Abstract
Context: Platonia insignis Mart. (Clusiaceae), commonly known as “bacuri,” is a timber and fruit native species of the Brazilian Amazon. Some plants of the Clusiaceae family have their pharmacological properties associated with the presence of xanthone and polycyclic polyprenylated acylphloroglucinols derivatives, which have antioxidant and anticarcinogenic activities.
Objective: The aim of this study was to assess the in vivo potential of extracts, fractions, and garcinielliptone FC isolated from of Platonia insignis seeds as a natural antioxidant.
Materials and methods: Male Wistar rats (250–280 g; 2 months old) were treated with Tween 80 0.05% dissolved in 0.9% saline (i.p, vehicle – control group), ethanol extract (EE), hexane extract (HE), dichloromethane fraction (DMF), ethyl acetate fraction (EAF), and garcinielliptone FC (GFC) isolated from P. insignis at doses 2 mg/kg (i.p.). All groups were observed for 24 h after the treatment. The antioxidant enzymatic activities [superoxide dismutase (SOD) and catalase (CAT)] were measured using spectrophotometric methods.
Results: There were no marked alterations in SOD and CAT activities in rat hippocampus after pretreatment with EE, HE, DMF, EAF, and GFC. However, the pretreatment with GFC induced a significantly increase of 13, 17, 19, and 13% in SOD activities when compared to EE, HE, DMF, or EAF groups, respectively.
Discussion and conclusion: Our findings strongly support the hypothesis that GFC isolated from P. insignis has a significant potential to be used as a natural antioxidant agent probably due to the modulation of enzymatic activity of hippocampal SOD.
Keywords::
Introduction
Endogenous antioxidant mechanisms inhibit or delay specific substrate oxidation by reactive oxygen species (ROS) and are important in preventing many diseases (CitationHalliwell et al., 1992; CitationFerreira et al., 2008; CitationFreitas et al., 2010). Their effectiveness can be improved by exogenous antioxidants of natural and synthetic origin that are present in the diet. Phenolics, also known as high and low molecular weight polyphenols, represent the main class of natural antioxidants. These bioactive compounds participate in defense against oxidative damage associated with their scavenging of harmful reactive species (CitationMujic et al., 2011).
The Clusiaceae family includes 40 genexa, divided into 1200 species, distributed in tropical regions of the world. Several species are well known for their hulls manufacture due to the quality of their wood and for the healing properties of their latex used traditionally against dermatoses (CitationMarti et al., 2009). This family is known to be a rich source of polycyclic polyprenylated acylphloroglucinols (PPAPs) with a large spectrum of biological activities (CitationCuesta-Rubio et al., 2005; CitationMarti et al., 2009). Besides, some plants of Clusiaceae family have their pharmacological properties associated with the presence of xanthone derivatives, which has antioxidant and anticarcinogenic activities (CitationSundaram et al., 1983; CitationChen et al., 1996; CitationIinuma et al., 1996; CitationSuksamrarn et al., 2003; CitationEe et al., 2008; CitationChomnawang et al., 2009; CitationHung et al., 2009).
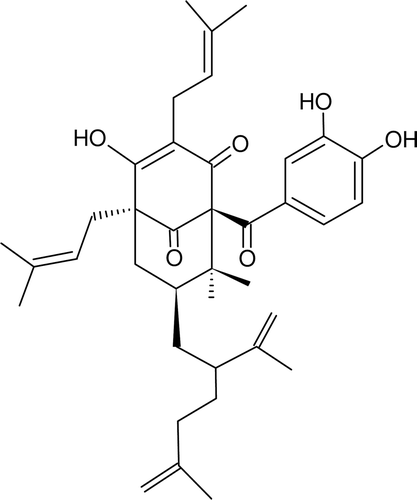
Among the Clusiaceae family, Platonia insignis Mart., commonly known as “bacuri,” is a timber and fruit native species of the Brazilian Amazon (CitationSundaram et al., 1983; CitationChen et al., 1996; CitationIinuma et al., 1996; CitationSuksamrarn et al., 2003; CitationEe et al., 2008; CitationChomnawang et al., 2009; CitationHung et al., 2009). The bacuri fruit has a thick-skin, and is 7–15 cm long and 5–15 cm in diameter, weighing 200–1000 g, containing a large quantity of resins. The pulp wrapping the seeds is white and bitter, with a pleasant smell and taste. The P. insignis fruit can be consumed raw or as juice, ice-cream, or jam (CitationAlves & Jennings, 1979). While, the P. insignis seed oil has been used to treat various skin diseases in both men and animals, the seed decoction is used in human diarrhea treatment (CitationAgra et al., 2007). In the search for biologically active constituents in Clusiaceae plants, we investigated bioactive constituents of the seeds of P. insignis and reported a novel tautomeric pair PPAPs, named garcinielliptone FC (), isolated from the seeds of P. insignis, were identified by extensive analysis of spectroscopic (CitationCosta Júnior et al., 2011). It was reported that phloroglucinols, especially PPAPs, are common constituents of members of the family Clusiaceae (CitationCiochina & Grossman, 2006).
Since previous investigations in our laboratory (CitationCosta Júnior et al., 2010) demonstrated an antioxidant and anticonvulsant effects of P. insignis, we investigated the effect of acute administration of P. insignis on oxidative free radical scavenging enzymes (superoxide dismutase, SOD; catalase, CAT) in order to assess the role of a possible antioxidant effect in the cognition-facilitating action of this medicinal plant.
The antioxidant effects ethanol extract (EE), hexane extract (HE), dichloromethane fraction (DMF), acetate ethyl fraction (EAF), and garcinielliptone FC (GFC) isolated from the seeds of P. insignis were assessed in in vivo tests.
Methods
The protocols for the animals experiments described in this study were performed in accordance with international (EEC Directive of 1986, 86/609/EEC) and national rules and institutional guidelines as prescribed by the ethical committee for animal experiments of the Federal University of Piauí (UFPI).
Plant material and chemistry study
The P. insignis fruits were collected at Barras, Piauí State, Brazil, in March 2009. A voucher specimen has been identified by botanist Roseli Farias de Melo Barros and deposited at the “Graziela Barroso,” Herbarium of Biology Department of Federal University of Piauí, Brazil (Voucher No.: ICN TEPB27164).
The seeds were dried at 55°C and powdered. Crushed seeds (848.2 g) were extracted with hexane (63%, w/w), followed by ethanol (EtOH) (5.8%, w/w) in a Soxhlet apparatus (8 h for each solvent). The ethanol extract (EtOH) was added to 100 mL of water and then partitioned with solvents of increasing polarity. The EtOH extract was fractionated with dichloromethane (8 × 100 mL) to obtain DMF (3.4%, w/w), acetate ethyl (7 × 100 mL) to obtain EAF (0.4%, w/w).
The new PPAP compounds, the known compound GFC, were isolated from the HE of P. insignis. GFC: yellow oil; EIMS m/z (%): 602 [M]+ (1), 465 (6), 341 (8), 231 (10),177 (3), 137 (20), 109 (11), 69 (100). Their structure and molecular formula (C38H50O6; m/z 603.3) was confirmed by ESI(+)/MSn and 1H NMR and 13C NMR data (CitationCosta Júnior et al., 2011). In the present work, the GFC was suspended in 0.05% Tween 80 distilled in 0.9% saline, and sonicated before use.
Animals and treatment protocols
Adult male Wistar rats (250–280 g; 2 months old) were maintained in a temperature controlled room (26 ± 1°C) with a 12 h light/dark cycle and food and water ad libitum (Nutrilabor, Campinas, Brazil). Animal care followed the official governmental guidelines in compliance with the Society Policy and was submitted by the Ethics Committee of the Federal University of Piauí, Brazil. All chemicals were obtained from Sigma Chemical Co. (St. Louis, MO, USA). All doses are expressed in milligrams per kilogram and were administered in a volume of 10 mL/kg injected intraperitoneally (i.p.).
A total of 57 rats were treated with either 2 mg/kg of EE, HE, DMF, EAF, or GFC from P. insignis (i.p.) or vehicle (saline/Tween 80 0.05% dissolved in 0.9% saline, i.p.): group 1, vehicle treatment serves as control (n = 12); group 2, DMF 2 mg/kg (n = 9); group 3, EAF 2 mg/kg (n = 9); group 4, EE 2 mg/kg (n = 9); group 5, HE 2 mg/kg (n = 9); and group 6, GFC 2 mg/kg (n = 9). After the treatment, the animals were recorded in 30 cm × 30 cm chambers to behavioral studies for 24 h. At the end of observations, the survivors were sacrificed by decapitation and their brains were dissected on ice to remove hippocampus for SOD and CAT determinations.
The drug dosages of EE (CitationCosta Júnior et al., 2010) and fractions (CitationCosta Júnior et al., 2011) were determined by previous studies in our laboratory. The drug doses used in this present study are not equivalent to those used by humans because rats have different metabolic rates.
Behavioral effects on adult rats pretreated with GFC and extracts from P. insignis
Behavioral screening of the rats was performed following parameters described by CitationAlmeida and collaborators (1999) and animals were observed at 24 h after i.p. administration of EE, HE, DMF, EAF, or GFC from P. insignis (2 mg/kg, i.p.). During the 24 h, they were observed for the occurrence of general signs of toxicity: piloerection, prostration, writhing, increased evacuation, grooming, dyspnea, sedation, analgesi,a and palpebral ptosis.
SOD and CAT activity determinations in the hippocampus of adult rats pretreated with GFC, fractions and extracts from P. insignis
The SOD and CAT activity determinations were estimated as previously described by CitationSantos and collaborators (2008). The hippocampus was ultrasonically homogenized in 1 mL 0.05 M sodium phosphate buffer, pH = 7.0. Protein concentration was measured by the method of CitationLowry and collaborators (1951). The 10% homogenates were centrifuged (800 × g, 20 min) and supernatants were used to assay SOD and CAT. The SOD activities in the EE (2 mg/kg; n = 9), HE (2 mg/kg; n = 9), DMF (2 mg/kg; n = 9), EAF (2 mg/kg; n = 9), GFC (2 mg/kg; n = 9), and control (n = 12) groups were assayed by using xanthine and xanthine oxidase to generate superoxide radicals (Flohe & Otting, 1984), and the results expressed as U/mg of protein.
The CAT activities were measured in the EE (2 mg/kg; n = 9), HE (2 mg/kg; n = 9), DMF (2 mg/kg; n = 9), EAF (2 mg/kg; n = 9), GFC (2 mg/kg; n = 9), and control (n = 12) groups by the method that uses H2O2 to generate H2O and O2 (CitationChance & Maehly, 1955). Results are expressed as mmol/min/mg of protein.
Statistical analysis
Results of SOD and CAT activity determinations were compared by one-way analysis of variance (ANOVA) followed by Student–Newman–Keuls t-test (p < 0.05) (Graphpad program Intuitive, Software for Science, San Diego, CA, USA).
Results
SOD activities in rat hippocampus pretreated with GFC, fractions, and extracts from P. insignis
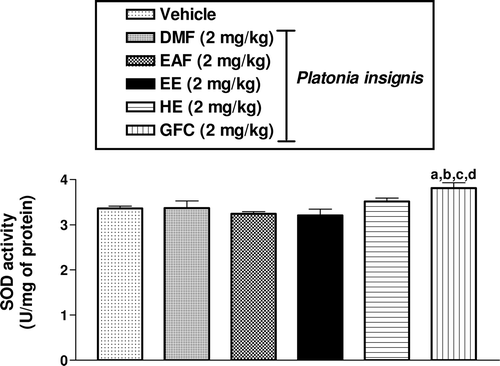
The SOD activities in rat hippocampus were not markedly altered in of EE, HE, DMF, EAF groups in comparison with the vehicle group (p > 0.05). On the other hand, there was a significant increase in SOD (13, 17, 19, and 13%) (p < 0.001) activity of rats pretreated with GFC (2 mg/kg) in comparison with vehicle, DMF, EAF, and EE groups, respectively ().
Figure 2. Effects of DMF, EAF, EE, HE, and GFC on SOD activities in rat hippocampus. Male rats (250–280 g; 2 months old) were treated with a single dose of DMF (2 mg/kg, intraperitoneal, i.p., n = 9), EAF (2 mg/kg, intraperitoneal, i.p., n = 9), EE (2 mg/kg, intraperitoneal, i.p., n = 9), HE (2 mg/kg, intraperitoneal, i.p., n = 9), GFC (2 mg/kg, intraperitoneal, i.p., n = 9), and the vehicle (Tween 80 0.05% dissolved in 0.9% saline) (i.p., n = 12, Control). Results are expressed as means ± S.E.M. for the number of animals shown above in parenthesis. Results were expressed as U/mg of protein. The Student–Newman–Keuls t-test was used for multiple comparisons of means of two groups of data. Differences in experimental groups were determined by two-tailed analysis of variance; ap < 0.05, when compared to vehicle group (ANOVA and Student–Newman–Keuls as post hoc t-test); bp < 0.05, when compared to DMF group (ANOVA and Student–Newman as post hoc t-test); cp < 0.05, when compared to EAF group (ANOVA and Student–Newman–Keuls as post hoc t-test); dp < 0.05, when compared to EE group (ANOVA and Student–Newman–Keuls as post hoc t-test).
CAT activities in rat hippocampus pretreated with GFC, fractions, and extracts from P. insignis
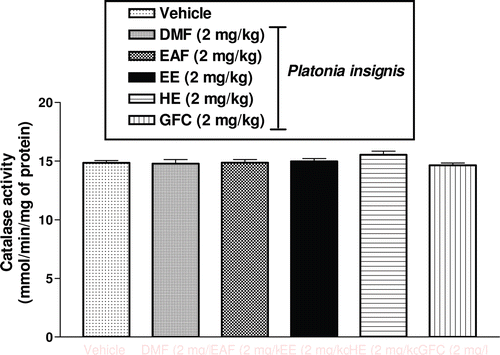
The CAT (p > 0.05) activities in rat hippocampus were not markedly altered in of EE, HE, DMF, EAF, and GFC groups in comparison with the vehicle group (p > 0.05). Correspondingly, there was no significant change in CAT activities of animals pretreated with extracts and fractions isolated from P. insignis (p > 0.05) ().
Figure 3 Effects. of DMF, EAF, EE, HE, and GFC on CAT activities in rat hippocampus. Male rats (250–280g; 2 months old) were treated with a single dose of DMF (2 mg/kg, intraperitoneal, i.p., n = 9), EAF (2 mg/kg, intraperitoneal, i.p., n = 9), EE (2 mg/kg, intraperitoneal, i.p., n = 9), HE (2 mg/kg, intraperitoneal, i.p., n = 9), GFC (2 mg/kg, intraperitoneal, i.p., n = 9), and the vehicle (Tween 80 0.05% dissolved in 0.9% saline) (i.p., n = 12, Control). Results are expressed as means ± S.E.M. for the number of animals shown inside in parenthesis. Results were expressed as mmol/min/mg of protein. The Student–Newman–Keuls t-test was used for multiple comparisons of means of two groups of data. Differences in experimental groups were determined by two-tailed analysis of variance.
Discussion
In present study, we investigated the effects of GFC, fractions, and extracts from P. insignis in the antioxidant enzymatic activities within hippocampus rats. The rats treated with the substances isolated from P. insignis presented behavioral alterations, such as increased ambulation, palpebral ptosis, and stimulation. These behavioral changes suggest a possible effect on CNS.
Recently, several research groups reported that PPAPs-type diterpenoids have shown antioxidant activity and reduction of ROS, and protection of subsequent cell death (CitationCiochina & Grossman, 2006; CitationLin et al., 2009). Our present study demonstrated that GFC isolated from P. insignis displays in vitro antioxidant effects (CitationCosta Júnior et al., 2011). In addition, in the present study, we investigated the influence of DMF, EAF, EE, or HE on antioxidant enzymatic activities of SOD and CAT in rat hippocampus. Our data shows a possible antioxidant effect of GFC through the scavenging of radical O2−. This consequent scavenging of O2− produces a decrease in the H2O2 levels genusested by superoxide dismutation hippocampus, causing increase of the SOD activity, suggesting this can be the antioxidant action mechanism of the GFC. Free radical formation elevations are frequently accompanied by an immediate compensatory increase in the activity of free radical scavenging enzymes (CitationTomé et al., 2010a).
It is well known that free radicals produce several diseases, such as Parkinson disease, Alzheimer type dementia, seizures, and others (CitationReuter et al., 2010; CitationTomé et al., 2010b). The production of free radicals and the activity of the scavenger enzymes against those radicals such as SOD are correlated with the life expectancies. We have demonstrated that GFC isolated from P. insignis seeds were capable of inhibiting and quenching free radicals, acting as reducing agents. Furthermore, phenolic compounds present in the plant kingdom are mainly responsible for the antioxidant potential of plants (CitationLarson, 1988).
The GFC demonstrated a significant scavenging effect on hydroxyl radicals and NO genusestion in vitro (CitationCosta Júnior et al., 2011). Our findings showed that GFC, DMF, EAF, EE, or HE did not produce alterations in the hippocampus CAT activity, since they did not protect to the hippocampus from neuronal damage induced by lipid peroxidation products. It is unlikely that the unaltered CAT activity is related to the mechanisms involved in maintenance of oxidative stress (CitationFreitas et al., 2005). These data suggest that hippocampus of rats pretreated with GFC, fractions, and extracts from P. insignis do not use CAT as the major free radical scavenging system.
Conclusions
Accordingly in this study, a significant relationship was found between the antioxidant activity and phenolic compounds, indicating that phenolic compounds could be major contributors to the antioxidant activity. Although the antioxidant activities found in vivo analyses were only indicative of the potential health benefit, these results remain important as the first step in screening antioxidant activity of P. insignis seeds. Thus, it can be concluded that GFC isolated from P. insignis seeds can be used as an accessible source of natural antioxidants.
Acknowledgments
The authors are grateful to Fundação de Amparo a Pesquisa do Estado do Piauí (FAPEPI), Instituto Federal do Piauí (IFPI), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Declaration of interest
The authors declare that there are no conflicts of interest.
References
- Agra MF, Freitas PF, Barbosa-Filho JM. (2007). Synopsis of the plants known as medicinal and poisonous in Northeast of Brazil. Rev Bras Farmacogn, 17, 114–140.
- Almeida RN, Falcão A, Diniz RST, Quintans-Júnior LJ, Polari RM, Barbosa-Filho JM, Agra MF, Duarte JC, Ferreira CD, Antoniolli AR, Araújo CC. (1999). Metodologia para avaliação de plantas com atividade no sistema nervoso central e alguns dados experimentais. Rev Bras Farmacogn, 80, 72–76.
- Alves S, Jennings WG. (1979). Volatile composition of certain amazonian fruits. Food Chem, 4, 149–159.
- Chance B, Maehly AC. (1955). Assay catalases and peroxidases. Methods Enzymol, 2, 764–768.
- Chen SX, Wan M, Loh BN. (1996). Active constituents against HIV-1 protease from Garcinia mangostana. Planta Med, 62, 381–382.
- Chomnawang MT, Surassmo S, Wongsariya K, Bunyapraphatsara N. (2009). Antibacterial activity of Thai medicinal plants against methicillin-resistant Staphylococcus aureus. Fitoterapia, 80, 102–104.
- Ciochina R, Grossman RB. (2006). Polycyclic polyprenylated acylphloroglucinols. Chem Rev, 106, 3963–3986.
- Costa Júnior JS, Freitas RM, Citó AMGL, Henriques JAP, Saffi J. (2010). Evaluation of effects of ethanol extract (EE) from Platonia insignis Mart. on pilocarpine-induced seizures. J Biol Sci, 10, 747–753.
- Costa Júnior JS, Ferraz ABF, Filho BAB, Feitosa CM, Citó AMGL, Freitas RM, Saffi J. (2011). Evaluation of antioxidant effects in vitro of Garcinielliptone FC (GFC) isolated from Platonia insignis Mart. J Med Plants Res, 52, 293–299.
- Cuesta-Rubio O, Piccinelli AL, Rastrelli L. (2005). Chemistry and biological activity of polyisoprenylated benzophenone derivatives. In: Atta-ur-Rahman, Editor, Studies in Natural Products Chemistry vol. 32, Elsevier, Amsterdam, 671–720.
- Ee GC, Daud S, Izzaddin SA, Rahmani M. (2008). Garcinia mangostana: a source of potential anti-cancer lead compounds against CEM-SS cell line. J Asian Nat Prod Res, 10, 475–479.
- Ferreira PMP, Farias DF, Oliveira JTA, Carvalho AFFU. (2008). Moringa oleifera: Bioactive compounds and nutritional potential. Rev Nutr, 21, 431–437.
- Flohé L, Otting F. (1984). Superoxide dismutase assays. Meth Enzymol, 105, 93–104.
- Freitas RM, Vasconcelos SM, Souza FC, Viana GS, Fonteles MM. (2005). Oxidative stress in the hippocampus after pilocarpine-induced status epilepticus in Wistar rats. Febs J, 272, 1307–1312.
- Freitas RM, Nascimento KG, Ferreira PM, Jordan J. (2010). Neurochemical changes on oxidative stress in rat hippocampus during acute phase of pilocarpine-induced seizures. Pharmacol Biochem Behav, 94, 341–345.
- Halliwell B, Gutteridge JM, Cross CE. (1992). Free radicals, antioxidants, and human disease: where are we now? J Lab Clin Med, 119, 598–620.
- Hung SH, Shen KH, Wu CH, Liu CL, Shih YW. (2009). alpha-mangostin suppresses PC-3 human prostate carcinoma cell metastasis by inhibiting matrix metalloproteinase-2/9 and urokinase-plasminogen expression through the JNK signaling pathway. J Agric Food Chem, 57, 1291–1298.
- Iinuma M, Tosa H, Tanaka T, Asai F, Kobayashi Y, Shimano R, Miyauchi K. (1996). Antibacterial activity of xanthones from guttiferaeous plants against methicillin-resistant Staphylococcus aureus. J Pharm Pharmacol, 48, 861–865.
- Larson RA. (1988). The antioxidants of higher plants. Phytochemistry 27, 969–978.
- Lin KW, Huang AM, Tu HY, Weng JR, Hour TC, Wei BL, Yang SC, Wang JP, Pu YS, Lin CN. (2009). Phloroglucinols inhibit chemical mediators and xanthine oxidase, and protect cisplatin-induced cell death by reducing reactive oxygen species in normal human urothelial and bladder cancer cells. J Agric Food Chem, 57, 8782–8787.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. (1951). Protein measurement with the Folin phenol reagent. J Biol Chem, 193, 265–275.
- Marti G, Eparvier V, Moretti C, Susplugas S, Prado S, Grellier P, Retailleau P, Guéritte F, Litaudon M. (2009). Antiplasmodial benzophenones from the trunk latex of Moronobea coccinea (Clusiaceae). Phytochemistry, 70, 75–85.
- Mujic A, Grdovic N, Mujic I, Mihailovic M, Zivkovic J, Poznanovic G, Vidakovic M. (2011). Antioxidative effects of phenolic extracts from chestnut leaves, catkins and spiny burs in streptozotocin-treated rat pancreatic [beta]-cells. Food Chem, 125, 841–849.
- Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. (2010). Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med, 49, 1603–1616.
- Santos LF, Freitas RL, Xavier SM, Saldanha GB, Freitas RM. (2008). Neuroprotective actions of vitamin C related to decreased lipid peroxidation and increased catalase activity in adult rats after pilocarpine-induced seizures. Pharmacol Biochem Behav, 89, 1–5.
- Suksamrarn S, Suwannapoch N, Phakhodee W, Thanuhiranlert J, Ratananukul P, Chimnoi N, Suksamrarn A. (2003). Antimycobacterial activity of prenylated xanthones from the fruits of Garcinia mangostana. Chem Pharm Bull, 51, 857–859.
- Sundaram BM, Gopalakrishnan C, Subramanian S, Shankaranarayanan D, Kameswaran L. (1983). Antimicrobial Activities of Garcinia mangostana. Planta Med, 48, 59–60.
- Tomé AR, Feng D, Freitas RM. (2010). The effects of alpha-tocopherol on hippocampal oxidative stress prior to in pilocarpine-induced seizures. Neurochem Res, 35, 580–587.
- Tomé Ada R, Ferreira PM, Freitas RM. (2010). Inhibitory action of antioxidants (ascorbic acid or alpha-tocopherol) on seizures and brain damage induced by pilocarpine in rats. Arq Neuropsiquiatr, 68, 355–361.