Abstract
Context: Enicostema axillare A. Raynal (Gentianaceae) has been used in the traditional Indian system of medicine as a depurative and for the treatment of skin diseases, tumors, intermittent fever, and helminthiasis.
Objective: E. axillare was investigated for antimutagenic and antioxidant effects.
Materials and methods: The antioxidant and antimutagenic activities of E. axillare fractions were determined by 2,2-diphenyl-1-picryl-hydrazyl (DPPH) radical scavenging assay and Ames test using Salmonella typhimurium tester strains TA98 and TA100 against direct-acting mutagens, such as sodium azide (NaN3), 4-nitro-O-phenylene diamine (NPD), and mutagen needing activation, such as 2-aminofluorene (2AF). Toxicity study of these fractions was also performed.
Results and discussion: The ethyl acetate fraction showed maximum antimutagenic effect by 88.25 and 84.46% (preincubation) and 85.13 and 84.47% (coincubation) of inhibition against NaN3 and NPD-induced mutagenicity, respectively. Inhibition of S9-dependent mutagens such as 2AF was higher than direct-acting mutagens by the ethyl acetate fraction of E. axillare. It showed 90.25 and 92.00% of inhibition in the preincubation and coincubation experiments. The ethyl acetate fraction showed higher total antioxidant capacity (24.79 ± 0.29 µg) and low IC50 value for DPPH radical scavenging assay (192.27 ± 3.67 µg). The overall effect of E. axillare fractions was found to be in the order: ethyl acetate > methanol > hexane in these assays. In subacute toxicity study, with oral administration of these fractions, no marked biochemical and histopathologic changes were observed.
Conclusion: The significant antimutagenic and antioxidant activities of E. axillare might provide a scientific validation for the traditional use of this plant.
Keywords::
Introduction
Cancer has become the number one cause of death in the world (CitationTominaga et al., 1994). In current scientific investigation, cancer induced or aided by chemically defined substances is of great interest. People are continuously exposed to varying amounts of chemicals that have been shown to have carcinogenic or mutagenic properties in experimental systems. Mutations can occur exogenously when carcinogenic agents are present in food, air, or water, and also endogenously when they are products of metabolism or pathophysiologic states such as inflammation. Exposure to environmental chemical carcinogens may contribute significantly to the causation of a sizable fraction, perhaps a majority, of human cancers (CitationWogan et al., 2004). Epidemiological studies indicated that many cancers are dependent on multiple mutational etiologies, as well as on inherited mutator phenotype.
Mutagens and carcinogens are the cause of innate metabolic defects in cellular system, triggering the morbidity and mortality in living organisms (CitationAqil et al., 2008). They must be metabolized to reactive intermediates that are capable of interacting covalently with DNA (CitationWeisberger, 1999). Mutagens that cause damage to DNA are involved in the initiation and promotion of various human diseases through generation of free reactive oxygen species (ROS). Excessive generation of ROS and other radicals can damage proteins, carbohydrates, polyunsaturated fatty acids, and DNA and lead to oxidative stress for certain cancers (CitationEbenharder & Grunhage, 2003). In recent years, the use of natural antioxidants present in food and other biological materials has attracted considerable interest due to their presumed safety and nutritional and therapeutic value (CitationAjila et al., 2007). Chemoprevention aimed at inhibiting or delaying the onset of carcinogenesis, particularly through natural products, is a rapidly growing area of cancer research (CitationPezzuto et al., 2005; CitationLakshmi et al., 2006).
Enicostema axillare A. Raynal (Gentianaceae) is commonly known as “Vellarugu” in Tamil. It is an herb, widely distributed throughout India, with subquadrangular stems, three-nerved simple leaves, and white flowers in auxiliary clusters (CitationMatthew, 1991). This plant has been used in the traditional Indian system of medicine as a depurative and for the treatment of skin diseases, intermittent fever, helminthiasis (CitationWarrier et al., 1995; Chopra et al., 2002), and for tumors (CitationRajan, 1991; CitationMudaliar, 2002). A recent ethnobotanical survey from our institute showed a high consensus in the use of this plant as a blood purifier; the traditional healers in Theni District, Tamil Nadu use this plant to treat infections and cancer (CitationPandikumar et al., 2011). Thus, the present study is aimed at evaluating the antimutagenic and antioxidant effects of the various fractions of E. axillare using in vitro assays.
Materials and methods
Chemicals
l-Histidine, d-biotin, and sodium azide (NaN3) were purchased from Hi-Media, Mumbai, India. 4-Nitro-O-phenylene diamine (NPD) and 2-aminofluorene (2AF) were purchased from Sigma Chemical, St. Louis, mo, USA. Kits for assessing serum biochemistry were purchased from Accurex Biomedical (P) Ltd., Thane, Maharashtra, India. All other chemicals used in the studies were of analytical grade.
Bacterial strains
Histidine-requiring strains of Salmonella typhimurium TA98 and TA100 were purchased from MTCC, Chandigarh, India. They were incubated in nutrient broth for 12 h and frozen permanents were prepared by freezing at −70°C in the presence of 10% dimethyl sulfoxide (DMSO). Fresh cultures were prepared by inoculating 40 µL of frozen permanents in 5 mL of nutrient broth and incubated for 12 h at 37°C. The cultures thus obtained were used for the experiments.
Plant material and preparation of the fractions
Whole plants of E. axillare were collected from Arakkonam, Vellore District, Tamil Nadu, India in November, 2009. The botanical identity of the plant material was confirmed by Dr. G. Jeya Jothi, taxonomist at Department of Botany, Loyola College, Chennai. Voucher specimens (ERISa-01-03) were stored in the herbarium of Entomology Research Institute for future reference. The coarsely powdered leaves of E. axillare (1 kg) were extracted with 3 L of methanol, by a cold percolation method. After 48 h, the filtrate was obtained and concentrated under reduced pressure. This concentrated extract (2 g) was packed on a silica gel column and eluted with hexane (500 mL), ethyl acetate (500 mL), and methanol (500 mL), successively. The solvents from the extracts were evaporated to dryness using a rotary evaporator and the residue was dissolved in DMSO.
Animals
Male Swiss albino mice (28 ± 1 g) and male Wistar rats (200 ± 5 g), bred in the animal house of Entomology Research Institute, Loyola College, Chennai, were used. The animals were maintained at 25 ± 1°C, with 12/12 h light/dark cycle and 55 ± 10% relative humidity. The animals were fed with normal pellet diet containing 74% carbohydrate, 21% protein, and 4% fat, purchased from Pranav Agro Industries Ltd., Maharashtra. This study protocols were reviewed and approved by the Institutional Animal Ethics Committee (Approval number: IAEC-ERI-LC-01/10).
Evaluation of the mutagenic and antimutagenic effects of the fractions
Mutagenic activity
TA98 and TA100 strains of S. typhimurium were incubated at 37°C for 48 h without any combination solutions of extracts and mutagens. Mutagenicity of E. axillare fractions, if any, against these bacterial strains was determined by incubating these strains in high concentration (3 mg/plate) of each fraction separately (CitationLoh et al., 2009). The strains incubated without fractions served as a control for this experiment. The number of spontaneous revertants was counted after 48 h, and the experiments were carried out in triplicate.
Antimutagenic activity
Direct acting mutagens
All the chemical mutagens were dissolved in DMSO except NaN3, which was dissolved in water (CitationLakshmi et al., 2006). Antimutagenicity of E. axillare fractions against direct-acting mutagens was determined by the method of CitationMaron and Ames (1983) with some modifications (CitationKaur et al., 2003). The antimutagenic capacity of the fractions was evaluated both by coincubation and preincubation modes of experiments. In the coincubation method, 2 mL of top agar containing 0.1 mL of bacterial culture (~1 × 109 cells/mL), 0.1 mL of nontoxic concentration of test compound at different range (1–3 mg/plate), 0.2 mL of 0.5 mM histidine-biotin, and 0.1 mL of mutagens NaN3 (0.0025 mg/plate) or NPD (0.02 mg/plate) were layered onto minimal glucose plates. In the preincubation experiments, a mixture of fractions and mutagens, each having a volume of 0.1 mL, was preincubated at 37°C with rapid shaking for 30 min and then added to the bacterial culture. The plates were incubated at 37°C for 48 h, and the number of revertant colonies was counted using a colony counter.
Mutagens requiring activation
The media and S9 mix were prepared according to the procedure of CitationMaron and Ames (1983). Liver microsomal fraction (S9) was prepared from rats that were treated with 0.1% phenobarbital in drinking water for 4 days (CitationAmes et al., 1973). After overnight fasting, the animals were killed by decapitation, the liver was removed, and the homogenate was prepared aseptically according to the method of CitationLakshmi et al. (2006). Bacterial culture (0.1 mL), 0.1 mL of mutagen, 0.5 mL of S9 mixture, and 0.1 mL of each fraction separately were vortex-mixed and poured onto minimal glucose plates. The plates were incubated at 37°C for 48 h, and the number of revertant colonies was scored. Bacterial survival was routinely monitored in all these experiments. The experiments were carried out in triplicates. Inhibitory activity was expressed as percentage decrease of reverse mutation.
where E1 is the number of revertants without extracts, E2 is the number of revertants with extracts, and SR is the number of spontaneous revertants. The experiments were performed in triplicate.
Evaluation of the antioxidant effect of the fractions
2,2-diphenyl-1-picryl-hydrazyl (DPPH) radical scavenging assay
The hydrogen atom or electron donor capacity of the extracts was tested by its ability to bleach DPPH radical according to the method of Gulcin (2007). Various concentrations of the fractions (100–500 µg) were added to 1 mL of 0.25 mM DPPH solution in ethanol. The tubes were incubated in dark for 30 min, and the absorbance was read at 517 nm. Each assay was carried out in triplicate. The percentage scavenging was calculated as the ratio of absorption of the sample relative to the control without the extract. DPPH radical scavenging activity was calculated using the following formula:
Antiradical effect of the fractions was defined as IC50, and it was calculated using nonlinear regression (CitationSlusarczyk et al., 2009).
Determination of total antioxidant capacity of the fractions
The total antioxidant activity of the fractions was determined by reduction of Mo (VI) to Mo (V) by the extracts and the subsequent formation of a green colored Mo(V)/phosphate complex in acidic pH. 100 µg of each fraction in 0.3 mL of distilled water was added to 3 mL of molybdate reagent containing 0.6 M sulphuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate. The tubes were incubated at 95°C for 90 min. Then the mixture was cooled to room temperature and the absorbance was measured at 695 nm. The results were expressed as equivalents of ascorbic acid.
Phytochemical analysis of the fractions
Estimation of the polyphenol content of the fractions
The amount of polyphenol in the fractions was determined by Folin-Ciocalteu method. Ten milligrams of the each fraction was dissolved in 1 mL of methanol: water: concentrated HCl (60:40:0.3). This stock solution (100 µL) was mixed with 2 mL of 2% Na2CO3 solution. After 2 min, 100 µL of 50% Folin-phenol reagent was added. The tubes were incubated for 30 min at room temperature and read at 750 nm. Gallic acid was used as standard. Each assay was carried out in triplicate. The results were expressed as milligram of gallic acid equivalent per 100 g of extracts.
Estimation of flavonoids content of the extracts
Total flavonoids (TFOs) content was determined following CitationYong et al. (2008). In a 10 mL eppendorf tube, 0.3 mL of stock solution, 3.4 mL 30% ethanol, 0.15 mL of 0.5 mol/L NaNO2, and 0.15 mL of 0.3 mol/L AlCl3·6H2O were added and mixed. After 5 min, 1 mL of 1 M/l NaOH was added, and the mixture’s absorbance was read at 506 nm. The TFOs concentration was calculated from a calibration curve using rutin as standard.
Toxicity study of E. axillare fractions
Acute toxicity study
The fractions of E. axillare were dissolved in a vehicle containing 0.2% polysorbate-80, 0.5% sodium carboxy methylcellulose, 0.9% sodium chloride, 0.9% benzyl alcohol, and 97.2% distilled water (CitationLee, 2001). Three animals were used for each group. The animals were appropriately grouped and placed in the experiment room for acclimatization. Food and water were removed from the cages in the morning. Then, the animals were treated orally with the vehicle or the fractions of E. axillare. At 0, 15, 30, 60, 120, and 180 min and 24 h after treatment of the fractions, behavioral alterations were observed on animals. For further assays, 1/10th-1/20th of the doses at which behavioral alterations were observed was considered safe (CitationOliveira et al., 2008). The fractions did not produce any toxic effect up to 2 g/kg b.w. concentration.
Subacute toxicity study
The first group served as the control group. Animals in Group I–III were administered with 200 mg/kg b.w of hexane, ethyl acetate, and methanol fractions of E. axillare, respectively. All applications were performed orally using a catheter for 21 days. The control group was given saline alone. At the end of 21 days, blood samples were collected into dry tubes from all animals under light ether anesthesia. Each tube was centrifuged at 3000 rpm for 10 min, and the serum was analyzed for various biochemical parameters.
Analysis of serum biochemical parameters
The measurements of uric acid, creatine, aspartate amino transferase (AST), alanine amino transferase (ALT), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), and gamma glutamyl transpeptidase (GGT), were performed using spectrophotometric methods (CitationTitez, 1995).
Histopathologic observations
A small piece of liver was fixed by 10% buffered formalin with PBS and then embedded into paraffin, sectioned for 5-µm thick, and mounted on the glass microscope slides using standard histopathologic techniques. The sections were stained with hematoxylin-eosin and examined by light microscopy.
Statistics
Results were expressed as mean ± SEM. Statistical significance was calculated by one-way ANOVA followed by Tukey’s test, and the values were considered as significant at p ≤ 0.05. IC50 values were calculated using Graph Pad Prism, Version 4.0 (Graphpad software, San Diego, CA, USA).
Results
Mutagenic effect of the fractions
The mutagenic and antimutagenic effects of E. axillare fractions are given in Tables (number of bacterial revertants obtained) and Figures (percentage inhibition of revertants). The extract of E. axillare at a concentration of 3 mg/plate did not show any effect on spontaneous revertants of both TA98 and TA100 S. typhimurium tester strain in the presence or absence of microsomal fraction (). It was confirmed that none of the fractions showed mutagenic activity.
Table 1. Effect of fractions of E. axillare to S. typhimurium spontaneous revertants in the presence (+S9) or absence of (−S9) microsomal fraction.
Antimutagenic effect of the fractions
Effect on direct acting mutagen
The antimutagenic potential of hexane, ethyl acetate, and methanol fractions of E. axillare against NaN3, NPD, and 2AF are presented in (TA98) and (TA100). Only the positive control (mutagens with bacterial strains) showed significantly higher number of revertants than the treatment groups. The percentage inhibitions of mutagenesis by the ethyl acetate and methanol fractions were comparatively higher compared with the hexane fraction. The ethyl acetate fraction showed 88.25% (TA98) and 88.85% (TA100; in the preincubation), as well as 84.68% (TA98) and 85.13% (TA100; in the coincubation) of inhibition against NaN3 induced mutagenicity at 3 mg/plate concentration followed by methanol and hexane fractions (). In case of NPD induced mutagenicity, the ethyl acetate fraction had shown 81.73% (TA98) and 84.46% (TA100; in preincubation), as well as 81.61% (TA98) and 84.47% (TA100) (in the coincubation) of inhibition followed by methanol and hexane fractions (). All these fractions did not show significant differences in the antimutagenic activities between coincubation and preincubation modes of experiments.
Table 2. Effects of fractions of E. axillare on mutagenesis of NaN3, NBT and 2-AF in TA98 strain of S. typhimurium.
Table 3. Effect of fractions of E. axillare on mutagenesis of NaN3, NBT and 2-AF in TA100 strain of S. typhimurium.
Figure 1. Percentage inhibition of mutagenesis by the preincubation and coincubation modes of experiments with E. axillare fractions against NaN3 induced mutagenicity on TA98 (A, preincubation and B, coincubation) and TA100 (C, preincubation and D, coincubation). ([GRAPHIC], hexane extract; [GRAPHIC], ethyl acetate extract; [GRAPHIC], methanol extract). Values carrying different alphabets vary significantly from each other (Tukey’s HSD; p ≤ 0.05).
![Figure 1. Percentage inhibition of mutagenesis by the preincubation and coincubation modes of experiments with E. axillare fractions against NaN3 induced mutagenicity on TA98 (A, preincubation and B, coincubation) and TA100 (C, preincubation and D, coincubation). ([GRAPHIC], hexane extract; [GRAPHIC], ethyl acetate extract; [GRAPHIC], methanol extract). Values carrying different alphabets vary significantly from each other (Tukey’s HSD; p ≤ 0.05).](/cms/asset/cc418dde-d946-4656-87b8-2b2abd03951b/iphb_a_618993_f0001_b.gif)
Figure 2. Percentage inhibition of mutagenesis by the preincubation and coincubation mode of experiments with E. axillare fractions against NPD-induced mutagenicity on TA98 (A, preincubation and B, coincubation) and TA100 (C, preincubation and D, coincubation). ([GRAPHIC], hexane extract; [GRAPHIC], ethyl acetate extract; [GRAPHIC] methanol extract). Values carrying different alphabets vary significantly from each other (Tukey’s HSD; p ≤ 0.05).
![Figure 2. Percentage inhibition of mutagenesis by the preincubation and coincubation mode of experiments with E. axillare fractions against NPD-induced mutagenicity on TA98 (A, preincubation and B, coincubation) and TA100 (C, preincubation and D, coincubation). ([GRAPHIC], hexane extract; [GRAPHIC], ethyl acetate extract; [GRAPHIC] methanol extract). Values carrying different alphabets vary significantly from each other (Tukey’s HSD; p ≤ 0.05).](/cms/asset/7f50b0cd-c936-44b2-9500-41d1593f6d4d/iphb_a_618993_f0002_b.gif)
Effect on mutagens requiring activation
It was observed that all fractions of E. axillare inhibited 2AF-induced revertants on both TA98 () and TA100 (), more significantly than those induced by direct-acting mutagens. The ethyl acetate fractions exhibited more inhibition of revertants induced by 2AF in the presence of S9 than those by methanol and hexane fractions. In this experiment also, the ethyl acetate fraction showed maximum inhibition of 90.25% (TA98) and 91.19% (TA100; in the preincubation), as well as 85.43% (TA98) and 92.00% (TA100) (in the coincubation) at 3 mg/plate concentration followed by methanol and hexane fractions (). These fractions showed relatively more antimutagenicity toward the S9-dependent mutagens, namely 2AF than the direct-acting mutagens such as NaN3 or NPD.
Figure 3. Percentage inhibition of mutagenesis by the preincubation and coincubation mode of experiments with E. axillare fractions against 2-AF induced mutagenicity on TA98 (A, preincubation; B, coincubation) and TA100 (C, preincubation; D, coincubation). ([GRAPHIC], hexane extract; [GRAPHIC], ethyl acetate extract; [GRAPHIC], methanol extract). Values carrying different alphabets vary significantly from each other (Tukey’s HSD; p ≤ 0.05).
![Figure 3. Percentage inhibition of mutagenesis by the preincubation and coincubation mode of experiments with E. axillare fractions against 2-AF induced mutagenicity on TA98 (A, preincubation; B, coincubation) and TA100 (C, preincubation; D, coincubation). ([GRAPHIC], hexane extract; [GRAPHIC], ethyl acetate extract; [GRAPHIC], methanol extract). Values carrying different alphabets vary significantly from each other (Tukey’s HSD; p ≤ 0.05).](/cms/asset/ee20cc0b-a644-4061-ad9b-3d8e81d3ca0a/iphb_a_618993_f0003_b.gif)
Antioxidant activity of the fractions
The DPPH assay constitutes a screening method currently used to provide basic information on the antiradical activity of the extracts. When a solution of DPPH is mixed with a substance that can donate a hydrogen atom, the reduced form of the radical is generated accompanied by loss of color and reduction in the absorbance. The phosphomolybdenum method of measuring total antioxidant capacity of the fractions is quantitative, because the antioxidant activity is expressed as the number of equivalents of ascorbic acid. In the present study, the ethyl acetate fraction had shown a significantly low IC50 value (192.27 ± 3.67 µg), followed by the methanol and hexane fractions. Likewise, the total antioxidant capacity of the ethyl acetate fraction was also significantly higher (24.79 ± 0.29), followed by the methanol and hexane fractions. The hexane fraction showed lowest antioxidant capacity ().
Table 4. Phytochemical analysis and antioxidant capacity of the fractions from E. axillare.
Phytochemical analysis of the fractions
In the present study, the methanol and ethyl acetate fractions of the E. axillare show a high amount of polyphenols and flavonoids ().
Serum biochemical parameters
The effects of oral administration of hexane, ethyl acetate, and methanol fractions of E. axillare on the serum biochemical levels of mice are shown in . When compared with the control, there was no significant difference in serum biochemical parameters of the animals treated with the fractions.
Table 5. Effect of E. axillare fractions on various serum biochemical parameters.
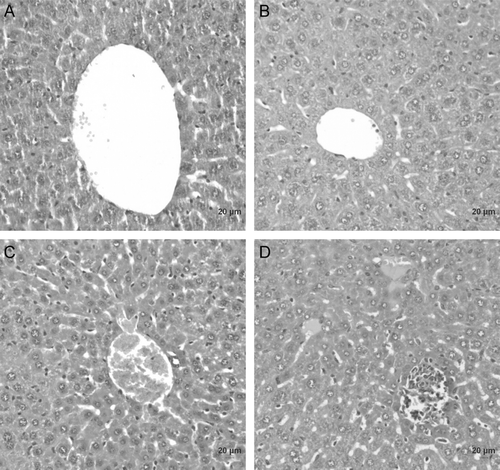
Histopathologic observations
Liver histopathologic sections are given in . No significant histopathologic differences were found between the treated and the control animals.
Discussion
Cancer is a multifactor and multimechanistic disease requiring a multidimensional approach for its treatment, control and prevention (CitationRavikumar et al., 2010). The incidence of cancer is on the rise with multiple risk factors that involve interplay between genetic and environmental components (CitationPerera, 1996; CitationDoll, 1996). Mutagenic and carcinogenic agents are omnipresent in the human environment, and it seems impossible to eliminate all of them. Moreover, several well-known mutagenic risk factors are closely connected with a modern lifestyle, and their entire eradication appears to be very burdensome, even unattainable (CitationCaillet et al., 2011). Therefore, there exists a need to reduce genotoxic effects of mutagenic and carcinogenic factors by the regular intake of antimutagenic agents (CitationHertog et al., 1993).
The antimutagenic properties elicited by plant species have an array of prospective applications in human health care. Plants have a long history of use in the treatment of cancer providing some of the currently used effective anticancer agents such as vinblastine, vincristine, mechlorethamine, prednisone, procarbazine, etoposide, teniposide, paclitaxel, bleomycin, cisplatin, and taxanes (CitationDevita et al., 1970; CitationWani et al., 1971; CitationStahelin, 1973; CitationWilliams et al., 1987). It was estimated that about 67% of pharmaceutical products approved between 1974 and 1994, for human cancer therapy were derived from natural sources (CitationRichard et al., 2005). Recent research has underlined the chemopreventive activity of several secondary plant metabolites (CitationNewman & Cragg, 2007).
Ethyl acetate and methanol fractions present in E. axillare showed significant antimutagenic and antioxidant effects. The significant anitmutagenic activity of each fraction against direct-acting mutagens suggests that these fractions may directly prevent the damage of DNA from mutagen. No significant variation was observed between preincubation and coincubation modes of experimentation. The chemical substances present in plants may act as antimutagens or anticarcinogens by blocking or trapping ultimate carcinogen electrophiles in a nucleophilic chemical reaction to form innocuous products (CitationKaur et al., 2002).
The ethyl acetate fraction showed significant antimutagenic effect against 2AF, which is S9-dependent mutagen on both TA98 and TA100 strains followed by methanol and hexane fractions. The activation of 2AF involves the formation of N-hydroxy-2-aminofluorene, a reaction catalyzed by the cytochrome P450 enzyme system. Antimutagenic property present in these fractions may interact with the specific enzyme system of microsomal cytochrome P448/P450 in the liver homogenates. Inhibition of 2AF-induced mutagenesis by these fractions indicated that they might interfere with the metabolic activation of promutagens, act as blocking agents, form adducts with ultimate metabolite and scavenge free radicals (CitationKaur et al., 2002). These fractions, when treated alone with these strains, did not show any mutagenic effect even at high concentration up to 48 h.
Antioxidants play a significant role on defense mechanisms against oxidative stress caused by ROS, which are highly reactive and potentially damaging transient chemical species that cause certain cancers (CitationAli et al., 2007). Low IC50 values and high antioxidant capacity of ethyl acetate and methanol fractions of E. axillare indicated significant defense action against free radicals from chemical species. The increased antimutagenic and antioxidant activities of E. axillare might be due to the rich content of polyphenols and flavonoids. A wide array of phenolic substances, particularly those present in dietary and medicinal plants, have been reported to possess substantial antimutagenic and anticarcinogenic activities (CitationOkuda et al., 1991; CitationKaur et al., 1997; CitationZhu et al., 1997; CitationEbringer et al., 1999; CitationLopes et al., 1999; CitationSurh, 1999). Phenolics and flavonoids might be the most likely candidates in the methanol and ethylacetate fractions providing the antimutagenic and antioxidant effects. A 21 day oral toxicity study of these fractions had not shown any ill effects on the physiology and behavior of the animals.
Mutagenic and carcinogenic agents are responsible for certain cancers through production of oxidative damage on macromolecules by free radicals. E. axillare has been used traditionally for treatment of tumors (CitationRajan, 1991; CitationMudaliar, 2002). In the present study, the ethyl acetate and methanol fractions of E. axillare revealed a significant free radical scavenging and antioxidant effect without any toxicity.
Conclusion
The significant antimutagenic and antioxidant activities of E. axillare might provide a scientific validation for the traditional use of this plant. The presence of high amount of phenolics and flavonoids might be the reason for their ability to protect against mutagenicity induced by NaN3, NPD, or 2AF through its antioxidant effect.
Acknowledgements
The authors thank Entomology Research Institute, Loyola College, Chennai, for financial assistance. They also thank the Dean, Tamilnadu Veterinary University, Chennai, for histopathologic analysis.
Declaration of interest
The authors report no conflict of interest.
References
- Ajila CM, Naidu KA, Bhat UJS, Rao P. (2007). Bioactive compounds and antioxidant potential of mango peel extract. Food Chem, 105, 982–988.
- Ali SS, Kasoju N, Luthra A, Singh A, Sharanabasava H, Sahu A, Bora U. (2007). Indian medicinal herbs as sources of antioxidants. Food Res Int, 41, 1–15.
- Ames BN, Durston WE, Yamasaki E, Lee FD. (1973). Carcinogens are mutagens: A simple test system combining liver homogenates for activation and bacteria for detection. Proc Natl Acad Sci USA, 70, 2281–2285.
- Aqil F, Zahin M, Ahmad I. (2008). Antimutagenic activity of methanolic extracts of four ayurvedic medicinal plants. Indian J Exp Biol, 46, 668–672.
- Caillet S, LessardS, Lamoureux G, Lacroix M, (2011). Umu test applied for screening natural antimutagenic agents. Food Chem, 124, 1699–1707.
- Chopra RN. (1965). Problems and prospects of a pharmacological career in India. Annu Rev Pharmacol, 10, 1–8.
- Devita VT Jr, Serpick AA, Carbone PP. (1970). Combination chemotherapy in the treatment of advanced Hodgkin’s disease. Ann Intern Med, 73, 881–895.
- Doll R. (1996). Nature and nurture: Possibilities for cancer control. Carcinogenesis, 17, 177–184.
- Edenharder R, Grünhage D. (2003). Free radical scavenging abilities of flavonoids as mechanism of protection against mutagenicity induced by tert-butyl hydroperoxide or cumene hydroperoxide in Salmonella typhimurium TA102. Mutat Res, 540, 1–18.
- Ebringer L, Krizková L, Polónyi J, Dobias J, Lahitová N. (1999). Antimutagenicity of lignin in vitro. Anticancer Res, 19, 569–572.
- Gülçin I. (2007). Comparison of in vitro antioxidant and antiradical activities of l-tyrosine and l-Dopa. Amino Acids, 32, 431–438.
- Hertog MGL, Hollman PCH, Katan MB, Kromhout D. (1993). Intake of potentially anticarcinogenic flavonoids and their determinants in adults in the Netherlands. Nutr Cancer, 20, 21–29.
- Kaur K, Arora S, Kumar S, Nagpal A. (2002). Antimutagenic activities of acetone and methanol fractions of Terminalia arjuna. Food Chem Toxicol, 40, 1475–1482.
- Kaur S, Arora S, Kaur S, Kumar S. (2003). Bioassay-guided isolation of antimutagenic factors from fruits of Terminalia bellerica. J Environ Pathol Toxicol Oncol, 22, 69–76.
- Kaur S, Grover IS, Kumar S. (1997). Antimutagenic potential of ellagic acid isolated from Terminalia arjuna. Indian J Exp Biol, 35, 478–482.
- Lakshmi B, Ajith TA, Jose N, Janardhanan KK. (2006). Antimutagenic activity of methanolic extract of Ganoderma lucidum and its effect on hepatic damage caused by benzo[a]pyrene. J Ethnopharmacol, 107, 297–303.
- Lee KM. (2001). Overview of drug product development. Curr Protoc Pharmacol 7, 1–10.
- Loh DS, Er HM, Chen YS. (2009). Mutagenic and antimutagenic activities of aqueous and methanol extracts of Euphorbia hirta. J Ethnopharmacol, 126, 406–414.
- Lopes GK, Schulman HM, Hermes-Lima M. (1999). Polyphenol tannic acid inhibits hydroxyl radical formation from Fenton reaction by complexing ferrous ions. Biochim Biophys Acta, 1472, 142–152.
- Maron DM, Ames BN. (1983). Revised methods for the Salmonella mutagenicity test. Mutat Res, 113, 173–215.
- Matthew KM. (1991). An Excursion Flora of Central Tamilnadu, India. New Delhi, India: Oxford and IBH Publisher and Distributor.
- Mudaliar KSM. (2002). Siddha Materia Medica – Vol. II (Plant kingdom), Chennai: Department of Indian Medicine and Homeopathy, 843–844.
- Newman DJ, Cragg GM. (2007). Natural products as sources of new drugs over the last 25 years. J Nat Prod, 70, 461–477.
- Okuda T, Yoshida T, Hatano T. (1991). Chemistry and biological activities of tannins in medicinal plants. In: Wagner H, Farnsworth NR, (eds.). Economic and Medicinal Plants Research. London: Academic press, pp. 129–165.
- Oliveira HC, dos Santos MP, Grigulo R, Lima LL, Martins DT, Lima JC, Stoppiglia LF, Lopes CF, Kawashita NH. (2008). Antidiabetic activity of Vatairea macrocarpa extract in rats. J Ethnopharmacol, 115, 515–519.
- Pandikumar P, Chellappandian M, Mutheeswaran S, Ignacimuthu S. (2011). Consensus of local knowledge on medicinal plants among traditional healers in Mayiladumparai block of Theni District, Tamil Nadu, India. J Ethnopharmacol, 134, 354–362.
- Perera FP. (1996). Molecular epidemiology: Insights into cancer susceptibility, risk assessment, and prevention. J Natl Cancer Inst, 88, 496–509.
- Pezzuto JM, Kosmeder J, Park EJ, Lee SK, Cuendet M, Gills JJ, Bhat K, Grubjesic S, Park HS, Mata-Greenwood E, Tan Y, Yu R, Lantvit DD, Kinghorn AD. (2005). Characterization of natural product chemopreventive agents. Totowa: Humana Press, 2, 3–37.
- Rajan SA, ed. (1991). Agathiyar attavanai vaagadam. Tanjore, Tamilnadu, India: Saraswathi Mahal Library, 193–194.
- Ravikumar YS, Mahadevan KM, Manjunatha H, Satyanarayana ND. (2010). Antiproliferative, apoptotic and antimutagenic activity of isolated compounds from Polyalthia cerasoides seeds. Phytomedicine, 17, 513–518.
- Richard J, Pietras K, Olga, Weinberg. (2005). Antiangiogenic steroids in human cancer therapy. eCAM, 2, 49–57.
- Slusarczyk S, Hajnos M, Skalicka-Wozniak K, Matkowski A. (2009). Antioxidant activity of polyphenols from Lycopus lucidus Turcz. Food Chem, 113, 134–138.
- Stähelin H. (1973). Activity of a new glycosidic lignan derivative (VP 16-213) related to podophyllotoxin in experimental tumors. Eur J Cancer, 9, 215–221.
- Surh Y. (1999). Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic substances. Mutat Res, 428, 305–327.
- Titez NW. (1995). Clinical Guide to Laboratory Tests – 3rd edition. Philadelphia: WB Saunders.
- Tominaga S, Aoki K, Fujimoto I, Kurihara M. (1994). Cancer Mortality and Morbidity. Tokyo, Japan: Japan Scientific Society Press.
- Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. (1971). Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc, 93, 2325–2327.
- Warrier PK, Nambiar VP, Ramankutty C. (Eds.) (1995). Indian Medicinal Plants – A Compendium of 500 species. Chennai, India: Orient Longman Publishers, 374–378.
- Weisberger JH. (1999). Antimutagens and anticarcinogens and effective worldwide cancer prevention. J Environ Pathol Toxicol Onc, 18, 85–93.
- Williams SD, Birch R, Einhorn LH, Irwin L, Greco FA, Loehrer PJ. (1987). Treatment of disseminated germ-cell tumors with cisplatin, bleomycin, and either vinblastine or etoposide. N Engl J Med, 316, 1435–1440.
- Wogan GN, Hecht SS, Felton JS, Conney AH, Loeb LA. (2004). Environmental and chemical carcinogenesis. Semin Cancer Biol, 14, 473–486.
- Yong SP, Soon TJ, Seong GK, Buk GH, Patricia AA, Fernando T, Drzewiecki J, Namiesnik J, Gorinstein S, (2008). Antioxidants and proteins in ethylene-treated kiwi fruits. Food Chem, 107, 640–648.
- Zhu M, Phillipson JD, Greengrass PM, Bowery NE, Cai Y. (1997). Plant polyphenols: Biologically active compounds or non-selective binders to protein? Phytochemistry, 44, 441–447.