQ, Qian ZM, Li SP. (2010). Inhibition of three selected beverage extracts on α-glucosidase and rapid identification of their active compounds using HPLC-DAD-MS/MS and biochemical detection. J Agric Food Chem, 58, 6608–6613.
- Matsuzaki T, Yamazaki R, Hashimoto S, Yokokura T. (1997). Antidiabetic effects of an oral administration of Lactobacillus casei in a non-insulin-dependent diabetes mellitus (NIDDM) model using KK-Ay mice. Endocr J, 44, 357–365.
- McCue P, Kwon YI, Shetty K. (2005). Anti-diabetic and anti-hypertensive potential of sprouted and solid-state bioprocessed soybean. Asia Pac J Clin Nutr, 14, 145–152.
- Nagayama K, Iwamura Y, Shibata T, Hirayama I, Nakamura T. (2002). Bactericidal activity of phlorotannins from the brown alga Ecklonia kurome. J Antimicrob Chemother, 50, 889–893.
- Nakamura H, Yamaguchi S, Hayashi T, Baba M, Okada Y, Tanaka J, Tokuda H, Nishino H, Okuyama T. (1997). Studies on the biological activities of marine algae (III): Antitumor promotion activity and inhibitory effects on aldose reductase. Natural Med, 51, 162–169.
- Nakamura T, Nagayama K, Uchida K, Tanaka R. (1996). Antioxidant activity of phlorotannins isolated from the brown alga Eisenia bicyclis. Fisheries Sci, 62, 923–926.
- Odaka H, Shino A, Ikeda H, Matsuo T. (1992). Antiobesity and antidiabetic actions of a new potent disaccharidase inhibitor in genetically obese-diabetic mice, KKA(y). J Nutr Sci Vitaminol, 38, 27–37.
- Okada Y, Ishimaru A, Suzuki R, Okuyama T. (2004). A new phloroglucinol derivative from the brown alga Eisenia bicyclis: Potential for the effective treatment of diabetic complications. J Nat Prod, 67, 103–105.
- Okazaki M, Saito Y, Udaka Y, Maruyama M, Murakami H, Ota S, Kikuchi T, Oguchi K. (2002). Diabetic nephropathy in KK and KK-Ay mice. Exp Anim, 51, 191–196.
- Shibata T, Kawaguchi S, Hama Y, Inagaki M, Yamaguchi K, Nakamura T. (2004). Local and chemical distribution of phlorotannins in brown algae. J Appl Phycol, 16, 291–296.
- Shibata T, Nagayama K, Kawaguchi S, Hama Y. (2006). Antioxidant and radical scanvenging activities of brown algal phlorotannis. ITE Lett Batter New Technol Med, 7, 69–72.
- Shim SY, Choi JS, Byun DS. (2009). Inhibitory effects of phloroglucinol derivatives isolated from Ecklonia stolonifera on Fc(epsilon)RI expression. Bioorg Med Chem, 17, 4734–4739.
- Singleton VL and Rossi JA. (1965). Colorimetry of total phenolics with phosphomolybdic- -phosphotungstic acid reagents. Am J Enol Viticult, 16, 144–158.
- Takada Y, Takata Y, Iwanishi M, Imamura T, Sawa T, Morioka H, Ishihara H, Ishiki M, Usui I, Temaru R, Urakaze M, Satoh Y, Inami T, Tsuda S, Kobayashi M. (1996). Effect of glimepiride (HOE 490) on insulin receptors of skeletal muscles from genetically diabetic KK-Ay mouse. Eur J Pharmacol, 308, 205–210.
- Takahashi S, Uchino H, Shimizu T, Kanazawa A, Tamura Y, Sakai K, Watada H, Hirose T, Kawamori R, Tanaka Y. (2007). Comparison of glycated albumin (GA) and glycated hemoglobin (HbA1c) in type 2 diabetic patients: Usefulness of GA for evaluation of short-term changes in glycemic control. Endocr J, 54, 139–144.
- The Expert Committee on the Diagnosis,and Classification of Diabetes Mellitus. (1997). Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care, 20, 1183–1197.
- Tomino Y. (2009). Lessons from the spontaneous mouse models for treatment of type 2 diabetic nephropathy and IgA nephropathy. Juntendo Igaku, 55, 228–234.
- Tsujita T, Takaku T. (2008). Mechanism of the inhibitory action of chestnut astringent skin extract on carbohydrate absorption. J Nutr Sci Vitaminol, 54, 416–421.
- Wang H, Du Y-J, Song H-C. (2010). α-Glucosidase and α-amylase inhibitory activities of guava leaves. Food Chem, 123, 6–13.
- Yao Y, Chen F, Wang M, Wang J, Ren G. (2008). Antidiabetic activity of Mung bean extracts in diabetic KK-Ay mice. J Agric Food Chem, 56, 8869–8873.