Abstract
Context: Da-Huang-Zhe-Chong pill (DHZCP), a classical traditional Chinese formula, consists of 12 crude drugs which have been widely used with significant therapeutic effects. Some drugs in this formula have toxicities that might result in some adverse effects of DHZCP.
Objective: The liver protection and toxicity of DHZCP were first evaluated against chronic carbon tetrachloride (CCl4)-induced liver injury in rats.
Materials and methods: The rats were treated by intraperitoneal injection of 10% CCl4 for 12 weeks. At the end of week 4, DHZCP at doses of 44 g/kg (high-dose group) and 22 g/kg (low-dose group) was intragastrically administered to CCl4-treated rats, once a day for four weeks. At the end of weeks 8 and 12, the general status of the rats, histopathology of liver, serum alanine aminotransaminase (ALT), aspartate aminotransaminase (AST), alkaline phosphatase (ALP) and total bilirubin (TBIL) levels were observed or determined and recorded. By correspondence analysis (CA) on biochemical markers and liver histopathological score (HS), the “dose-time-response” relationship of DHZCP on the hepatic injury rats was evaluated.
Results: The results showed that DHZCP exhibited a significant protective effect on liver injury by reversing the biochemical parameters and histopathological changes. However, this hepatoprotective effect may be weakened, or even be transferred to toxicity with the increase of the administration dose (44 g·kg−1·d−1) and time (more than 2 months) of this formula. These results were consistent with the histopathological observation and the serum levels.
Discussion and conclusion: Administration of proper dose and time of DHZCP could well play its hepatoprotective effect and even treat hepatitis, but the safety on liver should be considered when large-dose (44 g·kg−1·d−1) DHZCP is used for long time (more than 2 months). We suggest that the administration dose and time of DHZCP in clinical use should not be increased and prolonged, and simultaneously liver function should be regularly monitored.
Introduction
Liver diseases are a serious health problem. For the lack of reliable liver protective drugs in allopathic medical practices, traditional Chinese drugs (TCDs) play an important role in the management of these liver diseases. Numerous medicinal TCDs and their formula are used for these diseases in ethnomedical practices and in traditional medicine system in China. However, some TCDs and their formula, such as Radix aconiti, Radix puerariae, Rhizoma coptidis, Niu-Huang-Jie-Du pill, Xiao-Cai-Hu broth and Shen-Mai injection, etc., can result in liver injury (CitationDai et al., 2004; CitationLu & Peng, 2005). This is a handicap on the development of TCDs in treating liver diseases. Therefore, there is an urgent need to search for hepatoprotective drugs with high efficacy and safety.
Da-Huang-Zhe-Chong Pill (DHZCP) is a classical traditional Chinese formula comprising 12 crude drugs: Radix et rhizoma rhei (Da-Huang), Eupolyphaga seu steleophaga (Tu-Bie-Chong), Hirudo (Shui-Zhi), Gradfly (Meng-Chong), Grub (Qi-Cao), Resina toxicodendri (Gan-Qi), Semen persicae (Tao-Ren), Semen armeniacae amarum (Ku-Xing-Ren), Radix scutellariae (Huang-Qin), Radix rehmanniae (Di-Huang), Raidix paeoniae alba (Bai-Shao) and Radix glycyrrhizae (Gan-Cao) (CitationChina Pharmacopoeia Committee, 2005). This formula was first recorded in Jin-Gui-Yao-Lue (Synopsis of Golden Chamber) with widely pharmacodynamic actions for treating diseases like blood stasis, hepatic cirrhosis, cyclomastopathy, chronic active hepatitis (CAH) for nearly 2000 years (CitationChen & Tan, 2006; CitationCao et al., 2006). Since the 1980s, many reports have confirmed the significant therapeutic effects of DHZCP for treatment of chronic hepatitis B and cirrhosis (CitationJiao et al., 1999; CitationSun, 1998; CitationPan et al., 2004, 2005). But, Gradfly, Grub and Resina toxicodendri in this formula have certain toxicities (CitationChina Pharmacopoeia Committee, 2005), and also, rhubarb can cause liver and renal toxicity along with carcinogenicity for its active components of anthraquinones (CitationCao et al., 2006; CitationWang et al., 2009 a,b). The inapposite or indiscriminate use of large amounts of DHZCP by some people may result in adverse side effects, even become life-threatening. So, the major objective to evaluate the hepatoprotective and toxic effects of DHZCP is important for its clinical use.
In the present study, we evaluated the liver protection and toxicity of different doses of DHZCP by intragastrically administering it to CCl4-induced liver injury rats. By examining the general status of rats, histopathology of liver, biochemical parameters including serum ALT (alanine aminotransferase), AST (aspartate aminotransferase), ALP (alkaline phosphatase) and TBIL (total bilirubin) levels, combined with correspondence analysis, the hepatoprotective effect and toxicity of DHZCP on liver injury rats were investigated. Further, the “dose-time-response” relationship of it on liver injury rats was discussed to promote safe use of this kind of formula.
Materials and methods
Materials
Da-Huang-Zhe-Chong Pill (DHZCP) was prepared according to Chiniese Pharmacopoeia (CitationChina Pharmacopoeia Committee, 2005). Analytical-grade CCl4 was purchased from Beihua Fine Chemicals Co., Ltd. (Beijing, China) and dissolved in olive oil to a final concentration of 10% before use. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and total bilirubin (TBIL) kits were purchased from Nanjing Jiancheng Bioengineering Institute (China). Other chemicals and reagents were obtained from local sources and were all analytical grades.
Animals
Eighty, male and female Sprague-Dawley rats, 6–8 weeks old and weighing 150–180 g were obtained from the Vital River Lab Animal Technology Co., Ltd. (Beijing, China) (License No. SCXK 2003–0003). These animals were housed under controlled conditions before the commencement of the experiments. The conditions were as follows: light (12 h light/dark cycle, lights on at 7:00 am), controlled temperature (20 ± 2°C), humidity (60–70%), draughty, free access to food and water. Animal welfare and experimental procedures were carried out strictly in accordance with the institutional animal care guidelines approved by the Ministry of Science and Technology of China and the related ethical regulations.
Experimental procedure
All the animals were randomly divided into four groups: normal group (N, n = 20), CCl4 model group (M, n = 20), CCl4 + high- dose of DHZCP (44 g/kg, H, n = 20) group and CCl4 + low -dose of DHZCP (22 g/kg, L, n = 20) group. The rats [except the normal group, which received an equal volume of Isotonic Saline Solution (ISS)] were intraperitoneally injected with a solution of 10% CCl4 (5 mL/kg body weight, in olive oil, 1:10, v/v) for 12 weeks to induce chronic liver injury. All the rats in H and L groups were treated intragastrically with 20 mL/kg of 44 g/kg and 22 g/kg of DHZCP, respectively, once a day from the beginning of week 4 to the end of week 12, while the rats in N and M groups received an equal volume of water. At the end of week 8 (when the rats had received CCl4 for 2 months and DHZCP for 1 month) and week 12 (when the rats had received CCl4 for 3 months and DHZCP for 2 months), the rats had free access to water, but not food for 12 h, they were then sacrificed. Blood was taken from the femoral vein to prepare serum for biochemical assay and the liver was obtained for histopathological observations.
General observation
The general status of the rats, including mental status, weight change, food intake, etc, was first visually observed and recorded (CitationSuresh et al., 2008; CitationWang et al., 2009 a,c).
Biochemical assays
The blood sample (5.0 mL) was centrifuged at 3000 × g for 10 min to separate serum. ALT, AST, ALP and TBIL activities were determined with a commercial kit (Roche) by Roche Modular Autoanalyzer.
Histopathological studies
Portions of tissues from the same lobe of liver in each animal were immediately fixed in 10% formaldehyde, embedded in paraffin, cut into 4–5 μm-thick sections, stained with hematoxylin-eosin (H & E), and observed under a light microscope. Histological damage was expressed according to the “semiquantitative grading” standard (CitationLiu et al., 2001) using the following score system: 0, absent; 1, few; 2, mild; 3, severe, the average value were then calculated and expressed as mean ± S.D.
Statistical analysis
Data were expressed as mean ± S.D. Significant difference between groups was analyzed by Student’s t-test using SAS 8.0 software (SAS, USA). The Mann–Whitney rank sum test was used for the degree of histopathological liver injury. P < 0.05 was considered to be statistically significant.
Correspondence analysis (CA)
CA (CitationPark et al., 2007; CitationPusha et al., 2009; CitationKong et al., 2009) is an important multivariate statistical method for studying the relationship between investigated factor and multi-variables. Based on the idea of principal component analysis (PCA), CA can compress these multi-variables to two principal component (PC) variables Z1 and Z2, which have the main contributions to the total data set. Then, the PC variable aggregations [Z1, Z2] of every valid point are calculated and depicted in the form of 2D-projection. Thus, the internal change regularity of investigated factor can be directly represented. In this study, the “dose-time-response” relationships of the liver protection and toxicity of DHZCP on hepatic injury rats were investigated by CA on the biochemical markers (serum ALT, AST, ALP and TBIL levels) and liver histopathological scores (HS) using SAS 8.0 software (SAS, USA).
Results
General status
Rats in the normal (N) group and CCl4 + DHZCP groups appeared healthy with good mental status and increased weight, while the CCl4 (M) group showed decreased activity, less food intake, loose stool, piloerection, dark fur, and retarded weight gain or even weight loss. The animals in CCl4 + high- dose of DHZCP (H) group and CCl4 + low- dose of DHZCP (L) group had little mortality (), slight hepatocyte steatosis, liver fibrosis, cholestasis or small bile duct hyperplasia as compared with those of the CCl4 model (M) group.
Table 1. Histopathological changes in the liver of rats of the four groups.
Activities of DHZCP on serum ALT, AST, ALP and TBIL levels
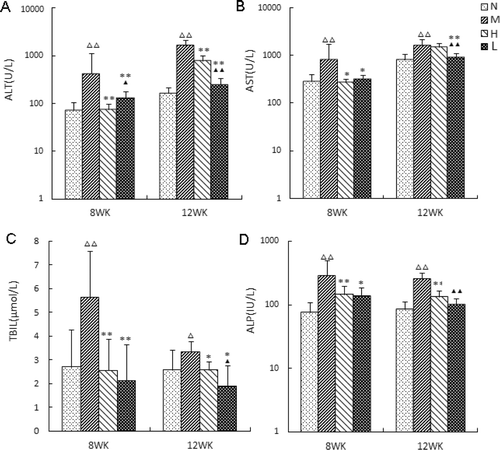
The serum activities of ALT, AST, ALP and TBIL were used as biochemical markers for the chronic hepatic damage and the contents of these four markers in the four groups at different times are shown in . At the end of week 8, compared to N group, the levels of ALT, AST and ALP in the M group were significantly elevated (p<F0.01) (ALT: from 71.60 ± 34.95 U/L to 426.90 ± 102.35 U/L, AST: from 288.50 ± 42.71 U/L to 826.50 ± 88.85 U/L, ALP: from 75.50 ± 33.52 IU/L to 281.60 ± 94.99 IU/L), and TBIL was also increased (p<0.05) from 2.70 ± 1.56 µmoL/L to 5.62 ± 3.95 µmoL/L, illustrating that the hepatocyte was damaged along with cholestasis. DHZCP administration with low and high doses (H and L groups) all significantly lowered transaminase level and cholestasis in rats compared with the corresponding levels in M group. The serum ALT level in low-dose group was higher (p<0.05) than the corresponding value in high-dose group, showing that high- dose of DHZCP had significant enzyme-lowering and liver-protecting effects on the CCl4-induced liver injury rats. At the end of week 12: the levels of ALT (1665.80 ± 37.71 U/L), AST (1629.20 ± 39.67 U/L), ALP (251.60 ± 26.40 U/L) and TBIL (2.34 ± 0.44 U/L) in M group were significantly elevated (p<0.01) from the corresponding values in N group (ALT: 166.29 ± 29.91 U/L, AST: 807.57 ± 59.567 U/L, ALP: 85.29 ± 28.14 U/L, TBIL: 2.56 ± 0.85 U/L), illustrating the notable hepatocyte damage. DHZCP administration in the H and L group could significantly lower transaminase level, protect liver and normalize gall bladder to cure jaundice. The rats treated with low dose of DHZCP showed a significant decrease in AST, ALT and ALP levels (p<0.01), and in TBIL level (p<0.05) compared with the corresponding values of rats treated with high- dose of DHZCP.
Figure 1. Activities of DHZCP on serum ALT, AST, TBIL and ALP levels in 4 groups of rats. N, normal group; M, model group, H, high-dose group, L, low-dose group. A–D: serum levels of ALT (alanine aminotransferase), AST (aspartate aminotransferase), alkaline phosphatase (ALP) and total bilirubin (TBIL) in 4 groups at weeks 8 and 12, respectively. Data are expressed as mean ± S.D. of 20 rats for each group. △p < 0.05, △△p < 0.01 compared with the normal group. *p < 0.05, **p < 0.01 compared with the CCl4 model group. ▴p < 0.05, ▴▴p < 0.01 compared with the CCl4 + high-dose of DHZCP group.
Histopathological observation
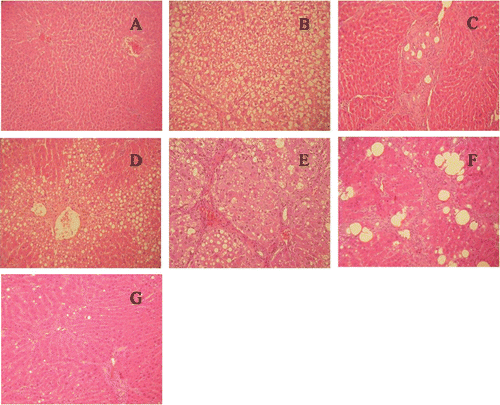
The histological features, as shown in and , indicated a normal liver lobular architecture and cell structure of the livers in the control animals (group N). There were no pathological changes in healthy livers of the normal group. At the end of week 8, in the CCl4-induced (M) group, liver pathological changes appeared characterized by swelling, hydropic degeneration, hepatocytes steatosis, small bile duct proliferation and cholestasis (), reflecting early biochemical and pathological changes in CCl4-induced liver injury. Histopathologcial changes induced by CCl4 were remarkably improved by high and low- dose of DHZCP (H and L groups) ( ), showing obvious difference from the M group except the hepatocytes steatosis, which is similar to the M group. The histopathological scores (HS) of liver pathological changes () were significantly low after DHZCP administration (p<0.05 for H group and p<0.01 for L group compared with the M group), but there were no notable differences between the H and L group. At the end of week 12, in M group, liver pathological changes () were much more severe than those at the end of week 8, which could well reflect the biochemical and pathological changes in CCl4-induced liver injury. These histopathologcial changes were remarkably improved by DHZCP of high and low- dose (H and L groups) (, ). The HS of liver pathological changes () in L group were significantly low, compared with the scores in H group (p<0.05). This shows that low- dose of DHZCP in L group could much more significantly improve the liver histopathologcial changes than high-dose DHZCP in H group, which, on the other hand, illustrated that DHZCP at high level would aggravate the liver histopathologcial damage by showing toxicity to the injured liver. The safety would be considered by successive administration of DHZCP at the dose of 44 g·kg−1·d−1 (H group) on the chronic liver injury rats for 2 months. The therapeutic action of DHZCP on CCl4-induced liver injury will be weakened or will be transferred to toxicity with the increase in administration dose and time of this formula. For the investigation of the “dose-time-response” relationships of DHZCP on hepatic injury rats, an important statistical method, CA, was introduced in the next part.
Figure 2. Light microscopic analysis (HE stain, × 200) of rat liver sections of normal rats and CCl4 treatment with or without DHZCP administration. (A) normal group; (B) CCl4-induced (M) group at the end of week 8; (C) high- dose of DHZCP at the end of week 8; (D) low- dose of DHZCP at the end of week 8; (E) CCl4-induced (M) group at the end of week 12; (F) high- dose of DHZCP at the end of week 12; (G) low-dose of DHZCP at the end of week 8.
CA for the “dose-time-response” relationship of DHZCP
The “dose-time-response” relationship of DHZCP on the hepatic injury rats were investigated by CA on the biochemical markers (serum ALT, AST, ALP and TBIL levels) and liver histopathological scores (HS) in each group and shown in . Each point, which was obtained by CA on the four markers and HS, was depicted in the rectangular coordinate system with Z1 as horizontal axis and Z2 as longitudinal axis to represent each investigated group. The point of each group was clearly distributed in this rectangular coordinate system based on the point of N8 (the normal group at the end of week 8) as zero. N8, M8, H8 and L8 represented the normal group, model group, high- dose of DHZCP group and low- dose of DHZCP group at the end of week 8 and N12, M12, H12 and L12 represented the normal group, model group, high- dose of DHZCP group and low- dose of DHZCP group at the end of week 12. The four groups at the end of week 8 were shown with solid rhombus, and the other four groups at the end of week 12 with solid triangle. The euclidean distance between each group was calculated and shown in .
Table 2. Euclidean distance between each group.
Figure 3. CA plot of principal components 1 and 2 (Z1, Z2) for the distribution of the investigated group. This plot was obtained by correspondence analysis (CA) using SAS 8.0 software on the biochemical markers and liver histopathological scores in each group. It could reflect the “dose-time-efficacy” relationships of DHZCP on hepatic injury rats. N8, M8, H8 and L8, which represented the normal group, model group, high- dose of DHZCP group and low- dose of DHZCP group at the end of week 8, were shown with solid triangles. N12, M12, H12 and L12, which represented the normal group, model group, high-dose of DHZCP group and low- dose of DHZCP group at the end of week 12, were shown with solid rhombuses.
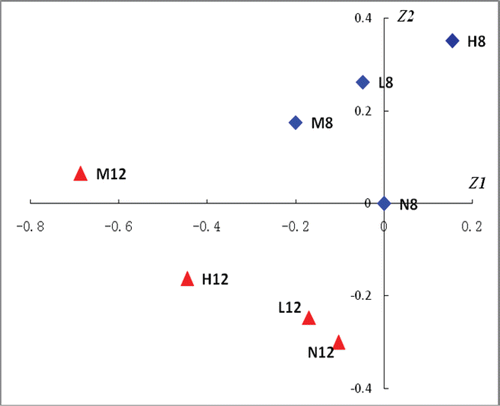
From and , the “dose-time-response” relationships of DHZCP on hepatic injury rats could be found: (1) the distance of M12 and N12 (0.6879) was larger than that of M8 and N8 (0.2656), showing that CCl4 could successfully induce the liver injury of rats and this injury would be aggravated by increasing the time of its use on rats; (2) at the end of week 8 (DHZCP administration for 1 month), the points L8 and M8 were distributed in the second quadrant and the distance between them was 0.1750, while H8 was alone distributed in the first quadrant and the distance of H8 and M8 was 0.3916, illustrating that high- dose (44 g·kg−1·d−1, which was 220-fold to the maximum dose 12 g·60 kg−1·d−1 in China Pharmacopoeia) of DHZCP had good therapeutic action on CCl4-induced liver injury of rats and this action was stronger than that of low- dose (22 g·kg−1·d−1) of DHZCP; (3) at the end of week 12 (DHZCP administration for 2 month), the points N12, H12 and L12 were distributed in the third quadrant and M12 was in the second quadrant. The distance of L12 and M12 (0.6027) was larger than that of H12 and M12 (0.3313), and the distance of L12 and N12 (0.0858) was the smallest. These illustrated that low and high- dose of DHZCP could significantly improve the CCl4-induced liver injury of rats at the end of week 12, and this improvement of low-dose (22 g·kg−1·d−1, which was 110-fold to the maximum dose 12 g·60 kg−1·d−1 in China Pharmacopoeia) DHZCP on this injury was better than that of high- dose of DHZCP; (4) the distance of H8 and H12 (0.7898) was the biggest among the distance of other groups, showing that the therapeutic action of high-dose DHZCP on CCl4-induced liver injury of rats will be weakened, even be transferred to toxicity with the increase of the administration dose and time of this formula. These results were consistent with the histopathological observations and the serum levels.
Discussion
Although TCDs have been attracting more attention in recent years because of its complementary therapeutic effects to western medicines, and its capability to deal with many essential problems that have not yet been solved by conventional medicinal practices, their adverse effects or toxicity of them should not be ignored (CitationTai et al., 1993; CitationDe Smet, 2004; CitationJordan et al., 2010). These adverse effects or toxicities may result from many factors including inherent toxicity, contamination with toxic materials, improper administration and large-dose or longtime use, etc. Discussion on the safety assessment of TCDs has got increased (CitationJordan et al., 2010). As a classical traditional Chinese formula, DHZCP has got quick development for treating liver diseases. However, there are few recent reports to show on the toxic and adverse effects of this formula.
In the present study, the hepatoprotective effect and toxicity of DHZCP on CCl4-induced liver injury rats were investigated based on examining the general status of the rats, histopathology of liver, biochemical parameters levels, and further, the “dose-time-response” relationship of it on liver injury was discussed combined with correspondence analysis. The results showed that DHZCP could improve the liver lesion of rats induced by CCl4. Administration of proper dose and time of DHZCP could well play its hepatoprotective effect and even treat hepatitis, but the safety on liver should be considered when a large- dose (44 g·kg−1·d−1) of DHZCP was used for a long time (more than 2 months). So, the safety should be considered in the long-term and large-dose use of DHZCP. Further research elucidating the action mechanism of these effects (protective effect and toxicity) will give an insight into the usefulness of this prescription on the chronic CCl4-induced liver injury. We suggest that the administration dose and time of DHZCP in clinical use should not be increased and prolonged, and simultaneously the liver function should be regularly monitored.
The study of “dose-time-response” relationship provided some useful references for the rational use of DHZCP and other medicinal TCDs and their formula clinically.
Acknowledgement
We are grateful to the support of National Basic Research Program of China (973 project) (No. 2007CB512607), National Nature Science Foundation (No. 30973947) and the National Key Technologies R&D Program of China (No. 2009ZX10005-017).
Declaration of interest
The authors have declared no conflict of interest.
References
- Lu BP, Peng B. (2005). Analysis and think on the liver injury of traditional Chinese drugs. J Chin Med Sci, 46, 630–632.
- Cao JL, Sun YQ, Fu Q, Li ZL, Xiao XH. (2006). Clinical study and prospect of Da-Huang-Zhe-Chong Pill. China Pharmacy, 17, 464–465.
- Chen KW, Tan Y. (2006). Clinical application development of Da-Huang-Zhe-Chong Pill. China Pharm, 15, 63–64.
- China Pharmacopoeia Committee. (2005). Pharmacopoeia of the People’s Republic of China, 1st Div. 2005 ed. Chinese Chemical Industry Press, Beijing.
- Dai YG, Xu JZ. (2004). Untoward reaction of traditional Chinese drugs on liver. Chin J Curr Practic Med, 3, 56–57.
- De Smet PA. (2004). Health risks of herbal remedies: an update. Clin Pharmacol Ther, 76, 1–17.
- Jiao SL, Shen DL, Zhang CD. (1999). Influence of Da-Huang-Zhe-Chong pill on the serum hyaluronidase, portal hypertension and hypersplenism of hepatic cirrhosis patients. Acta Shandong Univ Trat Chin Med, 3, 115–117.
- Jordan SA, Cunningham DG, Marles RJ. (2010). Assessment of herbal medicinal products: challenges, and opportunities to increase the knowledge base for safety assessment. Toxicol Appl Pharmacol, 243, 198–216.
- Kong WJ, Wang JB, Jin C, Zhao YL, Dai CM, Xiao XH, Li ZL. (2009). Effect of emodin on Candida albicans growth investigated by microcalorimetry combined with chemometric analysis. Appl Microbiol Biotechnol, 83, 1183–1190.
- Liu DH, Yong CY, Wu QH. (2001). Effect of Yi-Gan-Kan-Fu-Ling capsule on the chronic liver injury of rats. Chin J New Drugs Clin Rem, 12, 218–220.
- Pan ZH, Xie Y, He HW. (2004). Influence of Da-Huang-Zhe-Chong pill on hepatic stellate cell activation and proliferationrat of rats. Chin J Integra Trad Western Med, 6, 117–119.
- Pan ZH, Xie Y, He HW. (2005). Influence of Da-Huang-Zhe-Chong pill on hepatic stellate cell matrix metalloproteinase gene expression of rats. Chin J Integra Trad Western Med, 25, 1100–1103.
- Park M, Lee JW, Kim C. (2007). Correspondence analysis approach for finding allele associations in population genetic study. Comp Stat Data Anal, 51, 3145–3155.
- Pusha S, Gudi R, Noronha S. (2009). Polar classification with correspondence analysis for fault isolation. J Proc Control, 19, 656–663.
- Sun KW, Sun X, Liu WS. (1998). Study of anti-immunological liver fibrosis of Da-Huang-Zhe-Chong pill. China J Chin Mater Med, 23, 497–500.
- Suresh V, Asha VV. (2008). Preventive effect of ethanol extract of Phyllanthus rheedii Wight. on d-galactosamine induced hepatic damage in Wistar rats. j Ethnopharmacol, 116, 447–453.
- Tai YT, But PP, Young K, Lau CP. (1993). Adverse effects from traditional Chinese medicine. Lancet, 341, 892.
- Wang H, Feng F, Zhuang BY, Sun Y. (2009a). Evaluation of hepatoprotective effect of Zhi-Zi-Da-Huang decoction and its two fractions against acute alcohol-induced liver injury in rats. j Ethnopharmacol, 126, 273–279.
- Wang JB, Ma YG, Zhang P. (2009b). Effect of processing to the chemical contents and the hepatic and renal toxicity of rhubarb studied by canonical correlation analysis. Acta Pharm Sin, 44, 885–890.
- Wang J, Zhao Y, Xiao X, Li H, Zhao H, Zhang P, Jin C. (2009). Assessment of the renal protection and hepatotoxicity of rhubarb extract in rats. j Ethnopharmacol, 124, 18–25.