Abstract
Context: Holothuria polii (H. polii) Linnaeus (Holothuriidae), Actinopyga mauritiana (A. mauritiana) Quoy & Gaimard (Holothuriidae) andBohadschia vitiensis(B. vitiensis) Semper (Holothuriidae) are sea cucumbers inhabiting the coasts of Egypt. Their tegument and the cuvierian gland contained a substance called holothurin that was used in traditional medicine. These three species are abundant in the Egyptian coast, however there are no reports about their efficacy as antiparasitic agent.
Objective: The antischistosomal effect of the holothurin extracted from the three species of sea cucumber is investigated.
Materials and methods: The ethanol extract was made from the tegument of bothH. poliiandA. mauritianawhile it was made from the cuvierian gland ofB. vitiensis. The body wall (or cuvierian gland) of the sea cucumber was blended with 95% ethanol in a volume = 4 × tissue weight. Extraction was done at room temperature for one day then filtered. The ethanol was removed by evaporation using Rotavapour (BÜCHI 461 water bath REIII) at 40°C. Later the aqueous residue was placed in a vacuum oven at 20°C for about 48 h to remove water. The resulting dried mass was then stored at −4°C until use. The percentage yield and the LD50 were calculated for each extract. Each extract was administered orally toShistosoma mansoni infected mice in acute and chronic phases of infection. The dose of one-tenth of LD50 of each extract was administrated to mice (5.4, 62.2, and 10 mg/kg body weight/mouse forH. polii extract (HPE),A. mauritianaextract (AME), and cuvierian gland ofB. vitiensis, respectively) for 24 h. The effects of each extract on the worm burden and total egg count was studied. The effects of each extract on the worm tegument using scanning electron microscope (SEM) were investigatedin vivo andin vitro.
Results: The percentage yield of cuvierian gland extract (CGE) was higher (70%) than the tegument AME (33.4%) and HPE (9.3%). The 24 h LD50 of investigated sea cucumber ethanol extracts were 54.46, 627, and 100 mg/kg body weight/mouse for HPE, AME, and CGE. Oral administration of HPE caused decrease in male and female worm burden of 30-day infected mice to reach 60 and 90%, respectively. HPE decreased the egg count significantly in those mice with 30-day (1.75 egg counts/g tissue,p < 0.05) and 45-day (3.25 egg counts/g tissue,p < 0.05) infections. SEM studies of recovered worms from treated mice with all extracts showed different tegumental changes like formation of blebs, wrinkling, formation of numerous pores, and rupturing of some tubercles. These effects were more pronounced in those worms treatedin vitro represented by severe shrinkage of the tegument, deformation of spines, rupturing, and collapsing of tubercles.
Discussion and conclusion: Results support the hypothesis that holothurin is a promising antischistosomal agent.
Keywords::
Introduction
Sea cucumber is a worldwide echinoderm of the class Holothuroidea, with an elongated body and leathery skin, which is found on the sea floor. Sea cucumbers have long been used as a source of traditional medicines in Malaysia (CitationHassan et al., 1996). Modern research has shown that sea cucumber extracts have wound healing effects (CitationHassan et al., 1996), besides, they contain anticoagulant, antithrombotic compounds, cholesterol, lipid reducing compounds (CitationZancan & Mourao, 2004), anticancer compounds (CitationHatakeyama et al., 2002; CitationKariya et al., 2004), antiinflammatory (CitationYamonouchi, 1955; CitationNigrelli, 1972; CitationIdid et al., 2001) and an antifungal compounds (CitationSedov et al., 1990; CitationOmran, 2006). CitationYoshida et al. (2007) found that a lectin from sea cucumberCucumaria echinata Von Marenzeller (Cucumariidae) impaired the development of the malaria parasite when produced by transgenic mosquitoes.
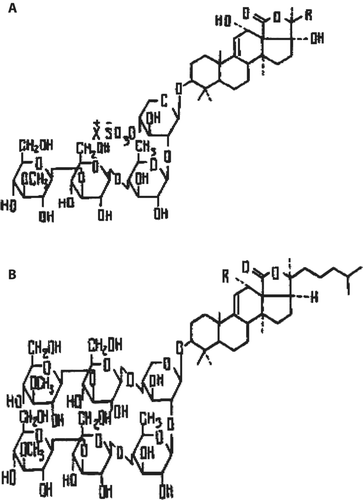
CitationYamonouchi (1955) was the first to coin the term holothurin to signify the biologically active compound produced by the body wall of holothuroid cucumbers. This compound is found also in the cuvierian tubules and the coelomic fluid. Holothurin was isolated from Pacific, Indo Pacific, and Atlantic species belonging to the Holothuriidae family and particularly from species ofHolothuria andActinopyga genera (CitationLevin et al., 1984). The structure of the holothurin extracted from the two latter species has been established by CitationKitagawa et al. (1978, Citation1982). Holothurin formed from triterpene glycosides possess a 12-α-hydroxy-9(11)-ene fragment in the glycone and disaccharide or tetrasaccharide part with anO-sulphate group (). This structure is restricted to species ofHolothuria andActinopyga genera (CitationStonik, 1986), while holothurin extracted fromBohadschia species contains glycosides without anO-sulphate group and with holost-9-(11)-en-12-α-3β-ol as a genuine glycone (). This type of glycoside was named bohadschioside (CitationStonik, 1986).
Figure 1. Structure of some glycosides from Holothurin family (CitationStonik 1986): A, Holothurin structure fromHolothuria andActinopyga genera. B, Bohadschiosides from genusBohadschia.
Schistosomiasis is a disease caused by an infection with parasitic blood flukes of the genusSchistosoma.This fluke is distributed across Africa, the Arabian Peninsula, South America, and some locations in the Caribbean. More than 2 billion people, about one third of the world’s population, are infected with these parasitic helminthes (WHO, Citation2005). Several schistosomocide drugs were administered to treat schistosomiasis, but praziquantel (PZQ) showed the successful results in the treatment because of its effectiveness and insignificant side effects (CitationAdam et al., 2005). However, after 30 years of PZQ usage, a decreased susceptibility to the drug and the emergence of drug-resistant strains was reported (CitationIsmail et al., 1999; CitationKing et al., 2000; CitationBotros et al., 2005; CitationRiad et al., 2009).
The present study investigated the antiparasitic activity of sea cucumber extract againstSchistosoma mansoni (S. mansoni)as a model of endemic parasite in Egypt. Three species of sea cucumber were usedHolothuria polii (H. polii),Bohadschia vitiensis (B. vitiensis), andActinopyga mauritiana (A. mauritiana). The efficacy of the holothurin was tested at the acute and the chronic phases of the infection and estimated by monitoring the worm burden, egg count, and the worm surface topographyin vivo andin vitro using scanning electron microscope (SEM).
Materials and methods
Experimental animals
Adult male albino mice,Mus musculus were purchased from Theodor Bilharz Research Institute, Egypt. Mice were weighing between 19–22 g at the beginning of the experiments. The mice were kept in the laboratory for 1 week before the experimental work and maintained on a standard diet and water availablead libitum. The temperature in the animal room was maintained at 23 ± 2°C with a relative humidity of 55 ± 2%. Mice were kept at regular light and dark cycle. The experimental protocol was approved by local ethics committee and animals research.
Mice infection
Collection ofS. mansoni cercariae and the infection of mice were carried out according to CitationChristensen et al. (1984). The mice were individually exposed to about 100 ± 10 cercariae by the tail immersion technique.
Praziquantel
This drug is a chemotherapeutic treatment of schistosome infection. It was purchased from Alexandria Company for drugs and chemicals (Alexandria, Egypt). PZQ was administered at days 30 and 45 in one oral dose of 685 g/kg (b.w.) (CitationEl-Bolkiny & Al-Sharkawi, 1997).
Collection of sea cucumbers
The sea cucumberH. polii was collected from the intertidal region at Abu-Kir coast (Mediterranean Sea) during July 2006, whileB. vitiensis andA. mauritiana were collected from the intertidal zone of Hurgada coast (Red Sea) in May 2006.
Preparation of crude extract
The ethanol extract was made from the tegument of bothH. poliiandA. mauritianawhile crude extract was made from the cuvierian gland ofB. vitiensis. All extracts were prepared according to CitationShimada (1969) with some modifications. Briefly, the body wall (or cuvierian gland) of the sea cucumber was blended with 95% ethanol in a volume = 4 × tissue weight. Extraction was done at room temperature for one day then filtered. The ethanol was removed by evaporation using Rotary evaporator (BÜCHI 461 water bath REIII) at 40°C. Later the aqueous residue was placed in a vacuum oven at 20°C for about 48 h to remove water. The resulting dried mass was then stored at −4°C until use.
Determination of the percentage of yield of different extracts
The percentage yield of each extract was calculated according to the following formula:
Determination of LD50 of different crude extracts
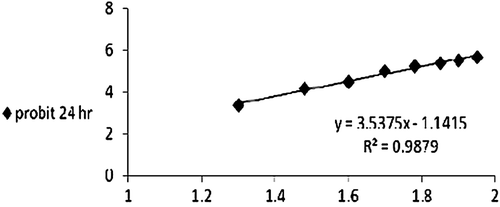
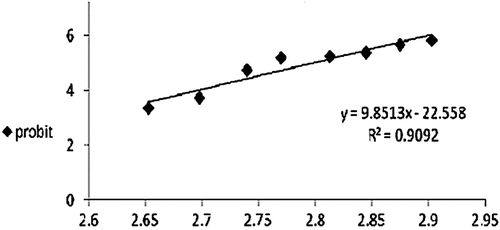
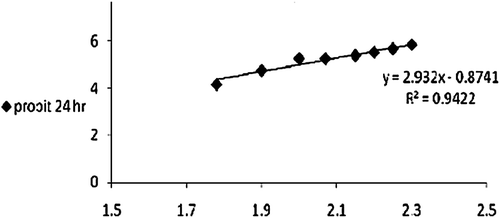
Series of concentrations of each extract that permit the computation of LD50 were prepared. Each concentration was made using pure clean water and the exposure time was 24 h. Data analysis was carried out using Finny program with reliability interval of 95% (CitationFinny, 1971).
Experimental design
Fifty-five mice were divided into three main groups:
Control uninfected (n = 5)
Infected with S.mansoni for 30 days (n = 25)
Infected with S.mansoni for 45 days (n = 25).
The second and the third groups were further divided into five subgroups (five mice per group) as follows:
| a. | Mice treated with 5.4 mg/kg b.w./mouse ofH. polii extract (HPE). | ||||
| b. | Mice treated with 62.2 mg/kg b.w./mouse ofA. mauritiana extract (AME). | ||||
| c. | Mice treated with 10 mg/kg b.w./mouse of cuvierian gland extract (CGE). | ||||
| d. | Mice treated with PZQ 685mg/kg b.w./mouse in a single oral dose. | ||||
| e. | Control infected mice. | ||||
Worm recovery
Worms were collected from control and treated mice by perfusion method as described by CitationChristensen et al. (1984). The collected worms from treated mice were examined by SEM to assess thein vivo effect of different extracts on their tegument, while those collected from the control mice were subjected toin vitro study.
In vitro study of the effect of different extracts onS. mansoni worm
Male and female worms were divided into three glass watches; in each, 7 worms in 1 ml saline (9%). One of each extract was added to each glass watch in doses the same as usedin vivo to show whether they was affected by endogenous factors of the mice which may decrease their effectiveness. The time taken by the worm untill death was calculated in each group.
Liver egg count
Total egg count was assessed as described by CitationPellegrino et al. (1962).
SEM studies
Thein vivo andin vitro effect of different extracts on the worm’s tegument was assessed using AMRAY 1200 Bor JEOL-JSM 5300 D.E.M. Specimens were washed many times vigorously by shaking in 9% saline, and then fixed in cold 2.5% glutraldahyde in phosphate buffer at 4°C overnight. Specimens were postfixed using 1% osmium tetraoxide solution in 0.1 M phosphate buffer for 1–3 h at 4°C. The specimens were then washed for three times in phosphate buffer and dehydrated in an ascending series of ethanol at room temperature. Specimens were mounted on the metal stubs, coated with gold using Spi-modul vac spatter coater and examined with AMRAY 1200 Bor JEOL-JSM 5300 D.E.M.
Statistics
The obtained results were statistically analyzed using Student’st-test (CitationKnapp & Miller, 1992) to determine the significant differences between treated and control specimens.
Results
The yield from the tegument ofH. poliiandA. mauritianawas 9.3 and 33.4%, respectively, from the cuvierian gland ofB. vitiensis it was 70%.
As shown the CGE showed the highest yield followed by AME and HPE.
To investigate the suitable dose of all investigated extracts, the LD50 was estimated for each extract as shown in , and and . The one-tenth the LD50 of each extract was administered to mice for 24 h. For HPE the dose used was 5.4 mg/kg b.w./mouse while those for AME and cuvierian gland ofB. vitiensis were 62.2 and 10 mg/kg body weight/mouse, respectively.
Table 1. Lethal dose values after 24 h exposure time to Sea Cucumber extracts.
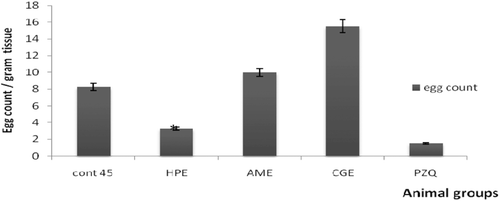
Oral administration of HPE to 30-day infected mice caused a significant decrease in male and female worm burden reached 60 and 90%, respectively (). This reduction of the worm burden was accompanied with a significant decrease in the liver egg count ().Administration of HPE to 45-day infected mice caused insignificant decrease in the worm burden (). But it caused a significant reduction in the liver egg count (). Both AME and CGE caused no significant changes on the worm burden and the egg count of day 30 and day 45 infected mice ().
Figure 5. The effect of different sea cucumber extracts on the worm burden of 30-days infected mice. *Significantp < 0.05. HPE,Holothuriapolii extract; AME,Actinopygamauritiana extract; CGE, cuvierian gland extract; PZQ, praziquantel; cont 30, Control infected mice for 30 days.
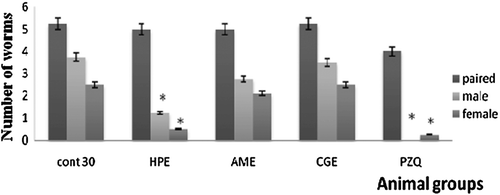
Figure 6. The effect of different extracts on the egg count of 30-days infected mice. *Significantp < 0.05. HPE,Holothuriapolii extract; AME,Actinopygamauritiana extract; CGE, cuvierian gland extract; PZQ, praziquantel; cont 45, Control infected mice for 45 days.
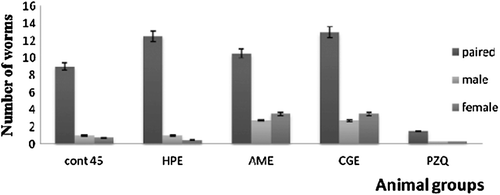
Figure 7. The effect of different sea cucumber extracts on the worm burden of 45-days infected mice. HPE,Holothuriapolii extract; AME,Actinopygamauritiana extract; CGE, cuvierian gland extract; PZQ, praziquantel; cont 30. Control infected mice for 30 days.
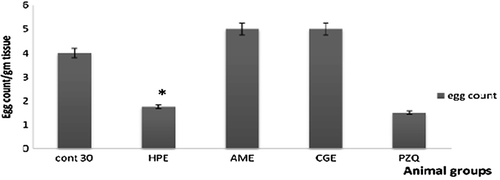
Figure 8. The effect of different extracts on the egg count of 45-days infected mice. *Significantp < 0.05. HPE,Holothuriapolii extract; AME,Actinopygamauritiana extract; CGE, cuvierian gland extract; PZQ, praziquantel; cont 45, Control infected mice for 45 days.
Scanning electron microscopy of male worms revealed that the tegument of the worm is smooth at the first third of the body while its remaining parts were sponge like and covered with tubercles armed with spines ( and ).
Figure 9. Photomicrographs of control and treatedSchistosomamansoni tegument. A, Control male worm showing os, vs and gpc. B, The tegument of the middle part of the control worm covered with Tb armed with s and sponge like Tg. C,In vivo treated worms with HPE showing a few blebs (B) and transverse folds (F). D,In vivo treated worm with HPE showing the RTg and deformed Tb. E,In vivo treated male worms with AME showing transverse folds (F) and (RTb). F,Invivo treated worms with CGE showing the tegument with numerous blebs (B) and rough tegument with transverse folds (F). G,In vitro treated worm with AME showing severe shrinkage of both Tg and vs. H,In vitro treated worm with AME showing CTb with deformed s. I,In vitro treated worm with CGE showing CTb and RTb. J,In vitro treated worm with HPE showing RTb and CTb. os, oral sucker; vs, ventral sucker; gpc, gynechophoric canal; Tb, tubercles; s, spines; Tg, tegument; HPE,Holothuriapolii extract; RTg, rough tegument; AME,Actinopygamauritiana extract; RTb, ruptured tubercles; CGE, cuvierian gland extract; CTb, collapsed tubercles.
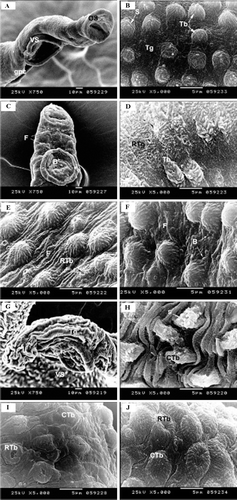
In vivo study of the effect of different extracts (, , and ) showed that all extracts had destructive effect on the tegument including rupturing of tubercles, folding of the tegument, formation of several pores, and blebs. These effects were more pronounced in those worms treatedin vitro represented by severe shrinkage of the tegument, deformation of spines, rupturing and collapsing of tubercles (, , and ).In vitro treatment with AME caused immediate death of the worm. While those treated with HPE and CGE died after 20 min and 30 min, respectively.
Discussion
The emergence of drug-resistant schistosome strains has brought great attention toward natural bioactive compounds. Several studies have been done to evaluate natural substances of plant origin as antischistosomal agents such asCurcuma longa (CitationRizk et al., 2000), myrrh (CitationMassoud, 1998; CitationSheir et al., 2001), garlic (CitationRiad et al., 2009), and ginger (CitationAl-Sharkawi et al., 2007; Mostafa et al., Citation2011). To the best of our knowledge the current study is the first trial to use this substance (holothurin) as an antischistosomal agent.
Among the investigated extracts, HPE showed decreased male and female worm burden to 60 and 90%, respectively, in 30-day infected mice. This reduction was accompanied with liver egg count reduction that reached 56%. Comparing these results with those obtained from PZQ treated mice revealed that HPE may be a promising antischistosomal agent. Another point that should be taken into consideration is that the effect of HPE is significant when administered at an early stage of infection (30 days of infection; the acute phase). Comparable findings were obtained by CitationRiad et al. (2007) who reported a significant reduction in the egg load after treating infected mice with aqueous garlic extract at acute phase.
Despite the significant effect of HPE during the early stage of infection, it did not reveal any significant change in the worm burden of 45 day-infected mice. It caused, however, 56% reduction in the liver egg count. Accordingly, it can be suggested that the reduction in the egg count is not due to the decrease in the worm burden but it might be due to its ability to decrease worm fecundity.
Recovered worms from treated mice with all extracts showed different tegumental changes like formation of blebs, wrinkling, formation of numerous pores, and rupturing of some tubercles. These tegumental alterations would impair the functioning of the defence system of the worm, so that it could easily be attacked by the host’s immune system (CitationXiao et al., 2000). Moreover, it results in the inability of the worm to adhere to the walls of the host blood vessels, causing the schistosome to be dislodged and moved by blood stream from mesenteric veins to the portal vein and intravenous hepatic capillaries and become lodged in the liver which is followed by the death and disintegration of the parasites (CitationMehlhorn et al., 1981; CitationRiad et al., 2009; CitationGnanasekar et al., 2009).
There is no available information about the effect of holothurin on the tegument. But there are other related studies about the effect of antischistosome drugs that reported tubercular disruption and loss of tubercular spines as a result of treatment (CitationAmin & Mikhail, 1989; CitationMostafa & Soliman 2002; CitationShaohong et al., 2006; CitationTaha & Soliman 2007; CitationRiad et al., 2009).
Although thein vivo administration of AME and CGE showed no effect on the worm burden or on the egg count, the SEM revealed severe destruction of the worm tegument as manifested by severe shrinkage of the tegument, collapsing and rupturing of tubercles beside deformation of spines, suggesting that these two extracts express strong schistosomocide. These defects could be attributed to the act of their metabolic pathways in infected mice that suppressed the activity of the extracts biological compounds or it may be attributed to the dose regimen or to the period of treatment that resulted in low serum concentration of the curative compound that reach mesenteric and portal vessels.
Conclusion
The results obtained suggest that sea cucumbersH. polii can be considered as a natural source for a new adjuvant antiparasitic drug with conventional therapy such as PZQ. Further study must be conducted to improve the effect of the other investigated extractsin vivo, and to examine their effects on the liver status of the infected mice.
Declaration of interest
The authors report no conflicts of interest.
Acknowledgments
We express our sincere thanks and appreciation to Prof. Amal Khalil Eskander and Prof. Mohamed Labib Salem for their kind revision of the manuscript.
References
- Adam I, Elwasila E, Homeida M. (2005). Praziquantel for the treatment ofSchistosomiasis mansoniduring pregnancy. Ann Trop Med Parasitol, 99, 37–40.
- Al-Sharkawi IM, El-Shaikh KA, Tabl GA, Ali JA. (2007). The effect of ginger onSchistosoma mansoniinfected mice. Delta J Sci, 31, 1–10.
- Amin AM, Mikhail EG. (1989). Schistosoma mansoni: Tegumental surface alterations following oxamniquine treatment of infected mice. J Egypt Soc Parasitol, 19, 815–826.
- Botros S, Sayed H, Amer N, El-Ghannam M, Bennett JL, Day TA. (2005). Current status of sensitivity to praziquantel in a focus of potential drug resistance in Egypt. Int J Parasitol, 35, 787–791.
- Christensen NO, Gotsche G, Frandsen F. (1984). Parasitological techniques for use in routine laboratory maintenance of schistosomes and for use in studies on the epidemiology of human and bovine Schistosomiasis. WHO Collaborating Centre for Applied Malacology: Danish Bilharziasis Laboratory, 40.
- El-Bolkiny YE, Al-Sharkawi IM. (1997). Intolererance of indomethacin by liver of mice withSchistosomiasis mansoni involvement. J Union Arab Biol, 7(A) Zool, 227–245.
- FinnyDJ. (1971). Estimation of the median effective dose.. In: Probit Analysis, 3rd ed. Cambridge University. Great Britain, 20–49.
- Gnanasekar M, Salunkhe AM, Mallia AK, He YX, Kalyanasundaram R. (2009). Praziquantel affects the regulatory myosin light chain ofSchistosoma mansoni. Antimicrob Agents Chemother, 53, 1054–1060.
- Hassan YN, Yeap KH, Shahimi MM. (1996). Effect of sea cucumber water extraction on systemic anaphylactic reaction. Malaysian J Med Sci, (Supp.) 3, 37–38.
- Hatakeyama T, Matsuo N, Shiba K, Nishinohara S, Yamasaki N, Sugawara H, Aoyagi H. (2002). Amino acid sequence and carbohydrate-binding analysis of theN-acetyl-d-galactosamine-specific C-type lectin, CEL-I, from the Holothuroidea,Cucumaria echinata. Biosci Biotechnol Biochem, 66, 157–163.
- Idid SZ, Jalaluddin DM, Ridzwan BH, Bukhori A, Nor Hazlinah S, Hoo CC. (2001). Effect of two extracts fromSticopus badionotus Slenka upon induced pleurisity in rat. Pakistan J Biol Sci, 4, 1291–1293.
- Ismail M, Botros S, Metwally A, William S, Farghally A, Tao LF, Day TA, Bennett JL. (1999). Resistance to praziquantel: direct evidence fromSchistosoma mansoni isolated from Egyptian villagers. Am J Trop Med Hyg, 60, 932–935.
- Kariya Y, Mulloy B, Imai K, Tominaga A, Kaneko T, Asari A, Suzuki K, Masuda H, Kyogashima M, Ishii T. (2004). Isolation and partial characterization of fucan sulfates from the body wall of sea cucumberStichopus japonicus and their ability to inhibit osteoclastogenesis. Carbohydr Res, 339, 1339–1346.
- King CH, Muchiri EM, Ouma JH. (2000). Evidence against rapid emergence of praziquantel resistance inSchistosoma haematobium, Kenya. Emerging Infect Dis, 6, 585–594.
- Kitagawa I, Yamanaka H, Kobayashi M, Nishino T, Yosioka I, Sugawara T. (1978). Saponins and sapogenols XXVII. Revised structures of holotoxin A and holotoxin B, two antifungal oligoglycosides from the sea cucumberStichopus japonicus Selenka. Chem Pharm Bull, 26, 3722–3731.
- Kitagawa I, Kobayashi M, Kyogoku Y. (1982). Marine natural products. IX. Structural elucidation of triterpenoidal oligoglycosides from the Bahamean sea cucumberActinopyga agassizi Selenka. Chem Pharmacol Bull, 30, 2045–2050.
- Knapp RG, Miller MC. (1992). Defining normality using the predictive value method In: Cinical Epidemiology and Biostatistics, 1st ed. National Medical Series (NMS), Egyptian edition CMASS publishing Co, 53–60.
- Levin VS, Kalinin VI, Stonik VA. (1984). Chemical characters and taxonomic revision of holothurianBohadschia graeffei (Semper) as refer to elucidation of a new genus. Biol Morya, 3, 33–38.
- Massoud A, Salama O, Bennett JL. (1998). Therapeutic efficacy of new antischistosomal drug, derived from myrrh, in active intestinal schistosomiasis complicated with hepatosplenomegaly. In: Tada I, Kojima S, Tsuji M, eds. Proceedings of the 9th International Congress of Parasitology (ICOPA IX), Chiba, Japan. Bologna: Monduzzi Editore, 619–623.
- Mehlhorn H, Becker B, Andrews P, Thomas H, Frenkel J. (1981). In vivo &in vitro experiments on the effects of praziquantel onSchistosoma mansoni. A light and electron microscopic study. Arzneimittelforschung, 31, 544–554.
- Mostafa OM, Soliman MI. (2002). Experimental use of black-seed oil againstSchistosoma mansoni in albino mice: II. Surface topography of adult worms. Egypt J Med Lab Sci, 11, 79–85.
- Mostafa OM, Eid RA, Adly MA. 2011). Antischistosomal activity of ginger (Zingiber officinale) againstSchistosoma mansoni harbored in C57 mice. Parasitol Res, 109, 395–403.
- Nigrelli F. (1972). The effect of holothurin on fish and mice with sarcoma-188. Zoologyica, 37, 89–90.
- Omran, NEE. (2006). Fungicidal and bactericidal effects of holothurin extracted from four species of sea cucumber inhabiting Mediterranean Sea and Red sea coast of Egypt. Proc 4th Int Con Biol Sci (Zool), 1–6.
- Pellegrino J, Oliveira CA, Faria J, Cunha AS. (1962). New approach to the screening of drugs in experimentalSchistosomiasis mansoniin mice. Am J Trop Med Hyg, 11, 201–215.
- Riad NHA, Taha HA, Mahmoud YI. (2009). Effects of garlic on albino mice experimentally infected withSchistosoma mansoni: A parasitological and ultrastructural study. Trop Biomed, 26, 40–50.
- Riad NH, Fares NH, Mostafa OM, Mahmoud YI. (2007). The effect of garlic on some parasitological parameters and on hepatic tissue reactions in experimentalSchistosomiasis mansoni. J App Sci Res, 3, 949–960.
- Rizk M, Hafez S, Farouk H. (2000). Measurement of urea cycle enzyme activities in mice under the influence of different stages ofSchistosoma mansoni infection andCurcuma longa treatment. J Egypt Ger Soc Zool, 32, 319–333
- Sedov AM, Apollonin AV, Sevast’ianova EK, Alekseeva IA, Batrakov SG, Sakandelidze OG, Likhoded VG, Stonik VA, Avilov SA, Kupera EV. (1990). [Stimulation of nonspecific antibacterial resistance of mice to opportunistic Gram-negative microorganisms with triterpene glycosides from Holothuroidea]. Antibiot Khimioter, 35, 23–26.
- Shaohong L, Kumagai T, Qinghua A, Xiaolan Y, Ohmae H, Yabu Y, Siwen L, Liyong W, Maruyama H, Ohta N. (2006). Evaluation of the anthelmintic effects of artesunate against experimentalSchistosoma mansoni infection in mice using different treatment protocols. Parasitol Int, 55, 63–68.
- Sheir Z, Nasr AA, Massoud A, Salama O, Badra GA, El-Shennawy H, Hassan N, Hammad SM. (2001). A safe, effective, herbal antischistosomal therapy derived from myrrh. Am J Trop Med Hyg, 65, 700–704.
- Shimada S. (1969). Antifungal steroid glycoside from sea cucumber. Sci, 83, 735–737.
- Stonik VA. (1986). Some terpenoid derivatives from echinoderms and sponges. Pure Appl Chem, 58, 423–436.
- Taha HA, Soliman MI. (2007). Antischistosomal activity of 3-substituted-5-(2-aryl-2-oxoethyl)-2, 4-dioxo-1, 3-thiazolidine (Ro-354). Inter J Agri Biol, 9, 87–93.
- WHO. 2005). Deworming for health and development. In: Report of the Third Global Meeting of the Partners for Parasite Control. Geneva: World Health Organization, 51.
- Xiao SH, Shen BG, Chollet J, Utzinger J, Tanner M. (2000).Tegumental changes in 21-day-oldSchistosoma mansoni harboured in mice treated with artemether. Acta Trop, 75, 341–348.
- Yamonouchi T. (1955). On the poisonous substances contained in holothurin. Publication of Seto Marine Biol Lab, 41, 183–202.
- Yoshida S, Shimada Y, Kondoh D, Kouzuma Y, Ghosh AK, Jacobs-Lorena M, Sinden RE. (2007). Hemolytic C-type lectin CEL-III from sea cucumber expressed in transgenic mosquitoes impairs malaria parasite development. PLoS Pathog, 3, e192.
- Zancan P, Mourão PA. (2004). Venous and arterial thrombosis in rat models: Dissociation of the antithrombotic effects of glycosaminoglycans. Blood Coagul Fibrinolysis, 15, 45–54.