Abstract
Context: The fruits of Feijoa sellowiana Berg. (Myrtaceae) have been used to treat goiter in traditional Turkish medicine.
Objective: To evaluate the in vivo antioxidant activities of different polarities of the fruit extracts in blood and tissue (liver, kidney, brain, and heart) antioxidant defense systems in standard pellet diet and in high fat diet consumed, male rats were assessed.
Materials and methods: The extracts (methanol, n-hexane, chloroform, ethyl acetate, n-butanol, and aqueous) were administered orally to male rats at 50 mg/kg doses daily for 4 weeks. The blood and tissue malondialdehyde (MDA), reduced glutathione (GSH) levels, plasma nitrate (NOx) level, total triiodothyronine (T3), thyroxine, cholesterol, triglyceride, protein, and glucose levels were determined, and erythrocyte superoxide dismutase (SOD) and catalase (CAT) activities; plasma antioxidant activity (AOA) were experimentally studied.
Results: Blood MDA level (7.81 ± 0.4) was significantly decreased; GSH level (29.65 ± 1.21) and AOA (1.52 ± 0.08) were increased in ethyl acetate extract as compared with control and the other extracts. In addition, all the extracts decreased MDA levels and increased GSH levels (except brain tissue homogenate) in the tissue homogenates. Erythrocyte SOD and CAT activity levels were unchanged in F. sellowiana extracts. However, the extracts had no effect on plasma NOx. In the histopathological examinations, any changes or damage in the vital organs were seen in animals.
Conclusion: The experimental data demonstrated that F. sellowiana extracts displayed remarkable antioxidant activity and decreased lipid peroxidation in rats; furthermore, no histopathological changes or damage have been observed in the vital organs of rats.
Introduction
The Myrtaceae is a family of approximately 130 genera and more than 3800 species, including Feijoa Berg. Named after Brazilian botanist de Silva Feijo, this genus of two species is grown for their showy, edible flowers, and guava-like fruits. Feijoa sellowiana Berg. [syn. Acca sellowiana (Berg.) Burret] is a bushy shrub native to South America, but it is now grown in many subtropical areas for its edible fruits or as an ornamental plant. Fruits appear only after a hot summer and may be damaged by autumn frosts. The fresh fruit is appreciated for its characteristic flavor and aroma that is similar to pineapple. Therefore, it is also called “pineapple guava.” The oval and glossy green leaves have a silvery underside. The flowers, carried on the new season’s growth, have red petals that are white underneath and almost overshadowed by prominent, dark red stamens (CitationWarren, 2004). Owing to its easy adaptability to subtropical regions, nowadays it is extensively cultivated in California and mainly in New Zealand where the fruits are popular. In the Mediterranean basin, this species was introduced at the end of 19th century, initially as an ornamental plant (CitationRuberto & Tringali, 2004).
F. sellowiana is naturalized in Turkey and known as “Kaymak agaci”; it has been extensively used in South Anatolia as a remedy for the treatment of goiter (CitationSamancı, 2010) and was also shown to possess potent antioxidant, antitumor, and antimicrobial activities (CitationVuotto et al., 2000; CitationBontempo et al., 2007).
The fruits and the leaves of F. sellowiana contains many basic components, especially volatile components, which are responsible for the strong feijoa-like character of the fruit (CitationRuberto & Tringali, 2004), flavonoids (CitationIelpo et al., 2000), tannins (CitationOkuda et al., 1980), terpenes, and steroidal saponins (CitationRuberto & Tringali, 2004). Isoflavonoids detected were namely daidzin, genistin, daidzein, genistein, formononetin, biochanin A, prunetin, and several incompletely characterized isoflavones (CitationLapcik et al., 2005). In addition to well-known components, α-tocopherol, stigmasterol, and β-carotene, some unreported long-chain esters of tyrosol and a novel galactolipid have also been isolated and characterized (CitationRuberto & Tringali, 2004). Like all exotic fruits, F. sellowiana contains large amounts of ascorbic acid, hydrocarbons, and minerals (CitationVuotto et al., 2000). High concentrations of iodine, 3 mg I/100 g, have also been found in the fresh fruits of F. sellowiana by using spectrophotometric method (CitationFerrara & Montesano, 2001).
Most animals and humans have an ability to conserve the iodine within their bodies if there is a deficiency of iodine consumed in food. If an inadequate intake continues, however, the ability to make thyroid hormone is slowly depleted. Many cellular processes occur to keep the thyroid as efficient as possible and the thyroid gland often enlarges in an attempt to maintain function. Subsequently, a goiter may form as the thyroid is stimulated to try to make more thyroid hormone. Basically, the changes in hormone levels (namely T4, T3, and TSH) are similar to those that occur in patients who develop low thyroid hormone blood levels (hypothyroidism) from an underlying disease, such as Hashimoto’s disease (CitationKayaalp, 1989).
Phenolic metabolites, such as flavonoids and tannins, speed up termination by catching free radicals and protect the cell membrane in the organism. The best-known components of the endogenous antioxidant system are superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase, reduced glutathione (GSH), and glutathione transferase. GSH, an intracellular reducing tripeptide, exhibits its potent antioxidant effect by preventing the antioxidant damage of free radicals (CitationDundar & Aslan, 2000; CitationAvci et al., 2006).
Depending on the traditional use of F. sellowiana in Turkey and in several other countries as well as its contents, the aim of this study is to investigate the possible subacute effects of the extracts prepared with methanol, n-hexane, chloroform, ethyl acetate, n-butanol, and water from F. sellowiana fruits on the antioxidant defense system, lipid peroxidation (LPO), thyroid functions, tissue morphology, and some biochemical parameters in male rats.
Materials and methods
Plant material
F. sellowiana fruits were collected from plants cultivated in the Mediterranean region (Mersin in South Anatolia) in 2008. Voucher specimen was authenticated by Assoc. Prof. I. Irem Tatli (Hacettepe University, Faculty of Pharmacy, Department of Pharmaceutical Botany, Sihhiye, Ankara, Turkey). A specimen of the original collection has been deposited in the Herbarium of Faculty of Pharmacy, Gazi University, Ankara, Turkey (GUE 2753).
Preparations of the extracts
The fruits (53 g) of F. sellowiana were extracted with 200mL of methanol for 5 h at 40°C under reflux and concentrated to dryness under reduced pressure. An aliquot of each concentrated methanol extract (10.46 g, w/w, 19.7%) was then suspended in water and the water-soluble portion was partitioned with n-hexane, chloroform, ethyl acetate, and n-butanol, individually. n-Hexane, chloroform, ethyl acetate, and n-butanol extracts as well as the remaining aqueous phase were then concentrated to dryness under reduced pressure and lyophilized in vacuo (0.35 g, w/w, 3.3%, n-hexane extract; 1.15 g, w/w, 10.9%, chloroform extract; 1.32 g, w/w, 12.6%, ethyl acetate extract; 2.36 g, w/w, 22.6%, n-butanol extract; and 4.08 g, w/w, 39.0%, aqueous extract). The main extract (methanol) and the extracts subjected to partition (n-hexane, chloroform, ethyl acetate, n-butanol, and the remaining aqueous) were used for activity assessments.
Animals, diets, and experimental protocols
Forty-eight Sprague-Dawley male rats (200–350 g) obtained from the animal breeding laboratories of the Experimental Animal Research and Application Center (Afyonkarahisar, Turkey) were used. Rats were divided into eight groups consisting of six animals each and acclimatized to animal room conditions for 2 days before the experiments under a 12-h light/12-h dark cycle at room temperature (25°C ± 3°C). Rats were fed with a standard rodent diet (Afyon Feed Industry, Afyonkarahisar, Turkey) with food and water ad libitum.
Methanol, n-hexane, chloroform, ethyl acetate, n-butanol, and aqueous extracts were given orally to test animals (50 mg/kg) by using a gastric gavage after suspending in a mixture of distilled H2O and 0.5% sodium carboxymethyl cellulose (CMC). There were two control groups in this study. The first control group animals (control) were maintained on rodent standard pellet diet and water ad libitum without any plant extract, while a second group (CMC control group) was given only CMC without any plant extract under the same experimental conditions.
At the end of 4 weeks, blood samples were collected from all the rats after the food was withdrawn the night before and the rats were sacrificed under a combination of ketamine and xylasine HCl anesthesia. Blood samples were obtained from each rat in the morning by using evacuated tubes containing sodium citrate as an anticoagulant. The blood samples were centrifuged at 1500g for 10 min at 4°C, and then the plasma were removed. Red blood cells were washed three times with 0.9% NaCl solution (phosphate buffer, pH: 7.2), and packed cells were hemolyzed by adding an equal volume of cold distilled water.
After blood sampling, the rats were subjected to complete necropsy. Brain, liver, lung, heart, spleen, kidney, adrenal glands, testes-epididymides, thymus, thyroid, and pancreas tissues were removed immediately and a piece of tissue was fixed in 10% buffered formalin for histopathologic examination and an enough portion of liver, kidney, heart, and brain tissue were washed in ice-cold saline and then homogenized 1:40 w/v in 0.1-M phosphate buffer, pH 7.4, containing 1-mM EDTA. After centrifugation at 3000g for 15 min at 4°C, the supernatant was extracted and was kept at −30°C in advance of assays.
The experimental protocols were approved by the Animal Care and Use Committee of Afyon Kocatepe University and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (AKUHADYEK-80-09).
Biochemical analysis
Blood samples were separated into plasma and erythrocytes. Blood malondialdehyde (MDA) level was estimated according to the method of CitationDraper and Hadley (1990), which is based on coupling MDA with thiobarbituric acid. Blood reduced glutathione (GSH) concentrations were assayed by colorimetric method of CitationBeutler et al. (1963). Erythrocytes were prepared according to CitationWitterbourn et al. (1975), and erythrocyte hemoglobin levels were determined as described by CitationFairbanks and Klee (1987). Cu-Zn SOD activity in erythrocytes was measured by the previously reported method of CitationSun et al. (1988). CAT in erythrocytes was measured spectrophotometrically as described by CitationLuck (1955). Plasma total antioxidant activity (AOA) and nitric oxide (NOx) were measured according to a method proposed by CitationKoracevic et al. (2001) and CitationMiranda et al. (2001). The levels of MDA (CitationOhkawa et al., 1979), GSH (CitationBeutler et al., 1963), SOD (CitationSun et al., 1988), and CAT (CitationAebi, 1974) were also measured in liver, kidney, brain, and heart tissue homogenates. Tissue protein content was assayed by the colorimetric method of CitationLowry et al. (1951). Plasma T3 (standard curve: 0−7.5 μg/L, code no: DSL-10–3100S) and T4 (standard curve: 50–500 μg/L, code no: DSL-10–3200) concentrations were determined by specific enzyme-linked immunosorbent assay tests (DSL diagnostic systems laboratories, Inc. Texas, USA). Plasma total cholesterol, triglyceride, protein, and glucose levels were measured using commercially available kits (TECO Diagnostics, California, USA). A Shimadzu UV-1601 visible spectrophotometer (Kyoto, Japan) was used for blood and tissue biochemical analysis.
Tissue preparation and histopathologic examination
Formalin-fixed tissues were trimmed, processed, embedded in paraffin, sectioned by 5-µm thickness, placed on glass microscope slides and then stained with hematoxylin and eosin (H&E). Hikmet Keles and H. Huseyin Demirel (histopathologists) blinded to the groups evaluated the HxE and then proliferating cell nuclear antigen (PCNA) stained sections under a light microscope by the use of an Image Processing and Analysis System (Olympus BX51 attached DP20 Digital Camera and Image Analyze System).
PCNA immunohistochemistry
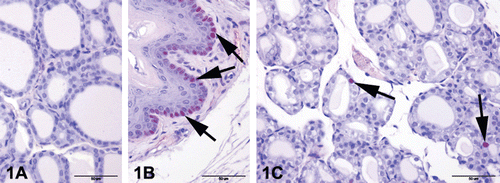
Thyroid tissues were selected for proliferation screening because of iodine content of F. sellowiana. PCNA immunostaining were performed for possible proliferative activity of thyroid gland. Firstly, 5-µm paraffin sections were mounted on silanized slides. PCNA immunostaining was carried out using the avidin−biotin−complex peroxidase method. After deparaffinization and rehydration, endogenous peroxides and nonspecific immunoglobulins were blocked with the super block system (ScyTEK/Logan, Utah, USA) for 10 min. Monoclonal mouse anti-rat PCNA (Clone PC10; Dako Carpinteria, CA, USA) was applied for 1 h; then UltraTek Anti-Polyvalent Biotinylated Antibody (ScyTEK/Logan, Utah, USA) was applied for 10 min and UltraTek HRP for 10 min. Color was developed by incubation with 3-amino-9-ethylcarbazole (AEC; Dako, Carpinteria, CA, USA) for 5 min. Finally, the sections were counterstained with Mayer’s hematoxylin for 3 min, and cover slips were applied with the aqueous mounting medium. Negative controls, in which the primary antibody was replaced with phosphate-buffered saline, did not show nonspecific staining (). As a positive control, esophagus tissues that are taken with thyroids were screened. In all sections except negative control slides, esophageal basal cells showed PCNA immunopositivity (); these positivities were confirmed by PCNA immunostaining.
Figure 1. Proliferating Cell Nuclear Antigen (PCNA) immunostaining of control and F. sellowiana extracts treated rats. Representative figures were stained with avidin−biotin−complex peroxidase method with Mayer’s hematoxylin counterstain. The original magnification was 400x and the scale bars represent 50 µm. (A) Negative control, primary antibody was replaced with phosphate-buffered saline, did not show any staining. (B) Positive control, esophageal basal cells showed PCNA immunopositivity (arrows). (C) Only a few PCNA immunopositivity were seen in the nucleus of tubular epithelium in all groups (arrows).
Statistical analysis of data
Data were expressed as mean standard error (± S.E.M.). Statistical differences between control diet and plant extracts given orally were evaluated by one-way ANOVA followed by Duncan post hoc tests. A difference in the mean values of p < 0.05 was considered to be significant.
Results and discussion
In this study, the effects of F. sellowiana extracts on the antioxidant defense system, LPO, and histopathological changes caused in brain, liver, lung, heart, spleen, kidney, adrenal glands, testes-epididymides, thymus, thyroid, and pancreas of male rats were investigated to verify the claimed traditional usage and to evaluate the potential risks and benefits of the treatment with F. sellowiana extracts in a scientific base.
The effects of F. sellowiana extracts on blood MDA and GSH concentrations, erythrocyte SOD and CAT activities, plasma NOx levels, and AOA are shown in ; the effects of the extracts on plasma T3, T4, cholesterol, triglyceride, protein, and glucose levels are given in . In addition, MDA and GSH concentrations, and SOD and CAT activities were also determined in liver (), kidney (), heart (), and brain () tissue homogenates.
Table 1. The effects of Feijoa sellowiana extracts on blood MDA and GSH concentrations, erythrocyte SOD and CAT activities, and plasma AOA and NOx levels in rats (mean ± S.E.M.).
Table 2. The effects of Feijoa sellowiana extracts on plasma T3, T4, cholesterol, triglyceride, protein, and glucose levels in rats (mean ± S.E.M.).
Table 3. The effects of Feijoa sellowiana extracts on MDA and GSH concentrations, SOD and CAT activities in liver tissue homogenate of rats (mean ± S.E.M.).
Table 4. The effects of Feijoa sellowiana extracts on MDA and GSH concentrations, SOD and CAT activities in kidney tissue homogenate of rats (mean ± S.E.M.).
Table 5. The effects of Feijoa sellowiana extracts on MDA and GSH concentrations, SOD and CAT activities in heart tissue homogenate of rats (mean ± S.E.M.).
Table 6. The effects of Feijoa sellowiana extracts on MDA and GSH concentrations, SOD and CAT activities in brain tissue homogenate of rats (mean ± S.E.M.).
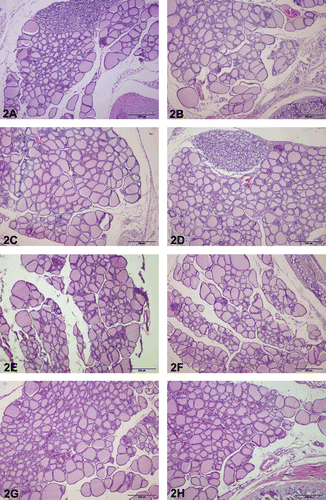
Moreover, histopathological screening of parenchymatous organs was carried out to detect possible harmful and/or toxic effects of different F. sellowiana extracts in rats. For these purposes, the possibility of hyperemia, edema, hemorrhage, degeneration, inflammation, necrosis and/or necrobiosis, hemosiderosis and/or other pigmentations and extramedullary hematopoiesis were investigated in liver, kidney, spleen, lung, heart, testes-epididymides, and brain sections; also lymphoreticular relation in spleen, atelectasia and emphysema in lung, dysfunction of spermatogenesis in testes and epididymides, intracellular depositions in brain (especially intraneuronal), liver, and kidney were screened with light microscope. In all experimental groups, light individual pathological conditions were observed generally in one organ or in maximum two systematically associated organs (such as lung and heart). But these findings do not include associated groups and are not considered as significant. In thymus immunohistochemistry, only a few PCNA immunopositivity, within the normal limits, were seen in the nucleus of tubular epithelium in all groups (). Any immunopositivity were detected in the parafollicular cells (C cells). In H&E-stained thyroid sections, any abnormalities were seen in the follicular, luminal, and parenchymal areas ().
Figure 2. Thyroid histology of control and F. sellowiana extracts treated rats. The glands showed no microscopic abnormalities. Normal thyroid architectures are seen as small follicles at the central portion of gland and large follicles at the periphery of the gland in all groups. Representative figures were stained with H&E. The original magnification was 100x, and the scale bars represent 200 µm. (A) Control group (any application). (B) Carboxymethyl cellulose group (0.5% sodium carboxymethyl cellulose). (C) Methanol extract group. (D) Hexane extract group. (E) Chloroform extract. (F) Ethyl acetate extract. (G) n-Butanol extract. (H) Aqueous extract.
Reactive oxygen species interact with other molecules within cells and cause oxidative damage to proteins, membranes, and genes. The reactive free radicals can oxidize biomolecules and may cause extensive LPO in biological membranes, which causes cell death and tissue injury. LPO is a complicated radical chain reaction leading to the formation of various products including lipid hydroperoxides, conjugated dienes, and MDA (CitationKucukkurt et al., 2008). Since membrane phospholipids are major targets of oxidative damage, LPO is often the first parameter analyzed for proving the involvement of free radical damage. Thus, the presence of MDA is considered as an indicator of free-radical damage through membrane LPO (CitationKüpeli et al., 2009). In this study, blood MDA level was significantly decreased in ethyl acetate extracts as compared with control and the other extract groups. In addition, all of the extracts also decreased MDA levels in tissue homogenates. This may suggest that F. sellowiana extracts include active components against LPO. The existence of antioxidants protects the biomolecules in the cell against the damage caused by free radicals. Such antioxidants involve enzymatic systems like SOD, glutathione peroxidase, and CAT, as well as nonenzymatic systems like glutathione and vitamins.
GSH is a main component of the cellular antioxidant defense, and it acts as an essential cofactor of antioxidant enzymes including GSH peroxidases (CitationHayes et al., 2005). Under oxidative stress, glutathione is consumed by glutathione-related enzymes to detoxify peroxides produced due to increased LPO (CitationCathcart, 1985). In this study, the ethyl acetate extract of F. sellowiana significantly increased GSH level in blood as compared with control and the other extract groups. Similarly, GSH levels were also increased in the extract groups in tissue homogenates except brain tissue homogenate. This increase in GSH levels suggests that the decreased LPO may be a consequence of enhanced GSH stores in tissues.
The antioxidant enzymes SOD and CAT limit the effects of antioxidant molecules on tissues and act as reactives in defense against oxidative cell injury by means of their being free radical scavengers (CitationKyle et al., 1987). SOD catalyzes the dismutation of two superoxide anions (O2−) into hydrogen peroxide and molecular oxygen. SOD protects the tissues from the harmful effects of superoxide radicals to a certain degree. CAT enzyme hydrolyzes H2O2 into H2O and 1/2 O2. These enzymes work together to eliminate active oxygen species. Small deviations in the physiological concentrations of these enzymes may have a dramatic effect on the resistance of cellular lipids, proteins, and DNA to oxidative damage (Mates & Sanchez-Jimenez, 1999). SOD and CAT activities may change according to the damage occurred in oxidant/antioxidant system (CitationSun et al., 1988). In this study, the levels of erythrocyte SOD and CAT activities did not change in F. sellowiana extract groups as compared with control groups. This might be related to the extracts including antioxidant substances such as vitamin C, flavonoids, and tannins and thus oxidant/antioxidant equilibrium maintained by the administration of the extracts.
Antioxidant capacity is an important parameter in the physiology of humans and all animals (CitationAvci et al., 2006). AOA level was increased in rats that were given F. sellowiana extracts. Also, the ethyl acetate extract significantly increased AOA levels compared with other extracts. High production of NO has been suggested as a cause of tissue injury (CitationBohloli et al., 2007). In this study, F. sellowiana extracts had no effect on plasma NOx suggesting that the extract treatment does not affect NO production.
The thyroid synthesizes two hormones, T3 and T4, primarily responsible for regulation of metabolism; an important component in the synthesis of thyroid hormones is iodine. Both T3 and T4 function to increase the basal metabolic rate of several cells and tissues, affect protein synthesis, and help regulate long bone growth. These hormones also regulate carbohydrate metabolism, affecting how human cells use energetic compounds. T3 and T4 indirectly increase blood glucose levels. Depressed T3 and T4 production is the trademark of hypothyroidism (CitationKayaalp, 1989). In this study, there is no proliferation, inflammation, or other pathologic findings in thyroid tissue and any biochemical abnormalities in total T3 and T4 levels with F. Sellowiana extracts.
Conclusion
In our studies, F. sellowiana enhanced the antioxidant activity while decreasing the LPO in rats. Especially, the ethyl acetate extract of F. sellowiana fruits, compared with other extracts, were found to have more beneficial effects on rat antioxidant defense system. In addition, there is no proliferation, inflammation, or other pathologic findings in thyroid tissue and any biochemical abnormalities total T3 and T4 levels. The antioxidant defense system was also strengthened at the same time. Therefore, iodine which will be provided by this plant will not lead to a harmful effect on the thyroid. F. sellowiana extracts did not show any histopathological changes or damage in the vital organs of rats. Further studies on this species may yield successful results and isolation of active constituents.
Acknowledgment
We are thankful to Dr. Hakan Eroglu (Hacettepe University, Faculty of Pharmacy, Department of Pharmaceutical Technology) for providing fruits from Mersin in 2008.
Declaration of interest
The authors report no declarations of interest.
References
- Aebi H. (1974). Catalase in vitro. In: Bergmeyer U, (ed.) Methods of Enzymatic Analysis. New York and London: Academic Press, 673–667.
- Avci G, Kupeli E, Eryavuz A, Yesilada E, Kucukkurt I. (2006). Antihypercholesterolaemic and antioxidant activity assessment of some plants used as remedy in Turkish folk medicine. J Ethnopharmacol, 107, 418–423.
- Beutler E, Duron O, Kelly BM. (1963). Improved method for the determination of blood glutathione. J Lab Clin Med, 61, 882–888.
- Bohloli M, Uzun H, Aytac E, Toklu AS, Paksoy M, Durak H, Ipek T. (2007). Hyperbaric Oxygen (HBO) Therapy after partial hepatectomy: An experimental study on oxidative stress in rats. Scand J Lab Anim Sci, 34, 131–40.
- Bontempo P, Mita L, Miceli M, Doto A, Nebbioso A, De Bellis F, Conte M, Minichiello A, Manzo F, Carafa V, Basile A, Rigano D, Sorbo S, Castaldo Cobianchi R, Schiavone EM, Ferrara F, De Simone M, Vietri M, Cioffi M, Sica V, Bresciani F, de Lera AR, Altucci L, Molinari AM. (2007). Feijoa sellowiana derived natural flavone exerts anti-cancer action displaying HDAC inhibitory activities. Int J Biochem Cell Biol, 39, 1902–1914.
- Cathcart RF 3rd. (1985). Vitamin C: The nontoxic, nonrate-limited, antioxidant free radical scavenger. Med Hypotheses, 18, 61–77.
- Draper HH, Hadley M. (1990). Malondialdehyde determination as index of lipid peroxidation. Meth Enzymol, 186, 421–431.
- Dundar Y, Aslan R. (2000). Oxidative Stress and Antioxidants in Medicine. Ankara, Turkey: Afyon Kocatepe University Publication, 29, Uyum Agency.
- Fairbanks VF, Klee GG. (1987). Biochemical aspect of haematology. In: Tiez NW, (ed.) Fundamentals of Clinical Chemistry. Philadelphia: W.B. Saunders, 803–804.
- Ferrara L, Montesano D. (2001). Nutritional characteristics of Feijoa sellowiana fruit: The iodine content. La Rivista di scienza dell’alimentazione. ISSN 1128–7969. 30, 353–356.
- Hayes JD, Flanagan JU, Jowsey IR. (2005). Glutathione transferases. Annu Rev Pharmacol Toxicol, 45, 51–88.
- Ielpo MT, Basile A, Miranda R, Moscatiello V, Nappo C, Sorbo S, Laghi E, Ricciardi MM, Ricciardi L, Vuotto ML. (2000). Immunopharmacological properties of flavonoids. Fitoterapia, 71 Suppl 1, S101–S109.
- Kayaalp O. (1989). Medical Pharmacology. Ankara, Turkey: Feryal Press.
- Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V. (2001). Method for the measurement of antioxidant activity in human fluids. J Clin Pathol, 54, 356–361.
- Kucukkurt I, Ince S, Fidan AF, Ozdemir A. (2008). The effects of dietary supplementation of different amount of Yucca schidigera powder (Sarsaponin 30®) on blood and tissue antioxidant defense systems and lipid peroxidation in rats. J Anim Vet Adv, 7, 1413–1417.
- Akkol EK, Avci G, Küçükkurt I, Keles H, Tamer U, Ince S, Yesilada E. (2009). Cholesterol-reducer, antioxidant and liver protective effects of Thymbra spicata L. var. spicata. J Ethnopharmacol, 126, 314–319.
- Kyle ME, Miccadei S, Nakae D, Farber JL. (1987). Superoxide dismutase and catalase protect cultured hepatocytes from the cytotoxicity of acetaminophen. Biochem Biophys Res Commun, 149, 889–896.
- Lapcik O, Klejdus B, Kokoska L, Davidova M, Afandi K, Kuban V, Hampl R. (2005). Identification of isoflavones in Acca sellowiana and two Psidium species (Myrtaceae). Biochem Syst Ecol, 33, 983–992.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. (1951). Protein measurement with the Folin phenol reagent. J Biol Chem, 193, 265–275.
- Luck H. (1955). Catalase. In: H.U., Bergmeyer, (ed.) Methods in Analysis. London: Academy Press, 885–894.
- Matés JM, Sánchez-Jiménez F. (1999). Antioxidant enzymes and their implications in pathophysiologic processes. Front Biosci, 4, D339–D345.
- Miranda KM, Espey MG, Wink DA. (2001). A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide, 5, 62–71.
- Ohkawa H, Ohishi N, Yagi K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem, 95, 351–358.
- Okuda T, Yoshida T, Hatano T, Yazaki K, Ashida M. (1980). Ellagitannins of the Casuarinaceae, Stachyuraceae and Myrtaceae. Phytochemistry, 21, 2871–2874.
- Ruberto G, Tringali C. (2004). Secondary metabolites from the leaves of Feijoa sellowiana Berg. Phytochemistry, 65, 2947–2951.
- Samancı H. (2010). Cultivation of Acca sellowiana. Ataturk Central Horticultural Research Institute Press, Yalova.
- Sun Y, Oberley LW, Li Y. (1988). A simple method for clinical assay of superoxide dismutase. Clin Chem, 34, 497–500.
- Vuotto ML, Basile A, Moscatiello V, De Sole P, Castaldo-Cobianchi R, Laghi E, Ielpo MT. (2000). Antimicrobial and antioxidant activities of Feijoa sellowiana fruit. Int J Antimicrob Agents, 13, 197–201.
- Warren W. (2004). Botanica, Könemann. Tandem Verlag GmbH, Italy.
- Witterbourn CC, Hawkins RE, Brain M, Carrel W. (1975). The estimation of red cell superoxide dismutase activity. J Lab Clin Med, 85, 337–341.