Abstract
Context: Drawbacks of presently available treatments for urolithiasis necessitate finding the treatment of hyperoxaluria specifically aimed at reduction in oxalate excretion. Interestingly, many Indian tribes use Bombax ceiba L. (Bombacaceae) fruits as a traditional medicine for the treatment of urinary stones.
Objective: The present study investigated the efficacy of B. ceiba fruit extracts as curative agents in experimentally induced calcium oxalate urolithiatic rats.
Materials and methods: Calcium oxalate lithiasis was induced in rats by oral administration of 0.75% ethylene glycol for 14 consecutive days. Treatments with aqueous and ethanol extract of B. ceiba fruit (400 mg/kg body weight) was performed in the same manner for further 14 consecutive days. Cystone (750 mg/kg body weight) was used as reference antiurolithiatic drug. The urinary excretion and kidney deposition of offending salt components, and serum biochemical parameters were investigated.
Results: Oral administration of ethylene glycol resulted in hyperoxaluria and increased renal excretion of calcium and phosphate. However, supplementation with aqueous and ethanol extracts of B. ceiba fruit significantly (p < 0.05) reduced the elevated urinary oxalate, showing a regulatory action on endogenous oxalate synthesis. The increased deposition of stone forming constituents in kidneys of calculogenic rats was also significantly lowered with curative treatment of aqueous and ethanol extract.
Discussion and conclusion: The results indicate that the fruit of B. ceiba is endowed with lithontriptic activity warranting further development for curative treatment of urolithiasis.
Keywords::
Introduction
Urolithiasis is a multifactorial process which starts with the formation of microcrystals in the urine and terminates as mature renal calculi. The major factors are supersaturation of urine with the offending salt and crystallization. Crystals retained in kidney become nucleus for stone formation (CitationBalaji & Menon, 1997). Majority of urinary calculi are made up of calcium phosphate (CaPh), calcium oxalate (CaOx), uric acid (urates) or magnesium ammonium phosphate. CaOx is the common component of calculi offered among more than 80% of stone formers population (CitationPrien & Prien, 1968; CitationBegun et al., 1991).
Due to the widespread clinical occurrence and the difficulty of treatment, CaOx urolithiasis has gained much importance over the other stone types. Hyperoxaluria is one of the main risk factors of idiopathic CaOx disease, wherein, the increased urinary oxalate promotes CaOx crystallization and stone formation. Factors that are likely to influence urinary oxalate excretion most are the hepatic synthesis of oxalate, the bioavailability and absorption of oxalate from the gastrointestinal tract and the renal handling of oxalate (CitationKrishnamurthy et al., 2003). Furthermore, oxalate is known to cause disruption of the renal cellular membrane integrity probably by inducing lipid peroxidation and thereby exaggerating the condition (CitationBijikurien & Selvam, 1987; CitationSaravanan et al., 1995).
Many remedies have been employed during the ages to treat urolithiasis and in most cases the management of urolithiasis is combined with surgical and medical approach using percutaneous nephrolithotomy (PCNL), extracorporeal shock wave lithotripsy (ESWL) and antibiotics (CitationRivers et al., 2000). However, the treatment selection depends on size, location and composition of stone, as well as, efficacy of each modality and associated morbidity, available equipments, physician skill, patient health and preference, and finally costs (CitationDenstedt & Singal, 1997). This has given rise to research on drugs from natural resources showing antiurolithiatic activity. A number of plants have been used in India and elsewhere which claim efficient cure of urinary stones (CitationMukherjee et al., 1984). In this regard, many plants have been studied systematically to establish the scientific validity to their use as an antiurolithiatic agent; however, the need for truly satisfactory, safe and efficacious drug persists.
There is plethora of evidence that Bombax ceiba L. (Bombacaceae), commonly known as “Cotton tree”, is used traditionally in the treatment of urinary stones by Indian tribes (CitationNadkarni & Nadkarni, 1976; CitationKirtikar & Basu, 1980). Stem, root, flower, fruit, and leaves of B. ceiba have been reported to contain many important phytoconstituents including alkaloids, glycosides, phytosterols, and triterpenoids (lupeol and β-sitosterol), proteins, phenolic compounds (naphthalene derivatives, mangiferin, shamimin, kaemferol, and quercetin) and tannins (CitationMehra & Karnick, 1968; CitationMukherjee & Roy, 1971; Harish & Gupta, 1972; CitationSeshadri et al., 1971, 1973).
In recent decades, the extracts of B ceiba have been extensively studied for many potential uses including moderate oxytocic activity and cardiac stimulant properties (CitationMisra et al., 1968), hypotensive and hypoglycaemic activity (CitationSaleem et al., 1999), analgesic and antioxidant activity (CitationDar et al., 2005; CitationVieira et al., 2009). An ethnobotanical study reported the use of B. ceiba as a traditional anti-inflammatory agent (CitationNamsa et al., 2009). Furthermore, the dried young fruits of B. ceiba are given in calculus affections and chronic inflammation and ulceration of the bladder and kidneys including strangury and other forms of dysuria (CitationKapoor, 1990), though the rationale behind its use is not scientifically established. In the present study, an effort has been made to ascertain the scientific validity to lithontriptic properties of B. ceiba using experimental hyperoxaluria model in rats.
Materials and methods
Plant material
The young fruits of B. ceiba were collected in April 2008 from local areas of Navi Mumbai in Maharashtra, India and authenticated by P. G. Diwakar at Botanical Survey of India (BSI), Pune, India. A voucher specimen of the plant was deposited in the BSI herbarium under number NBGBCP3. The fruits were dried in shade and ground to a coarse powder (approx. 40 mesh size).
Chemicals
Ethylene glycol (EG) was purchased from Merck Laboratories (Mumbai, India). Cystone tablets, a marketed product of Himalaya Drug Company (Bangalore, India), were used as reference antiurolithiatic drug (CitationMitra et al., 1998). Urocolor 10 test strips for routine urinalysis were purchased from Standard Diagnostics Inc. (South Korea). The biochemical estimation kits from Erba Diagnostics Mannheim GmbH (Germany) were used.
Preparation of extract
The ethanol extract (EtE, 10%) was prepared using 95% ethanol by Soxhlet method at temperature of 60°C to 70°C (yield 13.8%, 69.0 g) and aqueous extract (AqE, 10%) was prepared using purified water by maceration method for 7 days at room temperature (yield 22.1%, 110.5 g). The extracts were concentrated under vacuum and dried over anhydrous sodium sulphate. Both extracts were subjected to qualitative chemical analysis (CitationKhandelwal, 2002) followed by thin layer chromatographic (TLC) analysis. A suspension of AqE and EtE in 0.5% sodium carboxymethyl cellulose in distilled water was prepared for oral administration.
Antiurolithiatic activity
Animal selection
For acute toxicity studies, Wistar albino rats of either sex weighing between 150 and 200 g were selected and healthy adult male Wistar albino rats weighing between 150 and 200 g were selected for the antiurolithiatic activity. The animals were acclimatized to standard laboratory conditions (temperature: 22 ± 3°C) and maintained on 12:12 h light:dark cycle. They were provided with regular rat chow (Lipton India Ltd., Mumbai, India) and drinking water ad libitum. The animal care and experimental protocol was in accordance with NIH guide for the care and use of laboratory animals and approved by Institutional Animal Ethical Committee (IAEC).
Acute toxicity studies
The acute oral toxicity study was carried out as per the guidelines number 423 (CitationAnonymous, 2001) set by Organization for Economic Co-operation and Development (OECD) in accordance with Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). Based on the cut-off value of the median lethal dose (LD50), the therapeutically effective dose was derived.
Ethylene glycol induced urolithiasis model
A method of CitationAtmani et al. (2003) with suitable modifications was used to assess the antiurolithiatic activity. Rats were divided in five groups containing six in each. Group I served as normal control. Rats in group II to V were rendered hyperoxaluric by oral administration of 0.75% EG in drinking water till day 14. Group II rats was left untreated from day 15 till day 28 and served as negative control; while, curative treatments were administered in group III, IV and V using cystone (750 mg/kg body weight), AqE (400 mg/kg body weight) and EtE (400 mg/kg body weight), respectively. All doses were given once daily by oral route using gastric intubation method.
Collection and analysis of urine
At the end of study i.e. on day 28, all rats were kept in individual metabolic cages and urine samples of 24 h were collected. Routine urinalysis including quantitative determination of pH and specific gravity along with presence of occult blood, bilirubin, urobilinogen, ketone bodies, proteins, nitrite, glucose, and leucocytes in urine was carried out using Urocolor test strips. A drop of concentrated hydrochloric acid was added to the urine before being stored at 4°C. Urine was analyzed for calcium, phosphate and oxalate content (CitationFiske & Subbarow, 1925; CitationHodgkinson & Williams, 1972; CitationMustafa & Medeiros, 1985).
Microscopic examination of urine
Freshly collected urine samples (on day 28), were examined at ×50 magnification using compound microscope to ascertain the presence of characteristic crystals of CaOx and CaPh. Their photomicrographs were taken using a digital Mikrotek CCD camera supported with Avercap software (Avermedia Technologies, version 1.0.0.10).
Serum analysis
After the experimental period, rats were partially anaesthetized using ether anaesthesia and blood was collected from the retro-orbital vein. Finally, rats were sacrificed using higher doses of ether anaesthesia. Serum was separated from the blood sample by centrifugation at 10,000g for 10 min and analyzed for creatinine, uric acid and blood urea nitrogen (BUN) (CitationCaraway, 1963; CitationRaghuramulu et al., 1983).
Kidney homogenate analysis
The abdomen was cut open to remove both kidneys from each animal. Isolated kidneys were cleaned of extraneous tissue and preserved in 10% neutral formalin. One of the isolated kidneys was dried at 80°C in a hot air oven. A sample of 100 mg of the dried kidney was boiled in 10 mL of 1 N hydrochloric acid for 30 min and homogenized. The homogenate was centrifuged at 2000g for 10 min and supernatant was separated (CitationChow et al., 1975). The calcium, phosphate and oxalate content in kidney homogenate were determined (CitationFiske & Subbarow, 1925; CitationHodgkinson & Williams, 1972; CitationMustafa & Medeiros, 1985).
Histopathological examination of kidney
Another isolated kidney was then embedded in paraffin using conventional methods and cut into 5 µm thick sections and stained using hematoxylin-eosin dye and finally mounted in diphenyl xylene. The sections were observed under compound microscope at ×50 magnification for histopathological changes in kidney architecture and their photomicrographs were taken using a digital camera. By visualizing different fields, a general method of scoring was adopted to observe the extent of nephritic damage and the recovery. A minimum of 10 fields for each kidney slide were examined and assigned for severity of changes using scores on a scale of none (ND), mild (+), moderate (++) and severe (+++) damage (CitationSingh & Chopra, 2004).
Statistical analysis
Results were expressed as mean ± standard error of mean (SEM). Differences among data were determined using one way analysis of variance (ANOVA) followed by the Dunnett’s test (Graphpad Prism software for Windows, Version 4.00.255). Differences between the data were considered significant at p < 0.05.
Results
The qualitative chemical analysis revealed the presence of carbohydrates, glycosides, flavonoids, tannins and phenolic compounds in both extracts. From the acute oral toxicity studies in rats, the therapeutic dose was taken as 400 mg/kg body weight for both extracts.
In the present study, oral administration of EG for 14 consecutive days resulted in hyperoxaluria. The urinary excretion of oxalate was increased significantly (p < 0.01) in calculi-induced rats (, Group II) as compared to control (, Group I). Calcium and phosphate excretion was also increased in group II. The deposition of oxalate, calcium, and phosphate in the renal tissue, was increased in the calculi-induced rats. Administration of cystone significantly (p < 0.05) normalized the elevated urinary levels of oxalate, calcium, and phosphate (, Group III). The renal tissue deposition of these stone forming constituents was also significantly (p < 0.05) minimizing in this group. The curative treatment of AqE and EtE of B. ceiba fruit was also found to be beneficial in minimizing the elevated urinary and renal content of offending salt components. A notably significant reduction in urinary excretion of calcium (p < 0.01) and oxalate (p < 0.05) were exhibited by AqE and EtE treatment (, Group IV and V) comparable with the standard drug, cystone.
Table 1. Effect of Bombax ceiba fruit extracts on urinary and serum parameters in control and experimental animals.
The stones formed in kidneys remarkably damaged the kidney architecture as indicated by elevated serum levels of urea nitrogen (BUN), creatinine and uric acid (, Group II). However, the AqE and EtE of B. ceiba fruit were found to be effective in significantly (p < 0.01) lowering the elevated serum levels of creatinine and uric acid. The reduction in BUN exhibited by these groups was also significant at p < 0.05. Although the intergroup comparison between AqE and EtE treatments showed non-significant reduction in these accumulated waste products, the administration of EtE (, Group V) was found to be significant (p < 0.01) and comparable to that of cystone.
The results of routine urinalysis indicated the mean pH value of 7.58 in calculi-induced rats (, Group II). However, with the curative treatment with AqE and EtE of B. ceiba fruit lowering of the elevated urinary pH near to neutral values was observed. Glucosuria was found to be absent; while, the specific gravity of urine and changes associated with urinary excretion of bilirubin, urobilinogen, leucocytes, ketone bodies and nitrite were found to be non-significant among all experimental groups (). Further, the proteinuria was observed in calculi-induced group indicating the significant nephritic cellular damage. However, the curative treatment with AqE and EtE of B. ceiba fruit showed an adequate reduction in the urinary excretion of proteins comparable to the cystone treated rats (, group III).
Table 2. Effect of Bombax ceiba fruit extracts on miscellaneous urinary parameters in control and experimental animals.
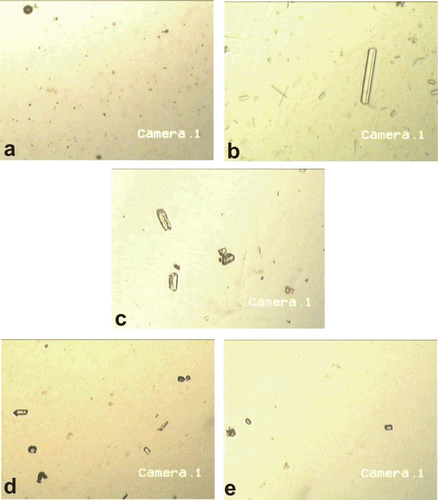
On microscopic examination, the urine of the control group was found to be devoid of any crystal or similar structures (). In calculi-induced rats, the urine sample showed abundant, large crystals with characteristic shape of CaOx and triple phosphate (). In comparison, the urine of cystone treated (), AqE and EtE treated rats ( and , respectively) showed less abundant crystal fragments having significantly reduced size. The pattern of dissolution of crystal exhibited by AqE and EtE of B. ceiba fruit was found to be comparable to the standard drug, cystone; however, smaller and discrete fragments of crystals were seen among these groups.
Figure 1. Microscopic examination (×50) of urine excreted by (a) the control rats, (b) rats receiving EG 0.75% only, (c) rats receiving EG 0.75% and treated with cystone, (d) rats receiving EG 0.75% and AqE of B. ceiba fruit, and (e) rats receiving EG 0.75% and EtE of B. ceiba fruit.
The histopathological examination of kidney also supported the serum biochemistry and urine microscopy results. In all the stone forming rats (), severe damage to the last part of the nephron, collecting system and peritubular interstitium was observed as compared to the normal rat kidney architecture (). The tubules appeared focally ectasic and surrounded by inflammatory infiltration (). Flattened epithelium with focal vacuolar degeneration and single cell necrosis bordered the tubules, which focally contained hyaline casts. Inflammatory infiltration was mainly composed of mature lymphocytes infiltrating tubular epithelium. Irregular crystals were present inside the tubules and in the peritubular interstitium, along the nephron and at papillary level. However, the AqE and EtE of B. ceiba treated groups ( and ) showed moderate recovery of renal histology comparable to that of cystone treated group () and showed normal glomeruli ().
Figure 2. Microscopic examination (H&E, ×50) of kidney section of (a) the control rats, (b) rats receiving EG 0.75% only, (c) rats receiving EG 0.75% and treated with cystone, (d) rats receiving EG 0.75% and AqE of B. ceiba fruit, and (e) rats receiving EG 0.75% and EtE of B. ceiba fruit.
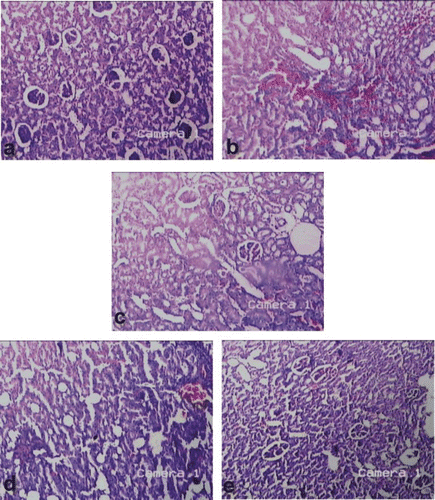
Table 3. Effect of Bombax ceiba fruit extracts on renal histology in control and experimental animals.
Discussion
Urinary chemistry is one of the important factors in determining the type of crystals formed and the nature of molecules occluded on to the surface of the crystals and stones. Substantially low urine volume, pH, mild hyperoxaluria, hypercalciuria, hypocitraturia, hyperuricosuria and hypomagnesuria are major risk factors in stone formers (CitationRobertson, 1999). Urine pH (<5.5 for uric acid stones and >6 for calcium stones) is a surrogate marker for determining the type of calculi. Particularly, in CaOx urolithiasis, the remarked basicity of the urine beyond pH 7.2 initiates the nucleation of phosphate and oxalate with calcium (CitationBalaji & Menon, 1997; CitationRivers et al., 2000).
In the present study, young male albino rats show increased pH (7.82) and form renal calculi composed mainly of CaOx in response to 14 days oral supplement of EG. Evidence in previous studies indicate that an increase in the urinary concentration of oxalate account for the biochemical mechanisms in this process (CitationSelvam et al., 2001; CitationHuang et al., 2002; CitationAtmani et al., 2003). The ready conversion of glycolate to oxalate renders the EG fed animals to a state of hyperoxaluria (CitationRichardson & Tolbert, 1961). Similar results have been obtained when rats were treated with EG and ammonium oxalate (CitationAdhirai & Selvam, 1997; CitationMuthukumar & Selvam, 1997).
In calculi-induced rats (Group II), the urinary excretion of calcium is also progressively increased, which may be attributed to defective tubular reabsorption in kidneys. However, the changes in urinary oxalate levels are relatively much more important than those of calcium (CitationRobertson & Peacock, 1980), since it is accepted that hyperoxaluria is far more significant risk factor in the pathogenesis of renal stones than hypercalciuria (CitationTisselius, 1996). Increased urinary calcium favors nucleation and precipitation of CaOx or apatite (CaPh) from urine and subsequent crystal growth (CitationLemann et al., 1991). However, in the present study the curative treatment with AqE and EtE of B. ceiba L. fruit lowers the levels of oxalate as well as calcium excretion.
A progressive increase in urinary phosphate as observed in calculi-induced rats (Group II), along with oxalate load seems to provide further an environment appropriate for calculi formation by forming a nidus of CaPh and triple phosphate crystals with epitaxial deposition of CaOx (CitationRoger et al., 1997). However, supplementation of B. ceiba fruit extracts restore phosphate level, thereby reducing the further risk of stone formation.
The kidney function is affected in urolithiasis, since lowering of the glomerular filtration rate (GFR) is observed due to the obstruction to the outflow of urine by calculi deposited along the urinary system. Thereby, the waste products particularly nitrogenous substances such as urea, creatinine and uric acid, accumulate in blood (CitationGhodkar, 1994). It is well established that the glycolate feeding causes increased lipid peroxidation and decreases levels of antioxidant potential in kidneys (CitationSumathi et al., 1993; CitationSaravanan et al., 1995). Oxalate, being the precursor molecule to induce lipid peroxidation, further causes renal tissue damage by reacting with polyunsaturated fatty acids in cell membrane (CitationErnster & Nordenbrand, 1967). In this scenario, marked renal damage is observed in calculi-induced rats (, Group II) ascribed by virtue of the elevated serum levels of creatinine and uric acid, and BUN (, Group II). Significant proteinuria and hematuria also substantiate the extent of nephritic damage (, Group II). However, the diuresis induced by the AqE and EtE of B. ceiba fruit (CitationGadge et al., 2009) causes to accelerate the process of dissolving preformed calculi and interrupts the process of crystal aggregation and deposition along the urinary system. The significant lowering of serum levels of accumulated waste products may be attributed to the enhanced GFR and the antioxidant property of B. ceiba (CitationDar et al., 2005; CitationVieira et al., 2009).
Results of phytochemical investigations from other researchers indicate the possible role of secondary plant metabolites (particularly saponins and triterpenoids) for possessing antiurolithiatic activity (CitationAnand et al., 1992; CitationBaskar et al., 1996). Lupeol, a pentacyclic triterpenes, which exists widely in Crataeva nurvala Buch Ham. (Capparidaceae) mainly as aglycone of triterpenoid saponins, have been found to inhibit glycolic acid induced the urolithiasis (Buhler et al., 1991; CitationVidya & Varalakshmi, 2000). On contrary, the phytochemical investigation in the present study revealed absence of saponins and triterpenoids in both extracts of B. ceiba fruit (CitationGadge et al., 2009).
Nevertheless, there are reports which correlate the antiurolithiatic activity of the lupeol with its diuretic potentials (CitationAnand et al., 1991; CitationMalini et al., 1995). Accordingly, the significant diuretic activity of B. ceiba fruit extracts could suggest the possible role of the plant in the present study as an antilithic agent (CitationGadge et al., 2009). Diuresis reduces the risk of stone formation by forbidding the saturation product of CaOx. Further, in detail phytochemical study of B. ceiba fruit extracts would elaborate the modus operandi of the plant as a diuretic and antilithiatic agent.
In conclusion, the presented data indicate that administration of the AqE and EtE of B. ceiba fruit to rats with experimentally induced urolithiasis reduced and prevented the growth of urinary stones, supporting folk information regarding antiurolithiatic activity of the plant. The mechanism underlying this effect is still unknown, but is apparently related to increased diuresis and lowering of urinary concentrations of stone forming constituents. The protective effect against oxalate induced lipid peroxidation may be contributory to the recovery of renal damage. These effects could conclude the antiurolithiatic property of Bombax ceiba fruit.
Acknowledgements
We express our thanks to P. G. Diwakar, Botanical Survey of India, Pune, India, for authentication of the plant material. The corresponding author is especially thankful to Indian Council of Medical Research, New Delhi, India, for providing the financial assistance in the form of SRF.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Adhirai M, Selvam R. (1997). Vitamin E pretreatment prevents cyclosporine A-induced crystal deposition in hyperoxaluric rats. Nephron, 75, 77–81.
- Anand R, Patnaik GK, Kulshrestha DK, Dhawan BN. (1991). Antiurolithiatic and diuretic activity of lupeol. Proceedings of 24th and 25th Pharmacology Society Conference; A-10.
- Anand R, Patnaik GK, Kulshrestha DK, Dhawan BN. (1992). Antiurolithiatic and diuretic activity of lupeol, the active constituent isolated from Crataeva nurvala (Buch Ham). Indian J Pharmacol, 24, 48–49.
- Anonymous. (2001). OECD series on testing and assessment, Number 24. Guidance document on acute oral toxicity testing. ENV/JM/MONO(2001)4. Paris: Environment directorate, OECD.
- Atmani F, Slimani Y, Mimouni M, Hacht B. (2003). Prophylaxis of calcium oxalate stones by Herniaria hirsuta on experimentally induced nephrolithiasis in rats. bju Int, 92, 137–140.
- Balaji KC, Menon M. (1997). Mechanism of stone formation. Urol Clin North Am, 24, 1–11.
- Baskar R, Malini MM, Varalakshmi P, Balakrishna K, Bhimarao R. (1996). Effect of lupeol isolated from Crataeva nurvala stem bark against free radical-induced toxicity in experimental urolithiasis. Fitoterapia, 67, 121–125.
- Begun FP, Knoll CE, Gottlieb M, Lawson RK. (1991). Chronic effects of focused electrohydraulic shock waves on renal function and hypertension. J Urol, 145, 635–639.
- Bijikurien T, Selvam R. (1987). Induction of lipid peroxidation by oxalate in experimental rat urolithiasis. J Biosci, 12, 367–373.
- Bühler H, Perschel FH, Hierholzer K. (1991). Inhibition of rat renal 11 beta-hydroxysteroid dehydrogenase by steroidal compounds and triterpenoids; structure/function relationship. Biochim Biophys Acta, 1075, 206–212.
- Caraway WT. (1963). Uric acid. In: Seligson D, ed. Standard Methods Clinical Chemistry. New York and London: Academic Press, 4, 239.
- Chow FC, Dysart MI, Hamar DW, Udall RH. (1975). Control of oxalate urolithiasis by DL-alanine. Invest Urol, 13, 113–116.
- Dar A, Faizi S, Naqvi S, Roome T, Zikr-ur-Rehman S, Ali M, Firdous S, Moin ST. (2005). Analgesic and antioxidant activity of mangiferin and its derivatives: the structure activity relationship. Biol Pharm Bull, 28, 596–600.
- Denstedt JD, Singal RK. (1997). Contemporary management of ureteral stones. Urol Clin North Am, 24, 68.
- Ernster L, Nordenbrand K. (1967). Oxidation and phosphorylation. In: Ronald WE, Maynard EP, eds. Methods in Enzymology.Vol. X. New York: Academic Press; 574–580.
- Fiske CH, Subbarow Y. (1925). The colorimetric determination of phosphate. J Biol Chem, 66, 375–381.
- Gadge NB, Jalalpure SS, Pawase KB. (2009). Preliminary phytochemical screening and diuretic activity of Bombax ceiba L. fruit extracts. Pharmacologyonline, 3, 188–194.
- Ghodkar PB. (1994). Chemical tests in kidney disease. In: Textbook of Medical Laboratory Technology. 1st ed. Mumbai: Bhalani Publishing House; 118–132.
- Gopal H, Gupta RK. (1972). Chemical constituents of Salmalia malabarica Schott and Endl. flowers. J Pharm Sci, 61, 807–808.
- Hodgkinson A, Williams A. (1972). An improved colorimetric procedure for urine oxalate. Clin Chim Acta, 36, 127–132.
- Huang HS, Ma MC, Chen J, Chen CF. (2002). Changes in the oxidant-antioxidant balance in the kidney of rats with nephrolithiasis induced by ethylene glycol. J Urol, 167, 2584–2593.
- Kapoor LD. (1990). Handbook of Ayurvedic Medicinal Plants. Boca Raton: CRC Press.
- Khandelwal KR. (2002). Practical Pharmacognosy, Techniques and Experiments. 9th ed. Pune: Nirali Prakashan.
- Kirtikar KR, Basu BD. (1980). Indian Medicinal Plants. 2nd ed., Vol. I. Dehradun: International Book Distributors.
- Krishnamurthy MS, Hruska KA, Chandhoke PS. (2003). The urinary response to an oral oxalate load in recurrent calcium stone formers. J Urol, 169, 2030–2033.
- Lemann J Jr, Worcestor EM, Gray RW. (1991). Hypercalciuria and stones. Am J Kidney Dis, 27, 386–391.
- Malini MM, Baskar R, Varalakshmi P. (1995). Effect of lupeol, a pentacyclic triterpene, on urinary enzymes in hyperoxaluric rats. Jpn J Med Sci Biol, 48, 211–220.
- Mehra PN, Karnick CR. (1968). Pharmacognostic studies on Bombax ceiba Linn. Indian J Pharm, 30, 284
- Misra MB, Mishra SS, Misra RK. (1968). Pharmacology of Bombax malabaricum (DC). Indian J Pharm, 30, 165–166.
- Mitra SK, Gopumadhavan S, Venkataranganna MV, Sundaram R. (1998). Effect of cystone, a herbal formulation, on glycolic acid-induced urolithiasis. Phytotherap Res, 12, 372–374.
- Mukherjee J, Roy B. (1971). Chemical examination of Salmalia malabarica Schott Endl. (syn. Bombax malabaricum DC.). J Indian Chem Soc, 48, 769–770.
- Mukherjee T, Bhalla N, Aulakh GS, Jain HC. (1984). Herbal drugs for urinary stones – Literature appraisal. Indian Drugs, 21, 224–228.
- Mustafa MA, Medeiros DM. (1985). Proximate composition, mineral content and fatty acids of cat fish (Ictalurus punctatus rafinesque) for different seasons and cooking methods. J Food Sci, 50, 585–588.
- Muthukumar A, Selvam R. (1997). Effect of depletion of reduced glutathione and its supplementation by glutathione monoester on renal oxalate retention in hyperoxaluria. J Nutr Biochem, 8, 445–450.
- Nadkarni AK, Nadkarni KM. (1976). Indian Materia Medica. 3rd ed., Vol. I. Popular Book Depot: Bombay.
- Namsa ND, Tag H, Mandal M, Kalita P, Das AK. (2009). An ethnobotanical study of traditional anti-inflammatory plants used by the Lohit community of Arunachal Pradesh, India. J Ethnopharmacol, 125, 234–245.
- Prien EL, Prien EL Jr. (1968). Composition and structure of urinary stone. Am J Med, 45, 654–672.
- Raghuramulu N, Madhavan NK, Kalyanasundaram S.(1983). In: A Manual of Laboratory Techniques. 1st ed. Hyderabad: National Institute of Nutrition; 34.
- Richardson TKE, Tolbert NE. (1961). Oxidation of glycolic acid to oxalic acid by glycolic acid oxidase. J Biol Chem, 57, 816–882.
- Rivers K, Shetty S, Menon M. (2000). When and how to evaluate a patient with nephrolithiasis. Urol Clin North Am, 27, 203–213.
- Robertson WG, Peacock M. (1980). The cause of idiopathic calcium stone disease: hypercalciuria or hyperoxaluria? Nephron, 26, 105–110.
- Robertson WG. (1999). A simplified procedure for assessing the biochemical risk of forming stones in patients with uric acid or calcium containing urinary calculi. In: Borghi L, Mesehi T, Briganti A, eds. Kidney Stones<. Cosenza, Italy: Editoriale Bios; 403–406.
- Roger K, Low MD, Stoller ML. (1997). Uric acid nephrolithiasis. Urol Clin North Am, 24, 135–148.
- Saleem R, Ahmad M, Hussain SA, Qazi AM, Ahmad SI, Qazi MH, Ali M, Faizi S, Akhtar S, Husnain SN. (1999). Hypotensive, hypoglycaemic and toxicological studies on the flavonol C-glycoside shamimin from Bombax ceiba. Planta Med, 65, 331–334.
- Saravanan N, Senthil D, Varalakshmi P. (1995). Effect of L-cysteine on lipid peroxidation in experimental urolithiatic rats. Pharmacol Res, 32, 165–169.
- Selvam R, Kalaiselvi P, Govindaraj A, Bala Murugan V, Sathish Kumar AS. (2001). Effect of A. lanata leaf extract and Vediuppu chunnam on the urinary risk factors of calcium oxalate urolithiasis during experimental hyperoxaluria. Pharmacol Res, 43, 89–93.
- Seshadri V, Batta AK, Rangaswamy S. (1971). Phenolic components of Bombax malabaricum (Root-Bark). Curr Sci, 40, 630–631.
- Seshadri V, Batta AK, Rangaswamy S. (1973). Phenolic components of Bombax malabaricum. Indian J Chem, 11, 825
- Singh D, Chopra K. (2004). The effect of naringin, a bioflavonoid on ischemia-reperfusion induced renal injury in rats. Pharmacol Res, 50, 187–193.
- Sumathi R, Jayanthi S, Kalpanadevi V, Varalakshmi P. (1993). Effect of DL alpha-lipoic acid on tissue lipid peroxidation and antioxidant systems in normal and glycollate treated rats. Pharmacol Res, 27, 309–318.
- Tisselius HG. (1996). Solution chemistry of supersaturation. In: Coe Fl Favus, MJ, Pak CYC, Parks JH, Preminger GM, eds. Kidney Stones: Medical and Surgical Management. Philadelphia: Lippincott Reven; 33.
- Vidya L, Varalakshmi P. (2000). Control of urinary risk factors of stones by betulin and lupeol in experimental hyperoxaluria. Fitoterapia, 71, 535–543.
- Vieira TO, Said A, Aboutabl E, Azzam M, Creczynski-Pasa TB. (2009). Antioxidant activity of methanolic extract of Bombax ceiba. Redox Rep, 14, 41–46.