Abstract
Context: Herbal remedies have been employed for the treatment and management of various ailments since the beginning of human civilization. Voacanga is an extensive genus of the family Apocynaceae and consists of small trees inhabiting the tropical and subtropical regions of Africa. Voacanga plants have been used in the treatment of leprosy, diarrhea, and generalized edema, convulsions in children as well as to treat cases of orchitis, ectopic testes and gonorrhea.
Objectives: The aim of this review is to present as much information as was established from the available scientific literature. The present review comprises the ethnopharmacological, phytochemical and therapeutic potential of the plant genus Voacanga.
Methods: The present review reports on 111 natural products as found in 44 references compiled from the major databases, viz., Chemical Abstracts, Science Direct, SciFinder, PubMed, Dr. Dukes Phytochemical and Ethnobotany, CIMER, and InteliHealth.
Results: An exhaustive survey of the literature revealed that indole alkaoids and steroids constitute the major classes of phytoconstituents of this genus. Pharmacological reports revealed that products derived from this genus have been used for the treatment of cancer, and for CNS, cardiotonic, antituberculosis, acetylcholinesterase (AChE), butyrylcholinesterase, antagonistic, anti-diarrheal activities.
Introduction
Plants belonging to the genus Voacanga (Apocynaceae) have been the subject of many investigations for their biologically active components. Some species of the genus Voacanga have long been used as medicinal plants. For example Voacanga africana Staph. is a tree plant occurring in all of West Africa and is used for the treatment of leprosy, diarrhea, generalized edema, convulsions in children and madness making them among the more prevalent ethno-medical applications of the plant (CitationTan et al., 2000). In Cameroon, the fruit bark and leaf extracts of V. africana are used to treat cases of orchitis, ectopic testes and gonorrhea. Information provided by practitioners of traditional medicine suggests that V. africana also possesses useful antiulcer properties (CitationTan et al., 2000). The gastric protective effect of the aqueous extract of the bark of V. africana against an HCl:ethanol solution was demonstrated. When further tested in the pylorus ligated rat model, the aqueous extract prevented gastric mucosal damage, but did not significantly reduce the acidity of the gastric juice (CitationTan et al., 2000). The isolation of interesting products, mainly indole alkaloids, from plants of the Voacanga genus prompted us to write the current comprehensive review in order to have a complete picture of the secondary metabolism of this genus.
Phytochemical investigation of genus Voacanga
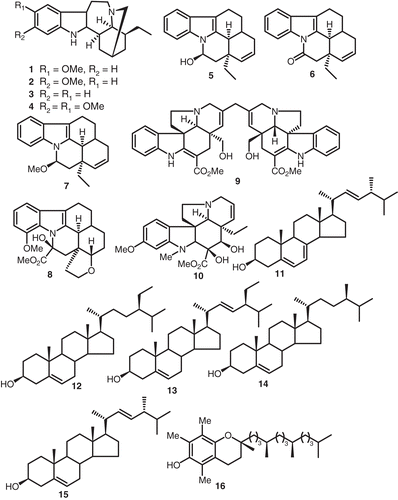
Phytochemical investigation of V. africana yielded ibogain (1), tabernanthin (2), (-)-ibogamine (3), and decarbomethoxyconopharyngine (4) (CitationJenks, 2002) ().
Ibogaine (1) was found to be effective in the treatment of withdrawal symptoms and craving in drug addicts. As the synaptic and cellular basis of ibogaine’s actions are not well understood, this study tested the hypothesis that both ibogaine and V. africana extract modulate neuronal excitability and synaptic transmission in the parabrachial nucleus using the nystatin perforated patch-recording technique (CitationKombian et al., 1997). Ibogaine (1) and V. africana extract dose dependently, reversibly, and consistently attenuate evoked excitatory synaptic currents recorded in parabrachial neurons. The ED50 of ibogaine’s effect is 5 µM, while that of V. africana extract is 170 µg/mL. At higher concentrations ibogaine (1) and V. africana extract induce inward currents or depolarization that were accompanied by increases in the evoked and spontaneous firing rate. The depolarization or inward current was also accompanied by an increase in input resistance and reverses polarity around 0 mV. The depolarization and synaptic depression were blocked by the dopamine receptor antagonist haloperidol. These results indicate that ibogaine (1) and V. africana extract depolarized parabrachial neurons with increased excitability and firing rate and also depress non-NMDA receptor-mediated fast synaptic transmissions. These results further revealed that the V. africana extract has one-hundredth the activity of ibogaine (1) in depressing synaptic responses. Thus, ibogaine (1) and V. africana extract may produce their central effects by altering dopaminergic and glutamatergic processes (CitationKombian et al., 1997).
Neurochemical studies indicate that ibogaine (1) interacts with several neurotransmitters and their receptors to produce its effects (CitationJenks, 2002). For example, ibogaine (1) has been shown to modulate both dopamine and serotonin responses by blocking their reuptake or by causing their release from cytoplasmic stores. In addition, ibogaine (1) also binds to several receptors in the CNS, including opioid receptors and the MK-801 binding site of the NMDA receptor complex. In behavioral studies, ibogaine (1) has psychoactive and memory-altering effects by itself and is tremorigenic at higher doses. In addition to these direct effects, ibogaine (1) also alters the actions of several drugs of abuse, including cocaine, amphetamine, morphine, alcohol and nicotine. In some studies, ibogaine (1) antagonizes or attenuates several behavioral effects of these drugs while either enhancing or not affecting them in other cases. It is, at present, not clear which of these actions of ibogaine (1) mediates its ‘‘antiaddictive’’ effects (CitationKombian et al., 1997). To begin to understand the cellular basis of these CNS effects of ibogaine (1), its actions on neuronal responses have been examined. Intravenous injection of ibogaine (1) has been shown to increase the firing rate of ventral tegmental area dopaminergic neurons. Consistent with its binding to the MK-801 binding site of the NMDA receptor complex, ibogaine (1) has been shown to block, in a voltage-dependent manner, currents induced by NMDA and to prevent glutamate-induced cell death in hippocampal cultures (CitationKombian et al., 1997). Furthermore, it has been shown to block NMDA-induced depolarization of frog spinal cord motorneurons. To our knowledge, no study has examined the action of ibogaine (1) and related alkaloids on synaptic transmission in the CNS, a likely locus for its behavioral effects. This study, therefore, examined the effects of ibogaine (1) and a total alkaloidal extract of V. africana on parabrachial nucleus (PBN) neuronal excitability and on non-NMDA receptor-mediated excitatory synaptic responses using in vitro electrophysiological recording techniques. The PBN was chosen for this study because the biophysical characteristics of cells in this nucleus, in addition to the excitatory afferent pathways and inputs to these cells, are quite well characterized, permitting selective activation of appropriate afferents (CitationKombian et al., 1997). Furthermore, excitatory synaptic transmission in this region has been thoroughly elucidated, thus making it attractive for examining the effects of substances on synaptic transmission (CitationKombian et al., 1997). Similarly V. africana also yielded Δ14-vincanol (5), Δ14-vincamone (6), and O-methyl-16-epi-Δ14-vincanol (7) (CitationPegnyemb et al., 1999) ().
Phytochemical investigation of Voacanga chalotiana Pierre ex Stapf yielded the indole alkaloid cuanzine (8), belonging to the eburnane series and which has a methoxy group in position 12 and an ether linkage between positions 15 and 18. Cuanzine (8) is endowed with vasodilating activity like vincamine and its derivative. The aim of this work was to investigate the cerebral antihypoxic activity of cuanzine (IdB 1119) and some of its semisynthetic derivatives (CitationMalandrino et al., 1993). Voacinol (9), a new and intriguing stereochemically symmetrical bisindole alkaloid, was isolated from Voacanga grandifolia leaves along with desacetylvindoline (10) (CitationGovindachari et al., 1987). Phytochemical investigation of V. africana produced ergosterol (11), β-sitosterol (12), stigmasterol (13), campesterol (14), brassicasterol (15), and dl-α-tocopherol (16) (CitationRafidison et al., 1987) ().
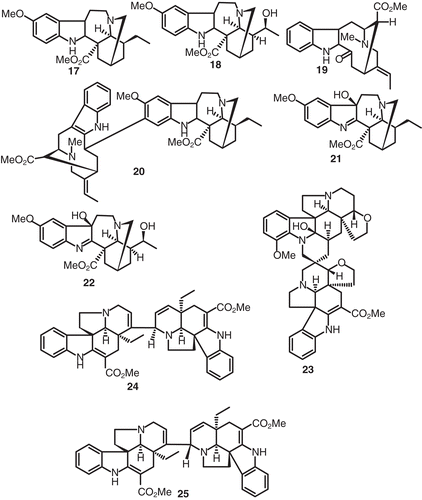
On the other hand, the alkaloids voacangine (17), voacristine (18), vobasine (19), voacamine (20), voacangine hydroxyindolenin (21), voacristine 7-hydroxyindolenine (22), and vobtusine (23) were isolated from the bark of V. africana growing in Guinea (CitationDiavara et al., 1984) ().
Voacamine (20) has been shown to possess remarkable cardiotonic activity with much less side effects of toxicity than the existing cardenolide-based drugs, thus leading to its favored clinical use for the treatment of heart failure. Later it was reported to display a general cytotoxicity and strong antimicrobial activity against Gram-positive bacteria (CitationRamanitrahasimbola et al., 2001). Interestingly, voacamine (20) as also found to enhance the cytotoxic response mediated by vinblastine with multidrug-resistant KB cells. As a scientific follow-up of the traditional use of Peschiera fuchsiaefolia in the treatment of malaria in Brazil, several bisindoles of the voacamine type have been found to be active in vitro against chloroquine-sensitive and resistant strains of Plasmodium falciparum (CitationDiavara et al., 1984). Recently it was shown that voacamine (20) exhibited in vivo activity with 25.4 and 43.4% inhibition of parasitaemia with concentrations of 2.5 and 10 mg/kg, respectively (CitationRamanitrahasimbola et al., 2001). In synchronized cultures, it was found to act on trophozoite and schizont stages of Plasmodium falciparum. Using the FMC29 strain of Plasmodium falciparum as parasite and the isobologram curve as a method to assess interaction in drug combination, it was shown to lack any chloroquine-enhancing activity and its in vitro antiplasmodial effect was not potentiated by the chemosensitizer malagashanine (CitationRamanitrahasimbola et al., 2001).
Vobtusine (23), quite a large alkaloid, was demonstrated to be a cardiac depressor. The intravenous LD50 of 23 for mice is approximately 34 mg/kg. On the other hand, 23 has little effect on the autonomic nervous system, and the hypotension it provokes is due to peripheral vasodilation and a direct action on the heart. In mice, intravenous injection of 25 mg/kg of 23 induces an early onset of agitation followed later by the mice becoming quiet, and this action is not antagonized by cresoxydiol, regitine, atropine, penthiobarbital, or trihexy-phenidyl. Larger doses of 23 may induce convulsions and death (CitationQuevauviller et al., 1965). Similary, V. africana also yielded the novel epimeric dimeric indole alkaloids voafrine A (24) and voafrine B (25) (CitationStoeckigt et al., 1983) (). The roots of Voacanga schweinfurthii Stapf yielded coronaridine (26), voacamidine (27), while extracts of the stem bark afforded perivine (28), and vobasinol (29) (CitationRichard et al., 1983) (). The spiro bisindole alkaloid vobtusamine (30) was isolated and identified from the root bark of V. chalotiana collected in Angola (CitationDanieli et al., 1983). Similarly further samples of V. africana yielded two more alkaloids, viz., the epoxy alkaloids 31 and 32 (CitationKunesch et al., 1981). In an earlier investigation assignment of a structure of a bisindoline alkaloid isolated from the root bark of V. chalotiana (Apocynaceae) was established as 3ϵ-hydroxyvobtusine (33) from spectral data (CitationDanieli et al., 1980). This alkaloid is amazingly identical to the vobtusamine (30) described vide infra.
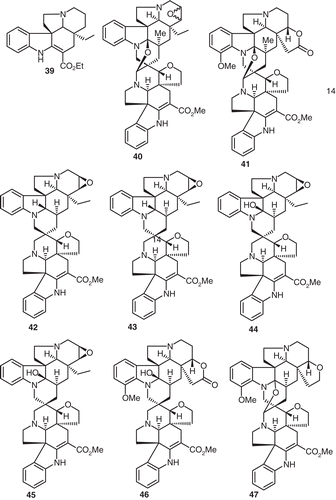
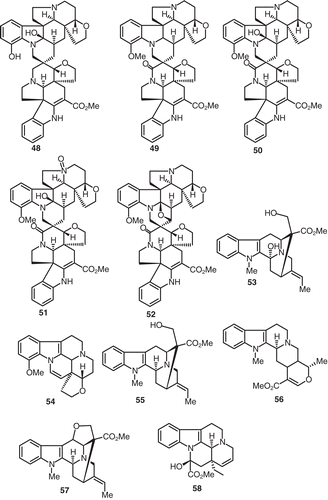
Indole alkaloids such as pachysiphine (34), minovincine (35), deoxyvobtusine lactone (36), vobtusine lactone (37), beninine(38) (),tabersonine (39), folicangine (40), subsessiline lactone (41), voafoline (42), isovoafoline (43), voafolidine (44), isovoafolidine (45), isovobtusine lactone (46), and amataine (47) have all been described in a thorough investigation of the extracts and their isolates from Voacanga thouarsii Roem. & Schult. (CitationKunesch et al., 1977) ().
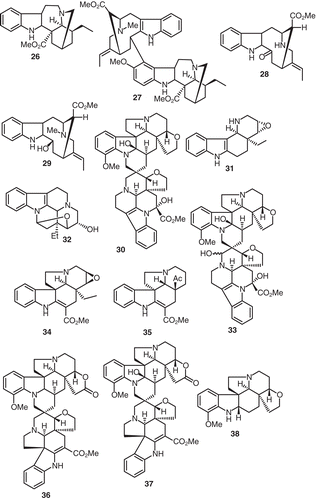
Phytochemical investigation of V. thouarsii resulted in the identification and isolation of the structurally demanding indole alkaloids O-demethylvobtusine (48), 2′-deoxy-8-oxo-vobtusine (49), 8-oxo-vobtusine (50), and 8-oxo-9′-oxide-vobtusine (51) (CitationRolland et al., 1975). Similarly, the equally demanding indole alkaloid, quimbeline (52), was reported to be isolated from V. chalotiana (CitationBombardelli et al., 1975) (). Another indole alkaloid given the name 3-hydroxyvoachalotine (53) was isolated from the root bark of V. chalotiana (CitationBombardelli et al., 1974). The benzene extract of the root bark of V. chalotiana, collected in Angola, produced the indole alkaloid, decarbomethoxyapocuanzine (54) (CitationBombardelli et al., 1974). Voachalotine (55), tetrahydroalstonine (56), dehydrovoachalotine (57), Δ14-vincamine (58) were also isolated from the root bark of V. chalotiana (CitationGabetta et al., 1974) ().
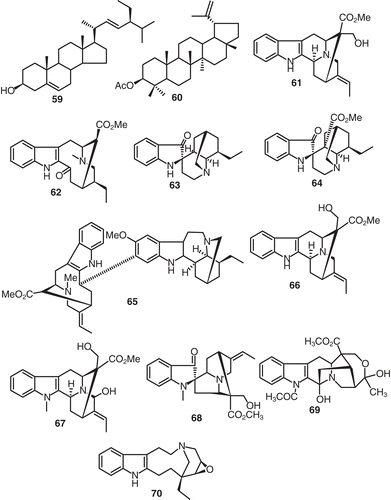
A chemical investigation of the extracts of V. grandifolia yielded the non-alkaloid compounds β-sitosterol (59), lupeol acetate (60), and the alkaloid rhazine (61) (CitationMajumbar & Dinda, 1974). Tabernaemontanine (62), which was isolated from Voacanga globosa Blanc., was found to be inactive in the chemotherapy of L-1210 lymphoid leukemia, Walker 256 carcinosarcoma in animals and in human epidermoid carcinoma of the nasopharynx in cell cultures (CitationLleander et al., 1972) (). Iboluteine (63), voaluteine (64), and decarbomethoxyvoacamine (65) were all found and identified to be present in the crude extract from the bark of V. thouarsii, var obtuse (CitationGoldblatt et al., 1970). Chemical investigation of V. chalotiana resulted in the isolation of (-)-polyneuridine (66), 21-hydroxy-voachalotine (67), voachalotine oxindole (68) and N-acetylvoamonine (69) (CitationBraekman et al., 1969). The ethanol extract of the leaves of V. africana yielded the interesting epoxyalkaloid named conoflorine (70) (CitationKunesch et al., 1968) ().
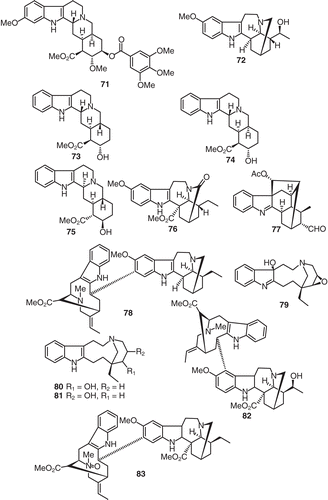
Additional investigations of the alkaloids of V. africana revealed the presence of temposerpine (71), (-)-iboxygaine (72), ψ-yohimbine (73), epi-3α-yohimbine (74), (-)-β-yohimbine (75), 3-oxovoacangine (76), perakine (77), and decarbomethoxyepivoacamine (78) () (CitationThomas & Biemann, 1968). The epoxy alkaloid, voaphylline hydroxyindolenine (79) was isolated from V. africana (CitationKunesch et al., 1967). Phytochemical investigation of V. africana gave the related epoxide ring-opened alkaloids viz., conoflorinol A (80), conoflorinol B (81) (CitationKunesch et al., 1967a). Another two bisindole alkaloids viz., 20′-hydroxyvoacamidine (82) (Geigy, Citation1967) and voacamine N-oxide (83) (CitationCarroll & Starmer, 1967) () and were isolated from V. africana.
The pharmacological actions of conopharyngine (84) which was isolated from Voacanga species were investigated and comparisons were drawn with the reported effects of closely related alkaloids (CitationCarroll & Starmer, 1967) (). Conopharyngine (84) possessed potent analgesic activity unaccompanied by a loss of coordination. However, this action was not like morphine. On smooth muscle preparations, 84 exerted general relaxant effects. Negative inotropic and chronotropic effects were observed on the isolated heart. However, on the anesthetized cat, hypotension and bradycardia were produced which were not abolished by atropinization or vagotomy (CitationCarroll & Starmer, 1967).
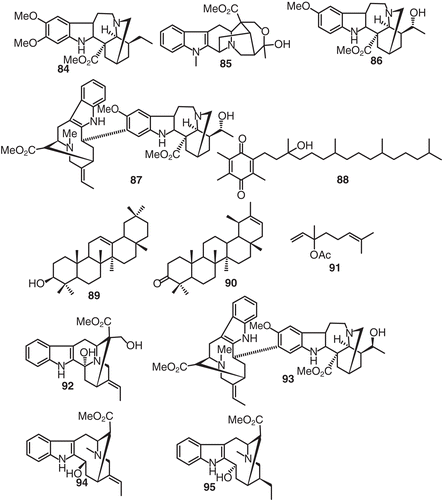
Chemical investigation of V. chalotiana led to the isolation of voacoline (85) (CitationLhoest et al., 1965). Two interesting alkaloids, viz., epivoacangarine (86) and epivoacorine (87) were isolated from Voacanga bracteata bark (CitationPoisson et al., 1965). Chemical investigation Voacanga papuana resulted in the isolation of the non alkaloids dl-α-tocopherolquinone (88), β-amyrin (89), lupenone (90), and dl-linalool acetate (91) () (CitationGuise et al., 1965). Voacarpine (92), was isolated as a minor alkaloid from V. chalotiana (CitationDenayer-Tournay et al., 1965) while the chemical investigation of the stem bark of V. schweinfurthii yielded the structurally quite demanding voacaline (93) (CitationFish & Newcombe, 1964). The final structural assignments s of the indole alkaloids isolated from Voacanga plants such as vobasinol (94) and tabernaemontaninol (95) () were apart from the usual NMR spectral investigations, confirmed by means of high resolution mass spectrometry (CitationBudzikiewicz et al., 1963).
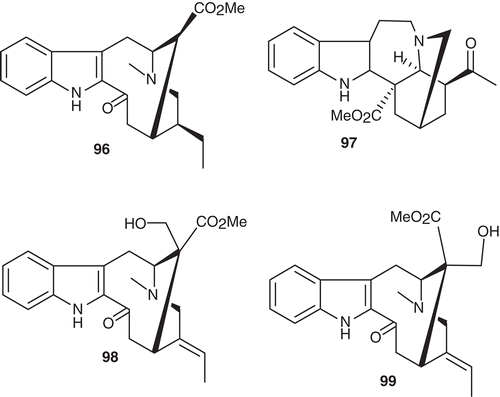
An interesting 2-acylindole type alkaloid, dregamine (96), has been isolated from V. africana (CitationRenner et al., 1963) while an alternative acyl type alkaloid, viz., voacryptine (97) has also been isolated from V. africana (CitationRenner & Prins, 1961). The two new epimeric alkaloids, voacafrine (98) and voacafricine (99) (), were isolated from the bark of V. africana (CitationRao & John, 1958).
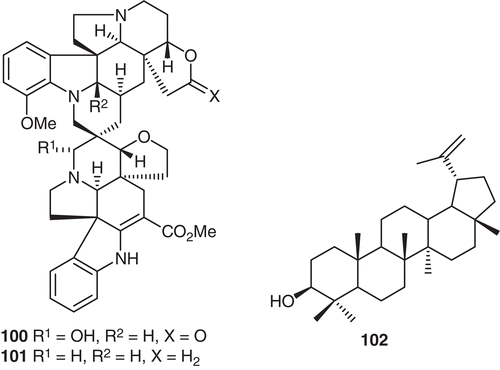
Globospiramine (100), a new spiro bisindole alkaloid possessing an extremely interesting spidospermae-Aspidosperma skeleton, together with the less oxidized analogue deoxyvobtusine (101), deoxyvobtusine lactone (36), vobtusine lactone (37) and the non-alkaloid lupeol (102) (), were isolated and identified from V. globosa through a bioassay-guided purification protocol (CitationMacabeo et al., 2011). The absolute stereochemistry of 100 was established by circular dichroism spectroscopy. In addition, a new biogenetic pathway for the formation of the spiro-Aspidosperma-Aspidosperma skeleton has been proposed.
The in vitro antituberculosis activity of the extracts, fractions and compounds 36, 37, 100–102 was evaluated against the Mycobacterium tuberculosis H37Rv strain using a microplate Alamar blue assay (MABA) with rifampin, isoniazid and moxifloxacin as positive controls. Compounds 36, 37, and 100 were shown to be the most active. Alkaloid 100 exhibited the highest inhibitory activity with MIC 4 μg/mL. Alkaloid 37 was observed to display insignificant activity compared with 101 and 36 (CitationMacabeo et al., 2011). Lupeol 102, a pentacyclic triterpenoid isolated from a non-polar fraction, showed no inhibitory activity. In addition, 100 was also found to be active against M. tuberculosis based on the low-oxygen recovery assay (LORA) displaying an MIC of 5.2 μg/mL. The LORA assay affords information about the capacity of a compound to inhibit a M. tuberculosis population that is on a non-replicating persistence physiological state and is responsible for the antimicrobial tolerance in many bacterial-allied diseases. In analyzing the structure of antitubercular alkaloid 100, it would seem that the presence of an hydroxyl group at C-3 and an unoxidized C-2′ plays a paramount role in providing enhanced inhibition properties, while the absence of oxygens at C-3 and C-18′ and the presence of a hydroxyl group at C-2′, results in decreased activity against M. tb H37Rv. These results extend the variety of indole alkaloids which show in vitro inhibitory activity against M. tuberculosis H37Rv (CitationMacabeo et al., 2011).
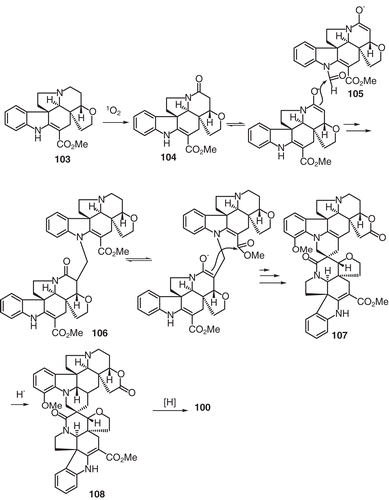
In the original biogenetic proposal of Morita and co-workers, it was postulated that a carbonylene reaction is the plausible initial reaction en route to biscarpamontamine B. However, such a route is considered here as highly unlikely, since little is known about carbonylene reactions with respect to plant biosynthetic pathways. To address this dilemma, it is proposed that modestanine (103) initially undergoes singlet oxidation to afford 3-oxomodestanine (104), which then undergoes enolization followed by condensation with N-formylaspidospermidine lactone (105) to afford the bisindole alkaloid 106 (CitationMacabeo et al., 2011). A subsequent round of enolization at the 3-oxo position followed by condensation with the acrylic side chain carboxyl ester would lead to anhydrovobtusine 107 which by stereoselective olefin reduction affords lactam 108. Finally, chemoselective ketone reduction to a carbinolamine would result in the bisindole alkaloid 100. All of the Aspidospermae-Aspidosperma spirocyclic bisindole alkaloids reported so far have oxidized functionalities at C-2′ and C-3 or only at C-2′. Hence, the identification of the globospiramine (100) in V. globosa demonstrates for the first time a different oxidation pattern being observed only at C-3. This concept highlights that variability in alkaloid structure is dependent on the Michael reaction, a subsequent enolization reaction which results in epimeric spirocyclic alkaloids, and reduction sequences (CitationMacabeo et al., 2011).
Compounds 36, 37, 100, and 101 were evaluated for their inhibitory activity against acetylcholinesterase (AChE) and butyrylcholinesterase activity (BChE) using the colorimetric Ellman’s assay. The alkaloid and pharmaceutically approved Alzheimer’s drug galanthamine served as the positive control. All compounds tested showed significant inhibitory activity on both AChE and BChE but with more pronounced activity against the latter enzyme. At a concentration of 10 μM, alkaloids 36, 37, 100, and 101 showed almost complete inhibition (greater or equal to 90%) (CitationMacabeo et al., 2011). The values obtained were not high enough to measure complete inhibition curves and it was not possible to precisely determine the IC50 values. Nevertheless, due to the higher inhibitory potencies, this was possible with BChE. All compounds turned out to be strong micromolar inhibitors. The bisindole alkaloids showed inhibitory activities with IC50 values lowest for deoxyvobtusine (36) at 6.2 μM followed by 101, 37 and 36. When inhibitory potencies were compared to galanthamine, alkaloid 101 displayed higher activity and revealed higher selectivity toward BChE over AChE. Previous investigations reported the Iboga alkaloids ibogaine and voacangine as common psychoactive agents of several Voacanga species (CitationMacabeo et al., 2011). Two additional monoterpenoid indole alkaloids from Ervatamia hainanensis have shown AChE inhibitory activity comparable to galanthamine. These results demonstrate for the first time the promise of spirocyclic Aspidospermae-Aspidosperma bisindole alkaloids as a new class of butyrylcholinesterase inhibitors and possibly, a new psychoactive compound type of the genus Voacanga. Based on the results of the present study, it was found that alkaloid-bearing plants are excellent sources of pharmacologically active alkaloid prototypes which should encourage greater efforts to discover potent bioactive leads. This report on the finding of bisindole alkaloids 100 and 101 as promising antituberculosis and memory-enhancing compounds, respectively, serves as a seminal study on the potential of the Philippine plant V. globosa as a source of bioactive phytomedicines (CitationMacabeo et al., 2011).
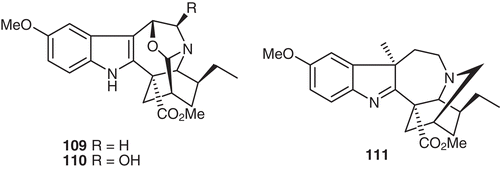
The screening of plant extracts and isolated alkaloids for agonistic or antagonistic activity on the CB1 receptor was performed using the aequorin/GPCR cell-based Ca2+ functional assay (CitationKitajima et al., 2011). CP55940 or rimonabant was used as the positive control for agonist or antagonist. As a result, it was found that the MeOH extract of V. africana root bark showed potent activity. This prompted researchers to clarify what the active principle in V. africana was. Extensive separation of the crude base material that was prepared from V. africana root bark resulted in the isolation of three active indole alkaloids: one iboga-vobasine-type bisindole alkaloid, voacamine (20), and two iboga-type monomer alkaloids, 3,6-oxidovoacangine (109) and 5-hydroxy-3,6-oxidovoacangine (110) () (CitationKitajima et al., 2011). These compounds exhibited relatively potent CB1 receptor antagonistic activity with IC50 values of 0.041, 0.199, and 0.141 μM, respectively, compared with that of rimonabant (IC50 0.004 μM). These are a new class of small molecules possessing CB1 receptor antagonistic activity. Interestingly, well-known co-existing alkaloids, such as voacangine (17), vobasine (19), and tabersonine (39), did not show this activity.
V. africana (Apocynaceae) is used as an anti-diarrheal medicine in West Africa. CitationLo et al. (2011) investigated the effect of an extract of V. africana and its constituents on smooth muscle contraction induced by capsaicin in mouse rectum, where transient receptor potential vanilloid type 1 (TRPV1)-immunoreactive fibers are abundant. Methanol and alkaloid extracts of the root bark of V. africana were found to inhibit capsaicin-induced contraction in a dose-dependent manner (30–300 μg/mL). Major constituents isolated from the alkaloid extract were then studied for their effects on the capsaicin-induced contraction. The main active constituents were found to be Iboga-type alkaloids, including voacangine (17), 3-oxovoacangine (76), voacristine (18), and 7α-voacangine hydroxyindolenine (111) () (CitationLo et al., 2011). The voacangine (17) concentration dependently (3–100 μM) inhibited the capsaicin-induced contraction. The capsaicin-induced contraction was almost completely inhibited by the TRPV1 antagonist, N-(4-tertiarybutylphenyl)-4-(3-chloro-pyridin-2-yl)tetrahydropyrazine-1(2H)-carbox-amide (BCTC). On the other hand, the Iboga-type alkaloids did not inhibit the contractions induced by 3 μM acetylcholine and 300 μM nicotine. These results suggest that Iboga-type alkaloids isolated from V. africana inhibit capsaicin-induced contraction in the mouse rectum, possibly via the inhibition of a TRPV1-mediated pathway. This inhibition may be involved in the antidiarrheal effect of V. Africana (CitationLo et al., 2011) ().
Table 1. Compound names and their sources.
Declaration of interest
The authors declare no conflicts of interest.
References
- Bombardelli E, Bonati A, Danieli B, Gabetta B, Martinelli EM, Mustich G. (1975). Structure of quimbeline, a new bisindole alkaloid from Voacanga chalotiana. Experientia, 31, 139–140.
- Bombardelli E, Bonati A, Danieli B, Gabetta B, Martinelli EM, Mustich G. (1974). Decarbomethoxy apocuanzine a new indole alkaloid from Voacanga chalotiana. Experientia, 30, 979–980.
- Bombardelli E, Bonati A, Danieli B, Gabetta B, Mustich G. (1974). New alkaloid from Voacanga chalotiana. Phytochemistry, 13, 2857–2859.
- Braekman JC, Tirions-Lampe M, Pecher J. (1969). Indole alkaloids. XX. Isolation and structural elucidation of four minor alkaloids from Voacanga chalotiana. Bull Soc Chim Belges, 78, 523–538.
- Budzikiewicz H, Djerassi C, Puisieux F, Percheron F, Poisson J. (1963). Mass spectrometry in structural and stereochemical problems. XXXVIII. Voacanga alkaloids. Structure of voacamine and of voacorine, observations on the mass spectra of vobasine and its derivatives. Bull Soc Chim France, 1899–1904.
- Carroll PR, Starmer GA. (1967). Studies on the pharmacology of conopharyngine, an indole alkaloid of the voacanga series. Br J Pharmacol Chemother, 30, 173–185.
- Danieli B, Lesma G, Palmisano G, Gabetta B. (1980). 3ϵ-Hydroxyvobtusine, a key-link between vobtusine and amataine. Heterocycles, 14, 201–203.
- Danieli B, Lesma G, Palmisano G, Tollari S, Gabetta B. (1983). Vobtusamine, the first spiro Aspidosperma-Eburnea alkaloid. J Org Chem, 48, 381–383.
- Denayer-Tournay M, Pecher J, Martin RH, Friedmann-Spiteller M, Spiteller G. (1965).Indole alkaloids. V. Structure of voacarpine. Bull Soc Chim Belges, 74, 170–186.
- Diavara D, Pyuskyulev B, Kuzmanov B. (1984). Alkaloid-bearing plants in the flora of Guinea. Alkaloids from Voacanga africana Stapf. Izvestiya po Khimiya, 17, 364–371.
- Fish F, Newcombe F. (1964). Alkaloids of Voacanga Schweinfurthii Stapf: Voacorine and voacangine. J Pharm Pharmacol, 16, 832–833.
- Gabetta B, Martinelli EM, Mustich G. (1974). Alkaloids of Voacanga chalotiana. Fitoterapia, 45, 32–36.
- GeigyJR, Neth. Appl. 1967, 9pp. CODEN: NAXXAN, NL, 6612608, 19670309, Patent written in Dutch. Priority: CH, 19650908. CAN 67:84862,AN 1967:484862
- Goldblatt A, Hootele C, Pecher J. (1970). Indole alkaloids. XXIII. Alkaloids of Voacanga thouarsii. Phytochemistry, 9, 1293–1298.
- Govindachari TR, Sandhya G, Chandrasekharan S, Rajagopalan K. (1987). Voacinol: A new bisindole alkaloid from Voacanga grandifolia (Miq Rolfe). J Chem Soc Chem Comm 15, 1137–1138.
- Guise GB, Rasmussen M, Ritchie E, Taylor WC. (1965). Some constituents of Rejoua aurantiaca and Voacanga papuana. Aust J Chem, 18, 927–931.
- Jenks CW. (2002). Extraction studies of Tabernanthe iboga and Voacanga africana. Nat Prod Lett, 16, 71–76.
- Kitajima M, Iwai M, Kikura-Hanajiri R, Goda Y, Iida M, Yabushita H, Takayama H. (2011). Discovery of indole alkaloids with cannabinoid CB1 receptor antagonistic activity. Bioorg Med Chem Lett, 21, 1962–1964.
- Kombian SB, Saleh TM, Fiagbe NI, Chen X, Akabutu JJ, Buolamwini JK, Pittman QJ. (1997). Ibogaine and a total alkaloidal extract of Voacanga africana modulate neuronal excitability and synaptic transmission in the rat parabrachial nucleus in vitro. Brain Res Bull, 44, 603–610.
- Kunesch N, Ardisson J, Poisson J, Halls TDJ, Wenkert E. (1981).Carbon-13 nuclear magnetic resonance spectroscopy of naturally occurring substances. LXXV. Natural products of degradation of the Aspidosperma alkaloid skeleton. Tetrahedron Lett, 22, 1981–1984.
- Kunesch N, Das B, Poisson J. (1967).Voacanga alkaloids. IX. Voaphylline hydroxy-indolenine, a new alkaloid extracted from the leaves of Voacanga africana. Bulletin de la Societe Chimique de France, 3551–3552.
- Kunesch N, Das BC, Poisson J. (1967A). [Voacanga alkaloids. VII. Structure of voaphylline]. Bull Soc Chim Fr, 6, 2155–2160.
- Kunesch N, Miet C, Troly M, Poisson J. (1968). [Alkaloids of Voacanga. 8. Alkaloids of leaves and seeds of Voacanga africana Stapf]. Ann Pharm Fr, 26, 79–86.
- Kunesch N, Rolland Y, Poisson J, Majumder PL, Majumder R, Chatterjee A, Agwada VC, Naranjo J, Hesse M, Schmid H. (1977). Alkaloids of Voacanga. XVIII. Alkaloids of Callichilia subsessilis. IV. Organic natural products. 165. The structure of bisindoline alkaloids of a novel type. Helv Chim Acta, 60, 2854–2859.
- Lhoest G, de Neys R, Defay N, Seibl J, Pecher J, Martin RH. (1965). Indole alkaloids. VII. Structure of voacoline. Bull Soc Chim Belges, 74, 534–550.
- Lleander GC, Salud EH, Rigor EC. (1972). Alkaloids of Voacanga globosa. Isolation and characterization of tabernaemontanine. Philippine J Sci, 98, 247–257.
- Lo MW, Matsumoto K, Iwai M, Tashima K, Kitajima M, Horie S, Takayama H. (2011). Inhibitory effect of Iboga-type indole alkaloids on capsaicin-induced contraction in isolated mouse rectum. J Nat Med, 65, 157–165.
- Macabeo AP, Vidar WS, Chen X, Decker M, Heilmann J, Wan B, Franzblau SG, Galvez EV, Aguinaldo MA, Cordell GA. (2011). Mycobacterium tuberculosisand cholinesterase inhibitors from Voacanga globosa. Eur J Med Chem, 46, 3118–3123.
- Majumbar PL, Dinda BN. (1974). Chemical investigation of the fruits of Voacanga grandifolia. J Indian Chem Soc, 51, 370.
- Malandrino S, Cristoni A, Sokolov SJ, Gabetta B, Pifferi G. (1993). Antihypoxic activity of cuanzine and derivatives. Pharmacol Res, 27, 121–122.
- Pegnyemb DE, Ghogomu RT, Sondengam BL. (1999). Minor alkaloids from the seeds of Voacanga africana. Fitoterapia, 70, 446–448.
- Poisson J, Puisieux F, Miet C, Patel MB. (1965). [Voacanga alkaloids: Structure of epivoacangarine, and epivoacorine alkaloids from the bark of the trunk of Voacanga bracteata Stapf]. Bull Soc Chim Fr, 12, 3549–3552.
- Puisieux F, Devissaguet JP, Miet C, Poisson J. (1967). [Alkaloids of Voacanga. V. N-Oxy-voacamine]. Bull Soc Chim Fr, 1, 251–253.
- Quevauviller A, Foussard-Blanpin O, Pottier J. (1965). Vobtusine an alkaloid of Voacanga africana. Comp Rendus Seances Soc Biol Ses Filiales, 159, 821–825.
- Rafidison P, Baillet A, Baylocq D, Pellerin F. (1987). Study of oil from Voacanga africana seeds. Oleagineux, 42, 299–302.
- Ramanitrahasimbola D, Rasoanaivo P, Ratsimamanga-Urverg S, Federici E, Palazzino G, Galeffi C, Nicoletti M. (2001). Biological activities of the plant-derived bisindole voacamine with reference to malaria. Phytother Res, 15, 30–33.
- Rao KV, John LS. (1958). Alkaloids of Voacanga africana. I. Voacafrine and voacafricine-two new alkaloids. J Org Chem, 23, 1455–1456.
- Renner U, Prins DA, Burlingame AL, Biemann K. (1963). Structure of the 2-acylindole alkaloids vobasine, dregamine, and tabernaemontanine. Helv Chim Acta, 46, 2186–2208.
- Renner U, Prins DA. (1961). [Voacanga alkaloids. IV. Structure of voacryptin and voacristine]. Experientia, 17, 106.
- Richard B, Delaude C, Massiot G, Le MenOlivier L. (1983). Alkaloids from Voacanga schweinfurthii var. puberula. J Nat Prod, 46, 283–284.
- Rolland Y, Kunesch N, Libot F, Poisson J, Budzikiewicz H. (1975). Alkaloids of Voacanga. XV. Structure of new alkaloids from the leaves of Voacanga thouarsii. Bulletin de la Societe Chimique de France, 11, 2503–2506.
- Stoeckigt J, Pawelka KH, Tanahashi T, Danieli B, Hull WE. (1983). Voafrine A and voafrine B new dimeric indole alkaloids from cell suspension cultures of Voacanga africana Stapf. Helv Chim Acta, 66, 2525–2533.
- Tan PV, Penlap VB, Nyasse B, Nguemo JD. (2000). Anti-ulcer actions of the bark methanol extract of Voacanga africana in different experimental ulcer models in rats. J Ethnopharmacol, 73, 423–428.
- Thomas DW, Biemann K. (1968). The alkaloids of Voacanga africana. Lloydia, 31, 1–8.