Abstract
Context: Nonalcoholic fatty liver disease (NAFLD) is increasingly prevalent in Egypt, in parallel with increasing obesity. NAFLD can lead to liver inflammation, fibrosis and cirrhosis. NAFLD appears tightly linked with metabolic syndrome (MetS).
Objective: Examine the impact of dietary fish oil on human patients with MetS and NAFLD.
Materials and methods: One hundred and forty patients were enrolled in the current study and classified into two groups: patients with both MetS and NAFLD and patients with MetS alone. Sixty-four patients were treated with daily supplementation of 2 g of fish oil for 6 months. Markers of hyperlipidemia and oxidative stress, hydrogen peroxide (H2O2) and malondialdhyde (MDA), as well as proinflammatory cytokines, tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), were analyzed.
Results: Patients without fish oil exhibited significant increases in triglycerides (TGs), low-density lipoprotein (LDL), H2O2 and MDA that were associated with significantly elevated TNF-α and IL-6 compared to controls. Furthermore, patients with both NAFLD and MetS showed significant increase in H2O2, MDA, TNF-α and IL-6 levels compared with MetS group (p < 0.05). Treatment with fish oil reduced serum level of TG, LDL-cholesterol (LDL-C), H2O2, MDA, TNF-α and IL-6 levels in patients and did not affect the control levels.
Discussion and conclusion: Patients with NAFLD had bad lipid profile through a mechanism that involved developed redox imbalance, characterized by boosted free-radical activity and lipid peroxidation enhancing the release of proinflammatory cytokines leading to increased MetS risk and liver damage. However, daily treatment of patients with fish oil for 6 months improved lipid profile and blocked the oxidative stress and cytokines release.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most common liver disease since its prevalence is estimated to be 20–30% in general population of Western countries (CitationBedogni et al., 2005). NAFLD is a chronic liver disease that covers a wide variety of clinical conditions, ranging from simple steatosis without any signs of inflammation to severe inflammatory activity with significant fibrosis or even cirrhosis. It is present mainly in severely obese patients. Currently, the importance of NAFLD and its relationship to metabolic syndrome (MetS) is increasingly recognized. In fact, it has been suggested that a fatty liver is a predisposing factor for the development of the MetS (CitationTargher & Arcaro, 2007; CitationMensink et al., 2008). MetS is a clustering of risk factors that greatly increases an individual’s probability for developing atherosclerotic cardiovascular disease, type 2 diabetes mellitus and chronic kidney disease. The predominant underlying risk factors appear to be abdominal obesity, atherogenic dyslipidaemia, hypertension, elevated plasma glucose, a prothrombotic state, and a proinflammatory state (CitationPaschos & Paletas, 2009).
Accumulating evidence suggests that deranged adipocyte metabolism and altered body fat distribution are important (CitationPuder et al., 2005). It acts by depressing mitochondrial β-oxidation of fatty acids in the liver by diverting acyl-CoA to triglyceride (TG) synthesis; it also inhibits hepatic TG secretion, while, in presence of excess body fat, free fatty acids (FFA) flux to the liver remains unchanged (CitationColicchio et al., 2005). Therefore, fatty liver, or hepatic steatosis, is no longer considered a benign manifestation (CitationWang et al., 2008). In addition, a growing body of evidence supports a role for the inflammatory cytokine such as tumor necrosis factor-α (TNF-α) to mediate obesity associated insulin resistance, liver injury, and fibrinogenesis (CitationCarter-Kent et al., 2008). The importance of TNF-α in human and animal fatty liver disease, caused by both genetic manipulation and overnutrition, has been shown convincingly. Furthermore, neutralization of TNF-α activity improves the fatty liver disease in animals.
Owing to the health consequences of NAFLD, interest in this disease has increased over the last years. Recent understanding of the molecular events of fatty liver has focused on oxidative stress and chronic inflammation in accumulated adipose tissue (Shoelson et al., 2006). However, the previous focus was, in particular, on diagnostics, prevalence and prognosis, metabolic aberrations, and drug therapy. Less attention has been paid to nutritional aspects. One of these nutritional supplements is fish oil. Fish oil contains two polyunsaturated fatty acids (PUFA), namely docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA). In addition, we have previously shown that DHA can obliterate the lethal cisplatin-induced nephrotoxicity and renal tissue injury by reducing systemic inflammation and oxidative stress in rats and mice (CitationEl-Mesery et al., 2009). As well as the risks of adverse events caused by the use of PUFA seems negligible (CitationDe Ley et al., 2007). Therefore, this study is designed to examine the impact of dietary fish oil on human patients with MetS and NAFLD by a mechanism that involved inhibition of free-radical formation and proinflammatory cytokines release.
Patients and methods
Patients’ characteristics and treatment
From July 2007 to May 2008, a total of 140 patients were enrolled in the current study. They were selected from the out-patient clinics of Internal Medicine department at Specialized Medical Hospital at Mansoura University. Patients were classified into two groups; one consists of patients with metabolic syndrome only and the other consists of patients with both fatty liver and metabolic syndrome. To be eligible for the study, adult patients were selected according to the following criteria: (a) no history of current or past alcohol drinking; (b) negative hepatitis B and C antigens and antibodies; (c) absence of diabetes (fasting blood glucose < 126 mg/dL); (d) no current use of anti-diabetic drugs or other medications potentially affecting liver function. Patients’ consent was obtained according to the regulations of the Egyptian Ministry of Health. As a baseline study, all prospective patients were subjected to patient history taking (personal, past and family history) as well as physical examinations. All subjects were interviewed for completion of a standardized questionnaire regarding personal medical history, current treatments and life-style behaviors.
Control group
This group consisted of 40 apparently healthy subjects (28 males and 22 females) with age range 38–60 years and their (mean age ± SD) was (50.52 ± 6.47). Twenty of the control subjects were given daily supplements of fish oils for three months.
Dietary records
Each participant answered a questionnaire about his or her usual daily intake of food and snacks, any changes with seasons, and changes in body weight and eating habits over the past 12 months. The participants were also asked to describe the food and snacks consumed the day before the visit. All subjects had no alcohol consumption. Each dietary questionnaire was evaluated by the dietician, who calculated intake of total amount of calories and the composition of the diet of each participant. Sixty-four of the patients were given daily supplements of 2 g fish oil (purchased from Sundown naturals Inc., Boca Raton, Fl, USA) for 6 months. The dose of fish oil was based on a previous study by CitationSpadaro et al. (2006).
Clinical parameters
Metabolic syndrome was diagnosed by the Adult Treatment Panel III (ATP III) definition. In accordance with this definition (CitationDeen, 2004), a person was classified as having the syndrome if he or she had at least two of the following four components: waist circumference >102 cm in men or >88 cm in women; TGs >150 mg/dL; HDL <40 mg/dL in men and <35 mg/dL in women or receiving treatment and blood pressure ≥130/85 mmHg or receiving treatment.
Chemicals
Phenol red, horse radish peroxidase, catalase, trichloroacetic acid, thiobarbituric acid, tetramethoxypropane were purchased from Sigma Aldrich, St. Louis, MO, USA and used in the current study for measurements of leucocytes hydrogen peroxide (H2O2) and serum malondialdehyde.
Analysis of clinical, laboratory and biochemical parameters
All tests were carried out on blood samples taken in the first part of the morning, after a 12–14 h fast and at a room temperature of 22–24°C. Serum TGs, cholesterol and HDL-cholesterol were determined by commercially available kits from Human Company. Serum LDL-cholesterol (LDL-C)was calculated by the equation of CitationFriedewald et al. (1972).
Leucocytic H2O2 concentration was measured as described previously by our group (CitationAl-Gayyar et al., 2007). This method depends on the amount of H2O2 released from leucocytes and was estimated by the HRPO method. The assay was based on the HRPO mediated oxidation of phenol red by H2O2, which resulted in the formation of a compound that could be read at 610 nm.
Serum MDA (malondialdhyde) was measured as described previously by our group (CitationAl-Gayyar et al., 2007). In brief, serum proteins are precipitated by the addition of trichloroacetic acid. Then thiobarbituric acid reacts with MDA to form thiobarbituric acid-reactive substance (TBARS) that is measured at 532 nm.
Serum TNF-α level was analyzed with commercially available ELISA kits from BioSource International Inc, Calif, USA. IL-6 was estimated by Pelikine Compact Human IL-6 ELISA Kit.
Data management and statistical analysis
Mean values ± SE was used for statistical computations using the computer software GraphPad InStat version 3.00, GraphPad Software, San Diego California USA. For group comparison, ANOVA and Tukey–Kramer Multiple Comparisons Test were calculated. Statistical significance was predefined as p ≤ 0.05.
Results
Patients’ classification
From July 2007 to May 2008, a total of 140 patients were clinically examined and a history of each patient was taken in the Department of Internal Medicine in Specialized Medical Hospital, Mansoura University, Mansoura, Egypt. They were classified into the following groups:
Group I: 48 patients with MetS only. The age of this group ranges between 37 and 61 years (mean age ± SD was 51 ± 7.15). They were subclassified into:
Group I patients with fish oil supplementation: consists of 22 patients.
Group I patients without fish oil supplementation: consists of 26 patients.
Group II: 92 patients with NAFLD and MetS. The age of this group ranges between 30 and 72 years (mean age ± SD was 49.24 ± 10.94). They were subclassified into:
Group II patients with fish oil supplementation: consists of 42 patients.
Group II patients without fish oil supplementation: consists of 50 patients.
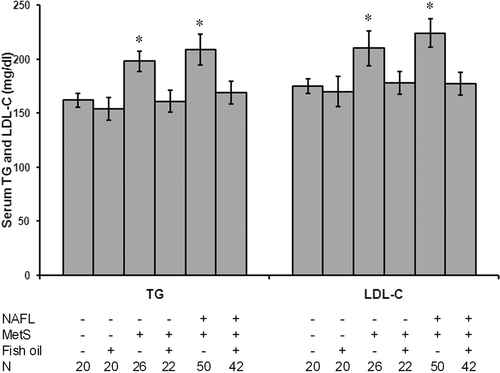
Fish oil significantly reduced serum level of TG and LDL-C in patients with NAFLD and MetS
Patients without fish oil treatment showed a significant increase in serum TG and LDL-C levels as compared with controls (p < 0.05). In addition, patients with both NAFLD and MetS showed significant increase in TG and LDL-C as compared with patients MetS alone (p < 0.05). The treatment of the patients with fish oil blocked the increase in serum level of both TG and LDL-C ().
Figure 1. Fish oil significantly reduced serum level of triglycerides (TG) and LDL-C in patients with NAFLD and MetS. All patients without fish oil treatment showed a significant increase in serum TG and LDL-C levels as compared with controls. Patients with both NAFLD and MetS showed significant increase in TG and LDL-C as compared with patients MetS alone. Treatment of the patients with fish oil blocked the increase in serum level of both TG and LDL-C. *Significant difference as compared with the control group at p < 0.05. N, number of subjects in the group.
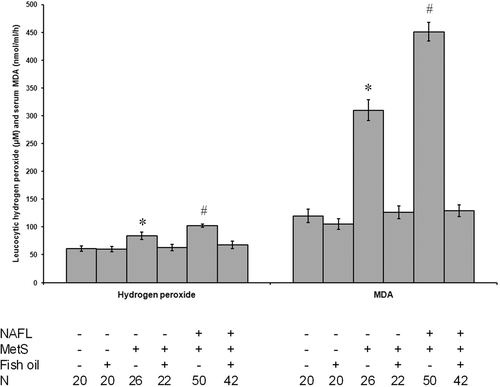
Fish oil reduced oxidative stress in patients with NAFLD and MetS
Patients without fish oil supplementation exhibited a significant increase in H2O2 and MDA levels as compared with the controls. Furthermore, patients with both NAFLD and MetS showed a significant increase in H2O2 and MDA levels as compared with the MetS group (p < 0.05). Treatment with fish oil blocked these effects in patients and did not affect the levels in controls ().
Figure 2. Fish oil reduced oxidative stress in patients with NAFLD and MetS. All patients without fish oil supplementation exhibited a significant increase in H2O2 and MDA levels as compared with the controls. Furthermore, patients with both NAFLD and MetS showed a significant increase in H2O2 and MDA levels as compared with the MetS group. Treatment with fish oil blocked these effects in patients and did not affect the levels in controls. *Significant difference as compared with the control group at p < 0.05. #Significant difference as compared with the rest of the groups at p < 0.05. N, number of subjects in the group.
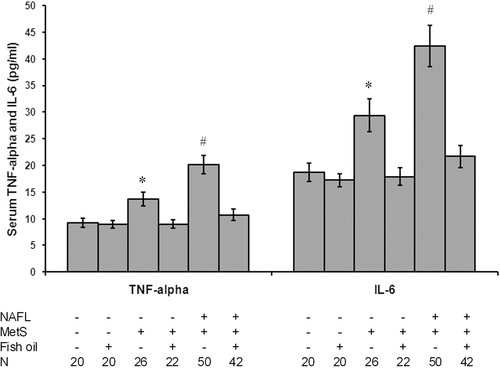
Fish oil blocked inflammatory cytokines release in patients with NAFLD and MetS
As shown in , patients without fish oil showed increased levels of proinflammatory cytokines as they showed a significant increase in TNF-α and interleukin-6 (IL-6) levels as compared with the controls (p < 0.05). Treatment with fish oil blocked these effects in patients and did not affect the levels in controls.
Figure 3. Fish oil blocked inflammatory cytokines release in patients with NAFLD and MetS. As shown in the figure, all patients without fish oil showed increased levels of proinflammatory cytokines as they showed a significant increase in TNF-α and IL-6 levels as compared with the controls. Treatment with fish oil blocked these effects in patients and did not affect the levels in controls. *Significant difference as compared with the control group at p < 0.05. #Significant difference as compared with the rest of the groups at p < 0.05. N, number of subjects in the group.
Discussion
We found that NAFLD worsens the lipid profile in patients with MetS. We believe that dietary fish oil supplementation is able to improve lipid profile through at least three mechanisms: (1) lowering LDL-C, (2) lowering oxidative stress, and (3) counteracting saturated fatty acid driven inflammation. Dietary fish oil shifts the fatty acid composition to be more polyunsaturated.
The MetS is characterized by different combinations of three or more of the following features: abdominal obesity, hypertension, hyperglycemia and serum dyslipidemia as defined by the criteria of the Third Report of the National Cholesterol Education Program Adult Treatment Panel III or by the updated criteria of the International Diabetes Federation (CitationFord, 2005). The results of this study reveal that patients with NAFLD and MetS showed a significant increase in serum TG and LDL-cholesterol as compared with patients with MetS alone. The lipid profile was improved by daily administration of 2 g of fish oil for 6 months. In this regard, it has been previously demonstrated that long chain fatty acids such as DHA can inhibit VLDL secretion (CitationBrown et al., 2010).
Experimental and clinical observations indicate oxidative stress as an important mechanism in obesity associated metabolic syndrome and in the development of NALFD (CitationPortincasa et al., 2005). Free fatty acids (FFA) that have accumulated within the hepatocytes undergo oxidation via mitochondria, microsomes, and peroxisomes, generating reactive oxygen species (ROS). In the present study, we found that patients with MetS and NAFLD showed a significant increase in H2O2 and MDA levels as compared with controls. Moreover, patients with both MetS and NAFLD showed significant elevation of these parameters as compared with patients with MetS alone. Treatment of patients with fish oil for 6 months blocked the oxidative stress. Chronic oxidative stress leads to depletion of the natural antioxidant pool, and results in excess reactive oxidative species within the hepatocyte over time. FFAs are shunted through peroxysomal β-oxidation, generating H2O2 that is subsequently converted to reactive hydroxyl radicals. Therefore, oxidative stress triggers lipid peroxidation which in turn initiates release of MDA. MDA binds to hepatocyte proteins initiating a potentially harmful immune response and stimulate neutrophil chemotaxis. Oxidative stress also activates transcriptional factor NFκB which in turn increases the production of proinflammatory cytokines promoting hepatocyte injury and apoptosis, neutrophil chemotaxis, and hepatic stellate cell activation (CitationDuvnjak et al., 2007).
TNF-α, IL-6 and other cytokines are synthesized and secreted by adipocytes, stromal vascular cells and endotoxin-activated macrophages. We found that patients with both MetS and NAFLD showed a significant increase in serum level of both TNF-α and IL-6. TNF-α level correlates well with liver disease severity, as demonstrated by reported studies (CitationIimuro et al., 1997). Much evidence supports the pathophysiological role of TNF-α in NAFLD, such as higher TNF-α mRNA expression in nonalcoholic steatohepatitis (NASH) patients with hepatic fibrosis, and increased prevalence of TNF-α polymorphisms in NAFLD patients (CitationCopaci et al., 2006). Furthermore, the 238 TNF-α gene polymorphism was found significantly more frequently in patients with fatty liver than healthy controls (CitationValenti et al., 2002). Moreover, elevated TNF-α production has been observed in cultures of peripheral blood cells collected from obese patients with NAFLD (CitationPoniachik et al, 2006).
The issue seems to be more complex with IL-6, a cytokine secreted by adipocytes, immune, and endothelial cells. Initial reports supported a hepatoprotective action of IL-6 in steatotic livers by suppressing oxidative stress and preventing mitochondrial dysfunction (CitationEl-Assal et al., 2004). However, this seems to be a paradoxical effect of short- and long-term IL-6 exposure, as the latter may sensitize the liver to injury and apoptotic cell death (CitationJin et al., 2006). It remains to be elucidated whether elevated IL-6 levels in chronic liver injury contribute to inflammation or represent an anti-inflammatory response. IL-6 has gained attention lately, after a small study in which IL-6 liver expression was markedly increased in NASH patients and positively correlated with inflammation and fibrosis (CitationWieckowska et al., 2008). In a study of morbidly obese patients, IL-6 was an independent predictor of steatosis and NASH (CitationGarcía-Galiano et al., 2007). Finally, serum IL-6 was found significantly elevated in NAFLD than controls, even after correction for age, sex, and BMI (CitationHaukeland et al., 2006).
There is an interactive relation between oxidative stress and proinflammatory cytokines. However, oxidative stress activates NFκB enhancing the production of proinflammatory cytokines, cytokine production by adipocytes has been suggested to enhance the production of ROS, which can induce subsequent liver damage via the intermediary step in which certain components of the mitochondrial respiratory chain are inhibited or altered (CitationBegriche et al., 2006). Such mitochondrial derangement would cause excessive heat production, accounting for the appearance of hypermetabolism and, potentially, a local energy deficit, which can lead to cellular degradation.
As for total fat intake, the effects of individual fatty acids on improving NAFLD have hardly been studied. We found that daily oral supplementation with 2 g of fish oil for 6 months significantly reduced cytokines production in patients with MetS and NAFLD. In fact, long chain ω-3 PUFA from fish oil have been shown to inhibit inflammation in cultured cells (Weatherhill et al., 2005). In agreement, CitationCapanni et al. (2006) therefore fed for 1 year 42 patients with NAFLD 1 g of fish oil daily. He found 24% improvement in patients in the fish oil group, whereas no changes were observed in the control group. In another study, a daily supplement of 2 g of fish oil for 6 months resulted in regression of steatosis (CitationSpadaro et al., 2006).
Therefore, we can conclude that patients with NAFLD had bad lipid profile through a mechanism that involved developed redox imbalance, which is characterized by boosted free-radical activity and lipid peroxidation enhancing the release of proinflammatory cytokines leading to increased MetS risk and liver damage. However, daily treatment of patients with fish oil for 6 months improved lipid profile and blocked the oxidative stress and cytokines release.
Acknowledgement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. We would like to express our appreciation to Mr. Mohammed M. Darwiesh, the technician in Dept. of Biochemistry, Faculty of Pharmacy, Mansoura University, for his continuous help.
Declaration of interest
The authors declared no conflict of interest.
References
- Al-Gayyar MM, Eissa LA, Rabie AM, El-Gayar AM. (2007). Measurements of oxidative stress status and antioxidant activity in chronic leukaemia patients. J Pharm Pharmacol, 59, 409–417.
- Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. (2005). Prevalence of and risk factors for nonalcoholic fatty liver disease: The Dionysos nutrition and liver study. Hepatology, 42, 44–52.
- Begriche K, Igoudjil A, Pessayre D, Fromenty B. (2006). Mitochondrial dysfunction in NASH: Causes, consequences and possible means to prevent it. Mitochondrion, 6, 1–28.
- Brown JM, Chung S, Sawyer JK, Degirolamo C, Alger HM, Nguyen TM, Zhu X, Duong MN, Brown AL, Lord C, Shah R, Davis MA, Kelley K, Wilson MD, Madenspacher J, Fessler MB, Parks JS, Rudel LL. (2010). Combined therapy of dietary fish oil and stearoyl-CoA desaturase 1 inhibition prevents the metabolic syndrome and atherosclerosis. Arterioscler Thromb Vasc Biol, 30, 24–30.
- Capanni M, Calella F, Biagini MR, Genise S, Raimondi L, Bedogni G, Svegliati-Baroni G, Sofi F, Milani S, Abbate R, Surrenti C, Casini A. (2006). Prolonged n-3 polyunsaturated fatty acid supplementation ameliorates hepatic steatosis in patients with non-alcoholic fatty liver disease: A pilot study. Aliment Pharmacol Ther, 23, 1143–1151.
- Carter-Kent C, Zein NN, Feldstein AE. (2008). Cytokines in the pathogenesis of fatty liver and disease progression to steatohepatitis: Implications for treatment. Am J Gastroenterol, 103, 1036–1042.
- Colicchio P, Tarantino G, del Genio F, Sorrentino P, Saldalamacchia G, Finelli C, Conca P, Contaldo F, Pasanisi F. (2005). Non-alcoholic fatty liver disease in young adult severely obese non-diabetic patients in South Italy. Ann Nutr Metab, 49, 289–295.
- Copaci I, Micu L, Voiculescu M. (2006). The role of cytokines in non-alcoholic steatohepatitis: A review. J Gastrointestin Liver Dis, 15, 363–373.
- De Ley M, de Vos R, Hommes DW, Stokkers P (2007). Fish oil for induction of remission in ulcerative colitis. Cochrane Database Syst Rev, 17: CD005986.
- Deen D. (2004). Metabolic syndrome: Time for action. Am Fam Physician, 69, 2875–2882.
- Duvnjak M, Lerotic I, Barsic N, Tomasic V, Virovic Jukic L, Velagic V. (2007). Pathogenesis and management issues for non-alcoholic fatty liver disease. World J Gastroenterol, 13, 4539–4550.
- El-Assal O, Hong F, Kim WH, Radaeva S, Gao B. (2004). IL-6-deficient mice are susceptible to ethanol-induced hepatic steatosis: IL-6 protects against ethanol-induced oxidative stress and mitochondrial permeability transition in the liver. Cell Mol Immunol, 1, 205–211.
- El-Mesery M, Al-Gayyar M, Salem H, Darweish M, El-Mowafy A. (2009). Chemopreventive and renal protective effects for docosahexaenoic acid (DHA): Implications of CRP and lipid peroxides. Cell Div, 4, 6.
- Ford ES. (2005). Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care, 28, 2745–2749.
- Friedewald WT, Levy RI, Fredrickson DS. (1972). Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem, 18, 499–502.
- García-Galiano D, Sánchez-Garrido MA, Espejo I, Montero JL, Costán G, Marchal T, Membrives A, Gallardo-Valverde JM, Muñoz-Castañeda JR, Arévalo E, De la Mata M, Muntané J. (2007). IL-6 and IGF-1 are independent prognostic factors of liver steatosis and non-alcoholic steatohepatitis in morbidly obese patients. Obes Surg, 17, 493–503.
- Haukeland JW, Damås JK, Konopski Z, Løberg EM, Haaland T, Goverud I, Torjesen PA, Birkeland K, Bjøro K, Aukrust P. (2006). Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J Hepatol, 44, 1167–1174.
- Iimuro Y, Gallucci RM, Luster MI, Kono H, Thurman RG. (1997). Antibodies to tumor necrosis factor α attenuate hepatic necrosis and inflammation caused by chronic exposure to ethanol in the rat. Hepatology, 26, 1530–1537.
- Jin X, Zimmers TA, Perez EA, Pierce RH, Zhang Z, Koniaris LG. (2006). Paradoxical effects of short- and long-term interleukin-6 exposure on liver injury and repair. Hepatology, 43, 474–484.
- Mensink RP, Plat J, Schrauwen P. (2008). Diet and nonalcoholic fatty liver disease. Curr Opin Lipidol, 19, 25–29.
- Paschos P, Paletas K. (2009). Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia, 13, 9–19.
- Poniachik J, Csendes A, Díaz JC, Rojas J, Burdiles P, Maluenda F, Smok G, Rodrigo R, Videla LA. (2006). Increased production of IL-1α and TNF-α in lipopolysaccharide-stimulated blood from obese patients with non-alcoholic fatty liver disease. Cytokine, 33, 252–257.
- Portincasa P, Grattagliano I, Palmieri VO, Palasciano G. (2005). Nonalcoholic steatohepatitis: Recent advances from experimental models to clinical management. Clin Biochem, 38, 203–217.
- Puder JJ, Varga S, Kraenzlin M, De Geyter C, Keller U, Müller B. (2005). Central fat excess in polycystic ovary syndrome: Relation to low-grade inflammation and insulin resistance. J Clin Endocrinol Metab, 90, 6014–6021.
- Spadaro L, Magliocco O, Spampinato D, Piro S, Oliveri C, Alagona C, Papa G, Rabuazzo AM, Purrello F. (2006). ω-3 Polyunsaturated fatty acids: A pilot trial in nonalcoholic fatty liver disease. J Hepatol, 44:S264.
- Targher G, Arcaro G. (2007). Non-alcoholic fatty liver disease and increased risk of cardiovascular disease. Atherosclerosis, 191, 235–240.
- Valenti L, Fracanzani AL, Dongiovanni P, Santorelli G, Branchi A, Taioli E, Fiorelli G, Fargion S. (2002). Tumor necrosis factor α promoter polymorphisms and insulin resistance in nonalcoholic fatty liver disease. Gastroenterology, 122, 274–280.
- Wang PW, Hsieh CJ, Psang LC, Cheng YF, Liou CW, Weng SW, Chen JF, Chen IY, Li RH, Eng HL. (2008). Fatty liver and chronic inflammation in Chinese adults. Diabetes Res Clin Pract, 81, 202–208.
- Weatherill AR, Lee JY, Zhao L, Lemay DG, Youn HS, Hwang DH. (2005). Saturated and polyunsaturated fatty acids reciprocally modulate dendritic cell functions mediated through TLR4. J Immunol, 174, 5390–5397.
- Wieckowska A, Papouchado BG, Li Z, Lopez R, Zein NN, Feldstein AE. (2008). Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am J Gastroenterol, 103, 1372–1379.