Abstract
Context: The red algae Gelidium crinale (Turner) Gaillon (Gelidiaceae), encountered along the Southeast and Northeast Brazilian sea coast, contains a sulfated galactan presenting a similar saccharide backbone compared to λ carrageenan. Inflammatory effects of other galactans were reported, but not for that obtained from G. crinale (SG-Gc).
Objective: To investigate the in vivo edematogenic effect of SG-Gc in comparison to λ carrageenan.
Methods: SG-Gc was isolated by ion exchange chromatography. Paw edema was induced by subcutaneous (s.c.) intraplantar injection of SG-Gc or λ carrageenan and evaluated by hydroplethysmometry. Data were expressed as the increase in paw volume subtracted from the basal volume or area under curve-AUC. To investigate the participation of early and late-phase inflammatory mediators, rats were treated with pyrilamine, compound 48/80, indomethacin, NG-nitro-l-arginine methyl ester (l-NAME), or pentoxifylline before SG-Gc.
Results: SG-Gc edematogenic effect was initiated at 0.5 h, peaked at 2 h (1.26 ± 0.05 mL) and lasted until 6 h (0.21 ± 0.03 mL), whereas the carrageenan-induced edema started at 1 h. The first phase (1–3 h) of SG-Gc-induced edema was 176 ± 15 (AUC) versus carrageenan (114.5 ± 14), whereas the second phase (3–5 h) was 95 ± 12 (AUC) versus carrageenan (117.5 ± 11). Treatment with compound 48/80, pyrilamine, indomethacin, L-NAME, and pentoxifylline inhibited the effect of SG-Gc by 32, 40, 69, 72, and 49%, respectively.
Discussion and conclusion: SG-Gc and λ carrageenan induce different profile of inflammatory response in the paw edema model, that involves histamine, cytokines, prostaglandins, and nitric oxide (NO), but with different degree of participation.
Keywords::
Introduction
Marine algae are organisms that contain a large number of sulfated polysaccharides (SP) with biological properties already explored. The literature has established an association between anti-inflammatory effects of fucans (CitationAruffo et al., 1991; CitationWeston & Parish, 1991; CitationBlondin et al., 1994; CitationTissot et al., 2003; CitationMedeiros et al., 2008; CitationSiqueira et al., 2011), the main representative sugar of brown algae (Phaeophyta), and proinflammatory/immunostimulant effects of galactans, the main representative sugar of red algae (Rodophyta). In this line, the sulfated polysaccharide obtained from the red seaweed Champia feldmannii Diaz-Pifferer (Lomentariaceae) stimulated leukocyte migration and paw edema paralleled with increase in vascular permeability in rats (CitationAssreuy et al., 2008) and antitumor effect in mice (CitationLins et al., 2009). The sulfated polysaccharide of the red seaweed Solieria filiformis Farias (Solieriaceae) also induced paw edema in rats (CitationAssreuy et al., 2010).
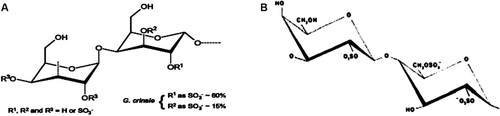
Carrageenans are the most common sulfated galactans from macroalgae, extensively investigated due to their inflammatory effects (CitationDi Rosa et al., 1971; CitationDamas, 1980; CitationPomin & Mourão, 2008). Among the main commercialized carrageenans (κ, ι, and λ), κ and ι form gelling polymers, whereas λ is a nongelling due to its content of three sulfate groups (2-sulfate in 3-β-d-Galp and 2,6-dissulfate in α-d-Galp) without the presence of 3,6-anhydro in α-galactopyranose (CitationMcCandless, 1981; CitationPerez et al., 1992) (). Alga of the Gelidium genus is excellent source of agar (CitationMurano et al., 1998). The red algae Gelidium crinale (Turner) Gaillon (Gelidiaceae) possesses wide distribution in tropical temperate waters (CitationSantelices, 1991) and is encountered in abundance along the Southeast and Northeast Brazilian sea coast. It contains an isolated sulfated galactan that presents a linear polysaccharide of alternating α-1→3 and 1→4-galactopyranose units with a variable sulfation pattern in which the 2,3-disulfated residues occur as ~15% of total galactan (). Similar to λ carrageenan, there is no evidence of 3,6-anhydro in α-galactopyranose (CitationPereira et al., 2005). The galactan of G. crinale was shown to present anticoagulant effect, as it inhibits thrombin and factor Xa by antithrombin and heparin cofactor II. It also presents antithrombotic effect via inhibition of venous and arterial thrombosis in rats (CitationPereira et al., 2005; CitationFonseca et al., 2008). However, its inflammatory role was not described yet.
Figure 1. Structures of the sulfated galactan of Gelidium crinale and λ carrageenan. (A)α- and β-galactopyranose with variable sulfatation pattern (β-D-galactopyranose-2-sulfate-O-α-D-galactopyranose-2,3 sulfate) (Source: Adapted from CitationPereira et al., 2005). (B) β-D-galactopyranose-2-sulfate-O-α-D-galactopyranose-2,6-sulfate (λ carrageenan) (CitationPerez et al., 1992).
Thus, the present study evaluated the edematogenic effect of the sulfated galactan from G. crinale (SG-Gc) in comparison to λ carrageenan and the participation of early and late phase inflammatory mediators.
Materials and methods
Animals
A total of 78 male Wistar rats, divided into 13 groups of 6 animals each (200–250 g), were maintained in a controlled environment (circadian cycle, 25°C, food and water ad libitum). Experiments were conducted in accordance with current guiding principles for the care and use of research animals (NIH guidelines) and approved by the Ethical Committee of the Estate University of Ceará, Brazil (UECE, under No. 10130208-8/40).
Isolation of SG-Gc
G. crinale was collected at Pacheco beach, Caucaia-CE, Brazil, and the voucher specimen (nº 35.579) deposited in the Prisco Bezerra Herbarium-Federal University of Ceará. SG-Gc was extracted and purified by ion exchange chromatography in diethylaminoethyl-cellulose (DEAE-cellulose) column (CitationPereira et al., 2005).
Evaluation of SG-Gc edematogenic effect and participation of inflammatory mediators
Paw edema was induced by subcutaneous (s.c.) intraplantar injection of SG-Gc (0.1, 0.3, 1 mg/kg) or carrageenan (300 μg/paw) and measured by a plethysmometer (LE 7500, PanLab, Barcelona, Spain) from 0.5–8 h. The control group received sterile saline. Data were expressed as the increase in paw volume (mL) displacement along the experiment subtracted from the basal volume, measured at zero time, or area under curve (AUC; arbitrary units) (CitationLanducci et al., 1995).
In order to investigate the participation of initial-phase inflammatory mediators, paw edema was induced by SG-Gc 30 min after intraperitoneal (i.p.) injection of pyrilamine (10 mg/kg), an antagonist of histamine H1 (CitationAshmawi et al., 2003), or after the 4-day i.p. treatment with compound 48/80 (0.6 mg/kg on Days 1–3 and 1.2 mg/kg on Day 4), a mast cell degranulator (CitationOhta et al., 2003). For the involvement of late-phase mediators, indomethacin (5 mg/kg; s.c.), inhibitor of the enzyme cyclooxygenase (CitationKankuri et al., 2001), pentoxifylline (90 mg/kg; s.c.), inhibitor of the enzyme phosphodiesterase (CitationVircheva et al., 2010) or NG-nitro-l-arginine methyl ester (l-NAME) (25 mg/kg; i.v.), inhibitor of the enzyme NOS (CitationMoncada et al., 1991), were administered 1 h before edema induction with SG-Gc.
All drugs were dissolved in sterile saline (NaCl 0.9%), except for indomethacin, that was initially dissolved in dimethylsulfoxide (DMSO) up to 10% of total volume and then in saline. Pyrilamine, compound 48/80, L-NAME, pentoxifylline, DEAE-cellulose, and λ carrageenan were purchased from Sigma Chemical Company, St. Louis, MO, US and indomethacin (Indocid®) from Merck Sharp & Dohme, Northumberland, UK.
Statistical analysis
Data are presented as mean ± SEM of six animals per group and differences analyzed using ANOVA followed by Bonferroni’s test. Statistical significance was set at p < 0.05.
Results
SG-Gc presents edematogenic activity
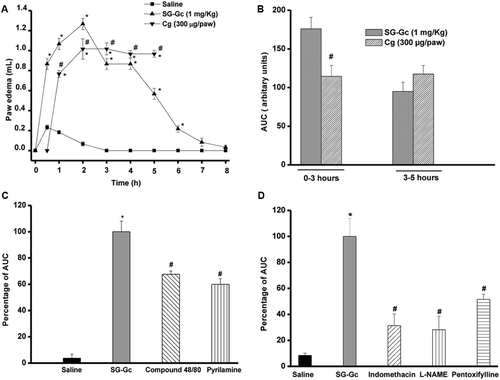
SG-Gc (1 mg/kg) elicited significant and dose-dependent paw edema, initiated at 30 min with a peak at 2 h (1.26 ± 0.05 mL versus saline: 0.06 ± 0.02 mL) and lasted until 6 h (0.21 ± 0.03 mL) compared to saline, that attained basal values (). At 0.1 mg/kg, SG-Gc was shown to be ineffective, being the response similar to saline. However, at 0.3 mg/kg SG-Gc induced paw edema with maximal effect at 30 min (0.5 ± 0.02 mL versus saline: 0.23 ± 0.02 mL) (data not shown in the figure). Conversely, carrageenan (300 μg/paw) induced paw edema, that was initiated only at 1 h, although with peak at 2 h (1.01 ± 0.10 mL versus saline: 0.46 ± 0.05 mL), and maintained until 5–6 h. The first phase (1–3 h) of the edema elicited by SG-Gc was 176 ± 15 (AUC) versus carrageenan (114.5 ± 14), whereas the second phase (3–5 h) was 95 ± 12 (AUC) versus carrageenan (117.5 ± 11) ().
Figure 2. Edematogenic effect of SG-Gc. Paw edema was induced by SG-Gc (1 mg/kg, s.c) (A–D)or (A, B) λ carrageenan (300 μg/paw) and measured at zero time and from 0.5–8 h by a plethysmometer. (C) Pyrilamine (10 mg/kg) or compound 48/80 (0.6 mg/kg on Days 1–3 and 1.2 mg/kg on Day 4) were injected i.p. 30 min before the injection of SG-Gc (1 mg/kg). (D) Indomethacin (5 mg/kg; ), L-NAME (25 mg/kg; i.v.) or pentoxifylline (90 mg/kg; ) were injected 1 h before SG-Gc injection (1 mg/kg). Mean ± SEM (n = 6). ANOVA and Bonferroni’s test. *p < 0.05 compared to saline and #p < 0.05 to SG-Gc.
Involvement of inflammatory mediators in the SG-Gc edematogenic activity
In the investigation of initial-phase mediators involved in the SG-Gc edematogenic effect (100 ± 8.04 AUC), the treatment of animals with compound 48/80 and pyrilamine showed inhibitory effects by 32 and 40%, respectively. In addition, the treatment with blockers of the late phase mediators indomethacin, l-NAME, and pentoxifylline reduced the SG-Gc-induced edema in 69, 72, and 49%, respectively.
Discussion
In general, polysaccharides of marine algae appear polydisperse in the gel permeation chromatography (GPC) and do not possess precise molecular masses. However, carrageenans (λ, κ, and ι), that present similar chemical structures compared to most polysaccharides of red alga, show molecular mass varying from 300–600 KDa (CitationStephen et al., 1995). In this study, the galactan isolated from the red marine alga G. crinale presented inflammatory activity in the experimental model of rat paw edema. Inflammatory effects of SP of red algae have already been demonstrated (CitationDi Rosa et al., 1971; CitationDamas, 1980; CitationPomin & Mourão, 2008; CitationAssreuy et al., 2008, Citation2010; CitationLins et al., 2009). The inflammatory effect of λ carrageenan, already demonstrated in literature, show different profile of response compared to SG-Gc. While carrageenan elicits paw edema, that is initiated after 1 h, that of SG-Gc starts earlier (at 30 min). Besides, SG-Gc induces a more effective paw edema compared to carrageenan in the first phase (0–3 h) of the time course. On the contrary, the late phase (3–5 h) response of both SG-Gc and carrageenan are similar in intensity. According to Di Rosa and collaborators (1971), inflammatory mediators of mast cell origin, such as histamine, serotonin, and nitric oxide (NO) formed by the neuronal isoform of nitric oxide sinthase (nNOS) play important role in the initial phase of carrageenan-induced edema. However, the late phase involves neutrophil infiltration and is sustained by the production of prostaglandin E2, cytokines (in particular, interleukin-1β [IL-1β]), and NO formed by the inducible NOS (iNOS) (CitationVinegar et al., 1969; CitationWedmore & Williams, 1981; CitationSalvemini et al., 1996).
The present study suggests that these inflammatory mediators are also involved in the edema elicited by SG-Gc, but probably at different degree of participation. In this line, pretreatment of animals with pyrilamine, an antagonist of histamine receptors (CitationAshmawi et al., 2003) prevented either the edematogenic effect of SG-Gc and carrageenan, suggesting direct activation of histamine receptors by these galactans. However, pretreatment of animals with the compound 48/80 prevented the edematogenic effect of SG-Gc but not that of carrageenan. The compound 48/80 is known to induce histamine and serotonin release from mast cells (CitationOhta et al., 2003). The same results were reported by CitationStochla and Maslinski (1982), pointing that SG-Gc, but not carrageenan induces histamine release, via mast cell activation, that precedes activation of histamine receptors. Moreover, the involvement of late phase mediators in the two types of edema were shown to be differentiated, according to several evidences using classical protocols of pharmacological blockage: (1) indomethacin, nonspecific inhibitor of the enzyme (cyclooxygenase) responsible for prostaglandins production (CitationKankuri et al., 2001), inhibited the edematogenic effect of SG-Gc by 69% () and that of carrageenan by 44% (CitationGarcia Leme et al., 1973); (2) l-NAME, nonspecific inhibitor of the enzyme NOS responsible for NO production (CitationMoncada et al., 1991), inhibited the edematogenic effect of SG-Gc by 72% () and that of carrageenan by 25% (CitationSalvemini et al., 1996); (3) pentoxifylline, inhibitor of the enzyme phosphodiesterase responsible for synthesis of the inflammatory cytokines tumor necrosis factor-α (TNF-α) and IL-1 inhibited the edematogenic effect of SG-Gc by 49% () and that of carrageenan by 40% (CitationVircheva et al., 2010).
Based in these data altogether and considering the chemical structures of SG-Gc and λ-carragenan that present similar saccharide backbones, but differ in the positions of the sulfated α-galactose units (CitationMcCandless, 1981; CitationPerez et al., 1992), it is possible to speculate that the observed differences in the edematogenic effects are associated to changes in the positions of sulfate groups. This association had already been described for the anticoagulant activity of other sulfated galactans (CitationFarias et al., 2000), but also for that obtained from G. crinale (CitationPereira et al., 2005). Thus, the present study suggests that SG-Gc may be used as an important tool in the investigation of initial-phase inflammatory processes.
In conclusion, this study demonstrated that SG-Gc presents inflammatory activity in the experimental model of rat paw edema with different profile of response compared to λ carrageenan. The mechanism of SG-Gc edematogenic effect seems to involve histamine, cytokines, prostaglandins, and NO.
Acknowledgments
The authors thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq, Fundação Cearense de Amparo a Pesquisa-FUNCAP and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-CAPES. The authors also thank Gabriela Fernandes Oliveira Marques Domingos for technical assistance. Assreuy and Pereira are senior investigators of CNPq and FUNCAP, respectively.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aruffo A, Kolanus W, Walz G, Fredman P, Seed B. (1991). CD62/P-selectin recognition of myeloid and tumor cell sulfatides. Cell, 67, 35–44.
- Ashmawi HA, Chambergo FS, Araújo Palmeira CC, de PaulaPosso I. (2003). The effects of pyrilamine and cimetidine on mRNA C-fos expression and nociceptive flinching behavior in rats. Anesth Analg, 97, 541–6, table of contents.
- Assreuy AM, Gomes DM, da Silva MS, Torres VM, Siqueira RC, Pires Ade F, Criddle DN, de Alencar NM, Cavada BS, Sampaio AH, Farias WR. (2008). Biological effects of a sulfated-polysaccharide isolated from the marine red algae Champia feldmannii. Biol Pharm Bull, 31, 691–695.
- Assreuy AM, Pontes GC, Rodrigues NV, Gomes DM, Xavier PA, Araujo GS, Sampaio AH, Cavada BS, Pereira MG, Farias WR. (2010). Vascular effects of a sulfated polysaccharide from the red marine alga Solieria filiformis. Nat Prod Commun, 5, 1267–1272.
- Assreuy AM, Pinto NV, Lima Mota MR, Passos Meireles AV, Cajazeiras JB, Nobre CB, Soares PM, Cavada BS. (2011). Vascular smooth muscle relaxation by a lectin from Pisum arvense: Evidences of endothelial NOS pathway. Protein Pept Lett, 18, 1107–1111.
- Blondin C, Fischer E, Boisson-Vidal C, Kazatchkine MD, Jozefonvicz J. (1994). Inhibition of complement activation by natural sulfated polysaccharides (fucans) from brown seaweed. Mol Immunol, 31, 247–253.
- Damas J. (1980). [Kinins-formation, hypotensive and oedematogen activities of four carrageenans (author’s transl)]. Pathol Biol, 28, 287–292.
- Di Rosa M, Giroud JP, Willoughby DA. (1971). Studies on the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J Pathol, 104, 15–29.
- Farias WR, Valente AP, Pereira MS, Mourão PA. (2000). Structure and anticoagulant activity of sulfated galactans. Isolation of a unique sulfated galactan from the red algae Botryocladia occidentalis and comparison of its anticoagulant action with that of sulfated galactans from invertebrates. J Biol Chem, 275, 29299–29307.
- Fonseca RJ, Oliveira SN, Melo FR, Pereira MG, Benevides NM, Mourão PA. (2008). Slight differences in sulfation of algal galactans account for differences in their anticoagulant and venous antithrombotic activities. Thromb Haemost, 99, 539–545.
- Garcia Leme J, Hamamura L, Leite MP, Rocha e Silva M. (1973). Pharmacological analysis of the acute inflammatory process induced in the rat’s paw by local injection of carrageenin and by heating. Br J Pharmacol, 48, 88–96.
- Kankuri E, Vaali K, Korpela R, Paakkari I, Vapaatalo H, Moilanen E. (2001). Effects of a COX-2 preferential agent nimesulide on TNBS-induced acute inflammation in the gut. Inflammation, 25, 301–310.
- Landucci EC, Antunes E, Donato JL, Faro R, Hyslop S, Marangoni S, Oliveira B, Cirino G, de Nucci G. (1995). Inhibition of carrageenin-induced rat paw oedema by crotapotin, a polypeptide complexed with phospholipase A2. Br J Pharmacol, 114, 578–583.
- Lins KO, Bezerra DP, Alves AP, Alencar NM, Lima MW, Torres VM, Farias WR, Pessoa C, de Moraes MO, Costa-Lotufo LV. (2009). Antitumor properties of a sulfated polysaccharide from the red seaweed Champia feldmannii (Diaz-Pifferer). J Appl Toxicol, 29, 20–26.
- McCandless EL. (1981). Biological control of carrageenan structure: effects conferred by the phase of life cycle of the carrageenophyte. Proc Int Seaweed Symp, 8, 1–18.
- Medeiros VP, Queiroz KC, Cardoso ML, Monteiro GR, Oliveira FW, Chavante SF, Guimaraes LA, Rocha HA, Leite EL. (2008). Sulfated galactofucan from Lobophora variegata: Anticoagulant and anti-inflammatory properties. Biochemistry Mosc, 73, 1018–1024.
- Moncada S, Palmer RM, Higgs EA. (1991). Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol Rev, 43, 109–142.
- Murano E, Jellús V, Piras A, Toffanin R. (1998). Cell wall polysaccharides from Gelidium species: Physico-chemical studies using MRI techniques. J Appl Phycol, 10, 315–322.
- Ohta Y, Kobayashi T, Inui K, Yoshino J, Nakazawa S. (2003). Protective effect of teprenone against acute gastric mucosal lesions induced by compound 48/80, a mast cell degranulator, in rats. J Pharmacol Sci, 93, 337–346.
- Pereira MG, Benevides NM, Melo MR, Valente AP, Melo FR, Mourão PA. (2005). Structure and anticoagulant activity of a sulfated galactan from the red alga, Gelidium crinale. Is there a specific structural requirement for the anticoagulant action? Carbohydr Res, 340, 2015–2023.
- Perez R, Kaas R, Campello F, Arbault S, Barbaroux O. (1992). La Culture Des Algues Marines dans le Monde. Ifremer, 614, 148–177.
- Pomin VH, Mourão PA. (2008). Structure, biology, evolution, and medical importance of sulfated fucans and galactans. Glycobiology, 18, 1016–1027.
- Salvemini D, Wang ZQ, Wyatt PS, Bourdon DM, Marino MH, Manning PT, Currie MG. (1996). Nitric oxide: A key mediator in the early and late phase of carrageenan-induced rat paw inflammation. Brit J Pharmacol, 118, 829–838.
- Santelices, B. (1991). Production ecology of Gelidium. Hydrobiologia, 221, 31–44.
- Siqueira RC, da Silva MS, de Alencar DB, Pires Ade F, de Alencar NM, Pereira MG, Cavada BS, Sampaio AH, Farias WR, Assreuy AM. (2011). In vivo anti-inflammatory effect of a sulfated polysaccharide isolated from the marine brown algae Lobophora variegata. Pharm Biol, 49, 167–174.
- Stephen AM, Dahl WJ, Sieber GM, van Blaricom JA, Morgan DR. (1995). Effect of green lentils on colonic function, nitrogen balance, and serum lipids in healthy human subjects. Am J Clin Nutr, 62, 1261–1267.
- Stochla K, Maslinski S. (1982). Carrageenan-induced oedema in the rat paw - histamine participation. Agents Actions, 12, 201–202.
- Tissot B, Montdargent B, Chevolot L, Varenne A, Descroix S, Gareil P, Daniel R. (2003). Interaction of fucoidan with the proteins of the complement classical pathway. Biochim Biophys Acta, 1651, 5–16.
- Vinegar R, Schreiber W, Hugo R. (1969). Biphasic development of carrageenin edema in rats. J Pharmacol Exp Ther, 166, 96–103.
- Vircheva S, Alexandrova A, Georgieva A, Mateeva P, Zamfirova R, Kubera M, Kirkova M. (2010). In vivo effects of pentoxifylline on enzyme and non-enzyme antioxidant levels in rat liver after carrageenan-induced paw inflammation. Cell Biochem Funct, 28, 668–672.
- Wedmore CV, Williams TJ. (1981). Control of vascular permeability by polymorphonuclear leukocytes in inflammation. Nature, 289, 646–650.
- Weston SA, Parish CR. (1991). Modification of lymphocyte migration by mannans and phosphomannans. Different carbohydrate structures control entry of lymphocytes into spleen and lymph nodes. J Immunol, 146, 4180–4186.