Abstract
Context: Chinese patent medicine Si-Mo-Tang oral liquid preparation (SMT) is composed of Aucklandia luppa Decne (Compositae), Citrus aurantium Linn (Rutaceae), Lindera aggregata (Sims) Kosterm (Lauraceae), and Areca catechu Linn (Arecaceae). Studies of SMT have been impeded due to the lack of quality control methods.
Objective: This study aimed to simultaneously determine three alkaloids including synephrine, arecoline, and norisoboldine in SMT for the first time.
Materials and methods: A strong cation exchange (SCX) high performance liquid chromatography (HPLC) method was developed to simultaneously determine synephrine, arecoline, and norisoboldine in SMT, and was compared with ion-pairing chromatography using regular reversed-phase chromatography columns. System suitability parameters of synephrine, arecoline, and norisoboldine using the SCX chromatography column were investigated.
Results: Results demonstrated that good separations were achieved on an Agilent SCX (250 × 4.6 mm, 5 µm) column at 35°C. The mobile phase consisting of methanol-0.2% phosphoric acid was delivered at a constant flow of 1.0 mL min−1 and the eluent was monitored at 215 nm. The HPLC method showed good linearity for the examined concentration ranges of 2.55–255.0, 1.30–208.0, and 2.06–201.6 µg mL−1 for synephrine, arecoline, and norisoboldine, respectively. The limits of quantification (S/N = 10) were 2.55, 1.30, and 2.06 µg mL−1, the limits of detection (S/N = 3) were 1.53, 0.78, and 1.21 µg mL−1, and average recoveries were 98.99, 95.63 and 99.04%, respectively, for synephrine, arecoline, and norisoboldine.
Discussion and conclusion: This method has been successfully applied to determine synephrine, arecoline, and norisoboldine in Chinese patent medicine SMT.
Introduction
Chinese patent medicine Si-Mo-Tang oral liquid preparation (SMT) is a prescription to clear out gastrointestinal dyspeptic disease and enhance the digestive function. This prescription is mainly composed of Aucklandia luppa Decne (Compositae), Citrus aurantium Linn (Rutaceae), Lindera aggregata (Sims) Kosterm (Lauraceae), and Areca catechu Linn (Arecaceae). The combination of the four drugs could work together to promote enterocinesia and soothe the swelling and pain (CitationZou et al., 1999). SMT has been mainly used for the treatment of lactivorous food retention in infants and abdominal flatulence in the elderly. It also can promote recovery of gastrointestinal functions after surgery in the abdomen and postpartum, enhance gastrointestinal peristalsis, promote secretion of digestive juice, and relieve gastrointestinal indigestion (CitationZou et al., 1999; CitationLiu et al., 2001).
A. luppas in the SMT contains costunolide and dehydrocostus lactone, which could stimulate large intestine excitation, strengthen the contraction, speed up the peristalsis, alleviate flatulence caused by abdominal distension, and thus promote gastric emptying and intestinal propulsion and exhibit antagonism against intestinal muscle spasms caused by acetylcholine and histamine (CitationZhu et al., 2000). C. aurantium and its major components synephrine and other alkaloids have certain excitatory effects on gastrointestinal smooth muscles mainly through the regulation of acetylcholine receptor, adrenergic receptor, and 5-HT receptor on cell membranes of the smooth muscles as well as direct effect on smooth muscle cells (CitationGuan, 2004). A. catechu and its major component arecoline can stimulate M-cholinergic receptors, increase gland and digestive juice secretion, and stimulate intestinal smooth muscles, which could cure food retention, abdominal distension, and constipation, etc. (CitationZhou et al., 2007; CitationNi et al., 2004; CitationZhang et al., 2010). L. aggregata plays a key role in the prescription. It not only enhances the pharmacological effects of other drugs, but also stimulates gastrointestinal smooth muscle and strengthens its contraction. It also accelerates blood circulation and promotes intestinal peristalsis (CitationGong & Zhu, 2008; CitationChou et al., 1999). L. aggregata contains the main active ingredients such as isoquinoline alkaloids, norisoboldine, laurolitsine, and reticuline, etc. (CitationChou et al., 2005). Of these, norisoboldine has been selected as a marker to assess the quality of L. aggregata in Chinese Pharmacopoeia 2010 Edition (CitationThe State Pharmacopoeia Commission of PR China, 2010a).
As part of the same project, the pharmacological results revealed that arecoline, synephrine, norisoboldine, and other alkaloids are the major active components in SMT (unpublished data). Thus, it is necessary to develop a simple, economical and effective method to determine the three main alkaloids in SMT for drug quality control. Liquid chromatography methods have been widely used in the analysis of Chinese herbal medicines, compound preparation, and biological specimens containing these ingredients. The methods for the determination of arecoline include high performance liquid chromatography (HPLC) (CitationHuang & McLeish, 1989), ion-pair reversed phase HPLC (CitationCox et al., 2004), high performance cation exchange chromatography (CitationChen et al., 2002), and liquid chromatography-mass spectrometry (LC-MS) (CitationDing & Peng, 2008). The major methods for the measurement of synephrine include capillary electrophoresis with electrochemical detection (CitationPeng & Ye, 2007; CitationAvulal & Khan, 2004; CitationChen et al., 2002), reverse phase HPLC (CitationHashimoto et al., 1992), flow injection chemiluminescence (CitationLi et al., 2006), and LC-MS (CitationPellegrini et al., 2007), etc. In addition, high performance liquid chromatography (HPLC) (CitationHan et al., 2009; CitationChen et al., 2009) and ultra-performance liquid chromatography-mass spectrometry (UPLC-MS) (CitationChen et al., 2011) methods have been developed for the determination of norisoboldine contained in L. aggregata and biological specimens. However, only a few HPLC methods have been developed for the determination of arecoline or synephrine in SMT (CitationYuan et al., 2009; CitationYao et al., 2009). In our previous studies, a combination method using HPLC fingerprint and multi-components analysis for quality consistency evaluation of SMT has been established for simultaneous determination of naringin, isonaringin, hesperidin, neohesperidin, norisoboldine, and potassium sorbate in SMT (CitationYi et al., 2011). However, due to the weak retention of arecoline and synephrine on regular reversed-phase columns, it was failed to determine arecoline and synephrine simultaneously.
In order to simultaneously determine arecoline, synephrine, and norisoboldine in SMT, a strong cation exchange column, which has specificity for alkaloids, was selected in this study for the separation of arecoline, synephrine, and norisoboldine in SMT based on the principle of high performance ion exchange chromatography. In addition, the method was compared with the one using the normal ODS reversed-phase column for the separation based on the principle of ion-pair reversed-phase HPLC.
Materials and methods
Chemicals and reagents
Synephrine (110727–200306) and arecoline hydrobromide (111684–200401) standards were provided by National Institute for the Control of Pharmaceutical and Biological Products, Beijing, China. Norisoboldine with purity >98% was provided by Shanghai Research Center of Standardization of Chinese Medicines, Shanghai, China. Phosphoric acid and methanol were of HPLC grade, and sodium dodecyl sulfate (SDS) and other reagents were of analytical grade. HPLC-grade water (>18 mΩ) was obtained from a Milli-Q water purification system (Bedford, MA). Twenty-six batches of SMT were provided by Hunan Hansen Pharmaceutical Co., Ltd. (Hunan, China) and Hunan Zhongda Wuma Pharmaceutical Co., Ltd. (Hunan, China).
Preparation of standard solutions
Standard stock solutions of arecoline hydrobromide (0.5 mg mL−1), synephrine (0.5 mg mL−1), and norisoboldine (0.4 mg mL−1) were prepared by dissolving accurately measured standards in methanol. The mixed standard working solutions containing 50 µg mL−1 arecoline hydrobromide, 50 µg mL−1 synephrine, and 40 µg mL−1 norisoboldine were obtained by sequential dilution of the mixture of the three standard stock solutions using methanol.
Preparation of sample solutions
Five bottles of SMT were mixed and filtered through 0.45 µm microporous membrane and the filtrate was collected as sample solution.
Instrumentation and chromatographic conditions
Instruments
An Agilent 1100 series LC system consisting of a G1312A binary pump, a G1322A degasser, a G1313A autosampler, a G1316A thermostatted column compartment, a G1315A DAD detector, and HP Chemstation Software (Agilent, Palo Alto) was used to achieve HPLC separation and data analysis. BP211D electronic analytical balance (Sartorius Co.) was also used.
Chromatographic conditions
Method 1: The separation was performed on an Agilent strong cation exchange (SCX) column (250 × 4.6 mm, 5 µm) at 35°C. The mobile phase consisting of methanol-0.17% phosphoric acid solution (2 mL of 85% phosphoric acid was diluted to 1000 mL with water and pH was adjusted to 3.8 using ammonia water) (65:35) was delivered at a flow rate of 1.0 mL min−1. Ten microliter of the sample solution was injected into the HPLC system and the eluent was monitored at 215 nm.
Method 2: The separation was performed on an Agilent ZORBAX SB-C18 column (250 × 4.6 mm, 5 µm) at 35°C. The mobile phase consisting of methanol-0.1% SDS (50:50) was delivered by a velocity gradient (0–25 min, 0.7 mL min−1; 25–43 min, 1.0 mL min−1; and 43–50 min, 0.7 mL min−1). Twenty microliter of the sample solution was injected into the HPLC system and the eluent was monitored at 230 nm.
Results and discussion
Chromatographic conditions and system suitability test
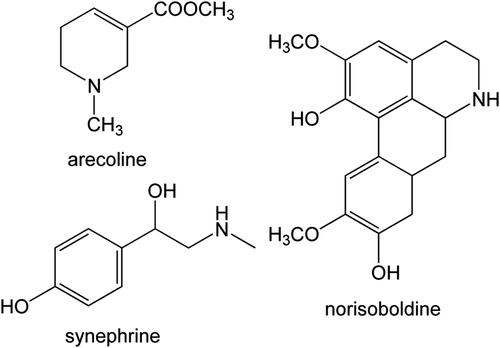
The structures of arecoline, synephrine, norisoboldine are shown in . Arecoline and synephrine belong to low molecular alkaloids with high polarity, which exhibit weak retention on regular reversed-phase columns and need the addition of ion-pairing reagents to the mobile phase to improve their retention (CitationCox et al., 2004; CitationChen at al., 2002; CitationHashimoto et al., 1992). Considering that the general application pH ranges for the reversed-phase columns are 2–8, in order to simultaneously determine arecoline, synephrine, and norisoboldine in SMT, the effect of pH values of the mobile phase on the retention of arecoline and synephrine was investigated. When the pH value of mobile phase was low, the peak came out very early, even without retention. Thus an Agilent SCX strong cation exchange column, which is specific for alkaloid separation, was selected to simultaneously determine arecoline, synephrine, and norisoboldine in SMT. As for the mobile phase, it was found that the reported mobile phase used to determine arecoline content from A. catechu (CitationThe State Pharmacopoeia Commission of PR China, 2010b) was suitable for the separation of arecoline, synephrine, and norisoboldine from SMT with some modifications on the composition of the mobile phase, but without modification on pH value. The chromatograms of arecoline, synephrine, and norisoboldine in SMT on the Agilent ZORBAX SB-C18 column and the Agilent SCX strong cation exchange column are shown in and , respectively. shows the chromatographic parameters including resolution, theoretical plate number, symmetry factor, and peak purity of arecoline, synephrine, and norisoboldine in SMT on SCX and ZORBAX SB-C18 columns, respectively.
Table 1. Chromatographic parameters of resolution, theoretical plate number, symmetry factor, and peak purity of arecoline, synephrine, and norisoboldine in SMT on SCX and ZORBAX SB-C18 columns.
Figure 2. HPLC chromatograms of reference standards (A) and SMT sample (B) separated on an Agilent ZORBAX SB-C18 column (250 × 4.6 mm, 5 µm) (1: synephrine; 2: arecoline; 3: norisoboldine).
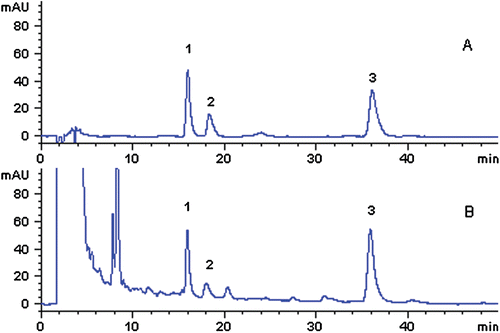
Figure 3. HPLC chromatograms of reference standards (A) and SMT sample (B) separated on an Agilent SCX column (250 × 4.6 mm, 5 µm) (1: synephrine; 2: norisoboldine; 3: arecoline).
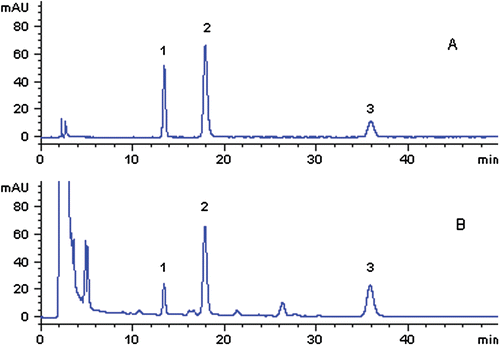
exhibited an acceptable separation of synephrine, arecoline and norisoboldine in SMT on an Agilent ZORBAX SB-C18 column with SDS being added to the mobile phase. When gradient elution was performed by changing the ratio of the mobile phase, baseline drift was serious and background interference was changed with the ratio of the mobile phase, resulting in unacceptable separation effects. Thus, in order to obtain stable baseline and good separation, velocity gradient elution with fixed mobile phase consisting of methanol-0.1% SDS = 50:50 was used.
These three alkaloids exhibited different retention times on the two different types of columns. On the SCX column, the retention times for synephrine, norisoboldine, and arecoline were 13.57, 18.46, and 35.96 min, respectively, while on the ZORBAX SB-C18 column, the retention times for synephrine, arecoline and norisoboldine were 15.90, 17.95, and 35.85 min, respectively, i.e., the retention times of norisoboldine and arecoline were reversed. Symmetry factors of these three alkaloids were between 0.64–0.74 on the ZORBAX SB-C18 column and 0.86–0.93 on the SCX column. The number of theoretical plates for synephrine and arecoline was improved on the SCX column compared with the ZORBAX SB-C18 column, which could improve the detection sensitivity. However, the number of theoretical plates was decreased for norisoboldine on the SCX column. The peak purity test indicated that the peak purity factors of those three alkaloids were all higher than their threshold limits separated on the SCX column, but not on the ZORBAX SB-C18 column ().
The maximum UV absorption wavelength was 215 nm for arecoline, and 283 nm for synephrine and norisoboldine. However, synephrine and norisoboldine also had some absorption at 215 nm. In method 2, arecoline and synephrine exhibited weak retention on the ODS column without the addition of SDS in the mobile phase, which could not meet the requirement of detection. However, due to the addition of SDS, the UV background of the mobile phase was increased and the interference at 215 nm was also increased, although the retention times of arecoline and synephrine were increased. With increased detection wavelength, baseline interference was gradually reduced, but the sensitivity of detection was also reduced. Thus 215 nm and 230 nm were selected as the detection wavelength for method 1 and 2, respectively, for the simultaneous detection of synephrine, arecoline and norisoboldine.
The results of system suitability tests showed that the SCX column was more suitable for the separation and detection of synephrine, norisoboldine, and arecoline in SMT than the ZORBAX SB-C18 column. Thus, the Agilent SCX column was selected for the determination of synephrine, arecoline and norisoboldine in SMT.
Robustness evaluation
Robustness test was mainly to examine the tolerability of the method itself to variable experimental conditions, i.e., the effects of column temperature and flow rate of the mobile phase on the separation of the chromatographic peaks.
Effect of column temperature
SMT sample solutions were analyzed at 30, 35 and 40°C, respectively, and the chromatograms were recorded. The column temperature between 30–40°C exhibited certain effect on the retention times of the peaks, but not on the separation of the peaks. The separation time was shortest when the column temperature was 40°C. Thus, a column temperature between 30–40°C could be selected.
Effect of flow rate
SMT sample solutions were analyzed at different flow rates of 0.8, 1.0, and 1.2 mL min−1, respectively, and the chromatograms were recorded. The results showed that the flow rates exhibited little effect on peak separation, but had some effects on the retention of the peaks. The separation time was short when the flow rate was high. Thus, a flow rate of 0.8–1.2 mL min−1 could be selected.
Method validation
Standard curves and linearity
Standards stock solutions of synephrine, arecoline hydrobromide, and norisoboldine were mixed and diluted with methanol to get standard working solutions with a serial concentrations of synephrine (2.55, 5.10, 25.5, 51.0, 102.0, 153.0, and 255.0 µg mL−1), arecoline hydrobromide (1.30, 2.60, 13.0, 26.0, 52.0, 154.0, and 208.0 µg mL−1), and norisoboldine (2.02, 4.03, 20.16, 40.32, 80.64, 120.96, and 201.6 µg mL−1). Ten microliter of these standard working solutions were injected into the HPLC system and analyzed under conditions as described in method 1.
Linear regression of chromatographic peak areas of synephrine, arecoline hydrobromide, and norisoboldine and corresponding concentrations was performed, resulting in regression equations of standard curves. The standard curves for synephrine, arecoline hydrobromide, and norisoboldine were y = 18027X + 12.397, r = 0.9999; y = 20543X + 10.220, r = 0.9999; and y = 46596X −1.189, r = 1, respectively. Good linearity was observed for synephrine, arecoline hydrobromide, and norisoboldine within the ranges of 2.55–255.0, 1.30–208.0, and 2.02–201.60 µg mL−1, respectively.
Limit of detection and limit of quantification
One microliter of the mixed standard working solution of synephrine, arecoline hydrobromide, and norisoboldine was diluted with methanol to different concentrations desired. Ten microliter was injected into the HPLC system. Limits of quantitation for synephrine, norisoboldine, and arecoline hydrobromide were 2.55, 2.02, and 1.30 µg mL−1, respectively with S/N = 10, whereas limits of detection for synephrine, norisoboldine, and arecoline hydrobromide were 1.53, 1.21, and 0.78 µg mL−1, respectively, with S/N = 3.
Precision
Mixed standard solutions of synephrine (25.5, 51.0, 102.0 µg mL−1), norisoboldine (20.16, 40.32, 80.64 µg mL−1), and arecoline hydrobromide (13.0, 26.0, 52.0 µg mL−1) at three concentrations were injected repeatedly for six times, with 10 µL each time, and the peak areas were recorded. Intra-day RSD% values at three concentrations were 0.20, 0.16, and 0.26 for synephrine, 0.22, 0.54, and 0.34 for norisoboldine, and 0.33, 1.03 and 1.14 for arecoline hydrobromide, respectively. Inter-day RSD% values obtained by continuous injection for three days were 1.00, 1.56 and 1.38 for synephrine, 1.27, 1.58, 1.56 for norisoboldine, and 0.81, 1.63, and 1.68 for arecoline hydrobromide at three concentrations. The intra-day and inter-day RSD values of synephrine, arecoline hydrobromide, and norisoboldine were all less than 2%, which met the requirements of sample analysis (CitationICH, 2005).
Sample stability
SMT sample was processed according to the above method and 10 µL sample solution was injected at 0, 2, 4, 8, 12, 24, 48 h. Peak areas were recorded and the stability of synephrine, arecoline, and norisoboldine was assessed by comparing the peak areas. The results showed that RSD% of the peak areas of synephrine, arecoline, and norisoboldine were 1.57, 0.53, and 1.95, respectively. Thus, these three components in SMT were stable within 48 h.
Repeatability
Six SMT sample solutions were prepared according to the above methods and 10 µL of each was injected into the HPLC system, with peak areas recorded. The RSD% of the peak areas of synephrine, arecoline, and norisoboldine were 1.60, 1.04, and 0.85%, respectively. Thus, this method exhibited good repeatability.
Accuracy and recovery
SMT (2.5 mL) (Lot # 090436), of which the contents of synephrine, arecoline, and norisoboldine were accurately determined, was put into 5 mL volumetric flasks, followed by the addition of standard stock solutions containing 80, 100, 120% of each component. Methanol was added to make the total volume of 5 mL and the solution was mixed, filtered and the filtrate was injected into the HPLC system. The accuracy and recovery were calculated. The average recoveries for synephrine, arecoline and norisoboldine were 98.99, 95.63 and 99.04% with RSD of 2.64, 1.07, and 1.51%, respectively ().
Table 2. Accuracy and recovery of synephrine, arecoline and norisoboldine at different concentrations (n = 3).
Sample determinations
Five bottles of SMT were mixed and filtered through 0.45 µm membrane. The filtrate (10 µL) and 10 µL of the standard solutions were injected into the HPLC system. The average contents of synephrine, arecoline, and norisoboldine in 26 batches of SMT samples from two manufactures were analyzed using one-point external method. The average contents of synephrine, norisoboldine, and arecoline were 0.024 ± 0.005, 0.041 ± 0.009, and 0.029 ± 0.007 mg mL−1, respectively (Calculated from dada in ).
Table 3. Determination of synephrine, arecoline and norisoboldine from 26 batches of SMT (n = 3).
Conclusion
Based on system suitability study, cation exchange high performance chromatography resulted in improved peak separation, symmetry, and theoretical plate number, and could detect synephrine, arecoline, and norisoboldine at low UV region (215 nm), resulting in improved sensitivity. The system robustness test and method validation demonstrated that the established method could simultaneously determine synephrine, arecoline, and norisoboldine in SMT.
Declaration of interest
This work was partially supported by the grants from National Basic Research Program of China (2009CB523002), National Action of Technology Personnel Servicing Enterprise Program of China (2009FJ5049, 2009GJD20014), Foundation of Hunan Science and Technology Committee (2009XK6032, 2009-152), Foundation of Hunan Educational Committee (09CY001).
References
- Avulal B, Khan IA (2004). Separation and determination of ephedrine enantiomers and synephrine by high performance capillary electrophoresis in dietary supplements. Chromatographia, 59, 71–77.
- Chen G, Zhang L, Zhao J, Ye J. (2002). Determination of hesperidin and synephrine in Pericarpium Citri Reticulatae by capillary electrophoresis with electrochemical detection. Anal Bioanal Chem, 373, 169–173.
- Chen JA, Lai YH, Wang XY, Yang WR (2002). High-performance cation-exchange chromatographic determination of the alkaloids in betel nut. J Chin Med Mater, 25, 27–28.
- Chen J, Chou G, Yang L, Wang C, Wang Z. (2009). Determination of norisoboldine in Radix Lindera by RP-HPLC. Zhongguo Zhong Yao Za Zhi, 34, 2774–2776.
- Chen JZ, Xu Y, Chou GX, Wang CH, Yang L, Wang ZT. (2011). Simultaneous determination of norisoboldine and its major metabolite in rat plasma by ultra-performance liquid chromatography-mass spectrometry and its application in a pharmacokinetic study. Biomed Chromatogr, 25, 367–372.
- Chou GX, Li QL, Wang ZT, Xu GJ, Dou CG, Qi YQ (1999). Effect on digestive system of Radix Linderae extract. Chin Wild Plant Resour, 18, 52–53,57.
- Chou GX, Norio N, Ma CM, Wang ZT, Masao H (2005). Isoquinoline alkaloids from Lindera aggregata. Chin J Nat Med, 3, 272–275.
- Cox S, Piatkov I, Vickers ER, Ma G. (2004). High-performance liquid chromatographic determination of arecoline in human saliva. J Chromatogr A, 1032, 93–95.
- Ding FL, Peng SL (2008). Determination of arecoline from areca nut by high performance liquid chromatography/mass spectrometry. Acad Period Farm Prod Proc, 4, 76–79.
- Gong SX, Zhu JP (2008). Clinic effect observation of Radix Linderae promoting gastrointestinal motility. Zhejiang Clin Med J, 10, 323.
- Guan FL (2004). The effect of hesperidin and synephrine on the isolated hastric smooth muscle cells. Chin Pharmacol Bull, 20, 1420–1425.
- Han Z, Su H, Chen N, Luan L, Wu Y. (2009). Simultaneous determination of four alkaloids in Lindera aggregate by high performance liquid chromatography. Zhongguo Zhong Yao Za Zhi, 34, 583–586.
- Hashimoto K, Yasuda T, Ohsawa K (1992). Determination of synephrine from Chinese medicinal drugs originating from Citrus species by ion-pair high-performance liquid chromatography. J Chromatogr A, 623, 386–389.
- Huang JL, McLeish MJ (1989). High-performance liquid chromatographic determination of the alkaloids in betel nut. J Chromatogr A, 475, 447–450.
- ICH (2005): Q2 (R1) Validation of Analytical Procedures: Text and Methodology, International Conference on Har-monization, Geneva, 1–13.
- Li Q, Huang C, Huang Y. (2006). Sensitive determination of synephrine by flow-injection chemiluminescence. Luminescence, 21, 43–48.
- Liu WQ, Wang J (2001). Clinical research of Simo Tang oral Liquid on functional dyspepsia and gastrointestinal dynamia. J Tianjin Coll Tradit Chin Med, 20, 9.
- Ni YD, Wang JH, Wang RJ (2004). Comparative study of semen Arecae and arecoline on gastrointestinal function. Pharmacol Clin Chin Mat Med, 20, 11–12.
- Pellegrini M, Marchei E, Rossi S, Vagnarelli F, Durgbanshi A, García-Algar O, Vall O, Pichini S. (2007). Liquid chromatography/electrospray ionization tandem mass spectrometry assay for determination of nicotine and metabolites, caffeine and arecoline in breast milk. Rapid Commun Mass Spectrom, 21, 2693–2703.
- Peng YY, Ye JN (2007). Determination of flavonoids and synephrine in fructus anrantii immaturus and fructus aurantii by capillary electrophoresis with electrochemical detection. J Instr Anal, 26, 694–697.
- The State Pharmacopoeia Commission of PR China (2010a). Pharmacopoeia of PR China, 2010 Edition, Beijing, 71.
- The State Pharmacopoeia Commission of PR China (2010b). Pharmacopoeia of PR China, 2010 Edition, Beijing, 342.
- Yao Y, He GX, Pei G, Guo JS, Yuan Y (2009). Determination of arecoline in Simo Tang oral solution with HPLC. J Tradit Chin Med Univ Hunan, 29, 27–29.
- Yi YN, Cheng XM, Liu LN, Hu GY, Cai GX, Deng YD, Huang KL, Wang CH (2011). Combinative method using HPLC fingerprint and multi-components analysis for quality consistency evaluation of a traditional Chinese patent medicine: Si-Mo-Tang oral liquid preparation. Chem Res Chin Univ, 27, 756–763.
- Yuan Y, Pei G, Guo JS, He GX, Yao Y, Jin TF, Chen YM (2009). Quantitation determination of synephrine in Simo Tang oral liquid by HPLC. Chin J Med Guide, 11, 165–166.
- Zhang XH, Gao F, Yan X, Cheng M, Liu C. (2010). Traditional Chinese medicine monomer arecoline promotes gastric motility in diabetic gastroparesis rats. World Chin J Diges, 18, 975–980.
- Zhou XZ, Zhang JY, Zhao CY, Li JS, Zhang ZF, Li JY, Wei XJ, Niu JR, Wang L (2007). The study of arecoline hydrobromide’s time-effect and dose-effect relationship on propulsive ratio of mice’s small intestine. Chin J Vet Drug, 41, 28–30.
- Zhu JZ, Leng ER, Chen DF (2000). Investigation on mechanism and effect of Radix Aucklandiae against gastrointestinal motility. Chin J Interg Tradit West Med Gastro-Spleen, 8, 236.
- Zou YX, Cheng ZY, Wu XC (1999). Experimental study of Simo Tang oral liquid on gastrointestinal functional influence. Hunan Guid J TCMP, 5, 36–38.