Abstract
Objective: Achillea species are endowed with multiple biological activities including antioxidant properties. However, no study has yet investigated the impact of extraction method and pH on the biological activities of these plants. The present study aimed to investigate the antioxidant and antimicrobial effects of methanol extracts from the aerial parts of the species Achillea biebersteinii Afan and Achillea wilhelmsii C. Koch (Asteraceae). In addition, the impact of extraction method and pH on these biological activities was evaluated.
Materials and methods: Methanol extracts of A. biebersteinii and A. wilhelmsii were prepared using classical maceration and high-intensity ultrasound methods. Ultrasound-assisted extraction was performed at three different pH values: 5.7, 6.3 and 6.9.
Results: Total phenolic compounds (range: 20.16–108.54 vs. 17.18–59.61 mg gallic acid equivalent/g sample in A. biebersteinii and A. wilhelmsii, respectively), total flavonoids (range: 8.33–12.97 vs. 7.79–9.41 mg catechin equivalent/g sample), 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity (IC50: 40.63–346.34 vs. 84.02–462.41) and reducing power (IC50: 504.44–4406.67 vs. 1710.00–5501.67) were significantly higher in A. biebersteinii vs. A. wilhelmsii and ultrasound-assisted vs. classical maceration extracts of both species. The aforementioned items were higher at pH = 6.3, followed by pHs of 6.9 and 5.7, respectively. Overall, A. biebersteinii extracts were more active against all of the tested microorganisms than A. wilhelmsii. Sensitivities of Gram-positive bacteria were higher for both Achillea extracts than the Gram-negative bacteria. No observable inhibitory activity was found from different extracts against Aspergillus niger.
Conclusion: The findings of the present study suggest that methanol extracts of A. biebersteinii and A. wilhelmsii possess antioxidant and antimicrobial activity, being higher in the former. Ultrasound-assisted extraction and pH of 6.3 have significant augmenting impact on the total phenolic and flavonoid content as well as antioxidant activities of both species.
Introduction
Plant-derived drugs constitute a considerable fraction of world’s total medicines. To date, numerous herbs have been reported for their medicinal properties (CitationTajkarimi et al., 2010; CitationMartinia et al., 2009). Moreover, global awareness about harmful side effects of chemical drugs has promoted investigation on the therapeutic effects of herbal products. Besides their various medicinal features, the antimicrobial and antioxidant effects of plants are well investigated (CitationMartinia et al., 2009; CitationDe et al., 2009; CitationEntezari et al., 2009; CitationTajkarimi et al., 2010).
Microorganisms, including bacteria and fungi, are the main causes of various human infections (CitationMatasyoh et al., 2009) and are often responsible for the loss of food safety and quality. These microorganisms are increasingly the focus of investigations due to the outbreaks of food-borne diseases. Importance of herbal antimicrobial agents can be more appreciated, because about 30% of people in industrialized countries suffer from a food-borne disease each year (CitationNedorostova et al., 2009). Furthermore, it has been estimated that 70% of bacterial infections in US hospitals involves strains that are resistant to at least one drug (CitationCushnie et al., 2005). Natural antimicrobials, especially those obtained from herbal materials, have received great attention in recent decades because they are able to control microbial growth, reduce the need for antibiotics, delay microbial spoilage and decrease development of antibiotic resistance by pathogenic microorganisms (CitationTajkarimi et al., 2010).
In addition, it has been indicated that free radicals generated in human body can cause oxidative damage, resulting in critical diseases such as atherosclerosis, coronary heart disease, aging and cancer (CitationBajpai et al., 2009). Natural antioxidants obtained mainly from herbal materials are effective agents in the prevention of these adverse effects of free radicals.
The genus Achillea is one of the most famous genera of the Asteraceae family. Species included in this genus are widespread around the world and many of them display medicinal properties. There are different species belonging to Achillea in different parts of Iran. Achillea species have been used in folk medicine for a long time (CitationBozin et al., 2008; CitationTajik et al., 2008). Some applications of Achillea in folk medicine include treatment of pain, inflammation, headache and spasmodic diseases (CitationTajik et al., 2008). The therapeutic importance of this genus is so high that it has been included in national pharmacopoeias of some European countries (CitationTajik et al., 2008). In recent years, several studies have been reported dealing with antioxidant and antimicrobial properties of Achillea (CitationKonyalioglu and Karamenderes, 2005; CitationBozin et al., 2008).
Once the medicinal, including antimicrobial and antioxidant, effects of a given plant extract are confirmed, it is reasonable to seek the optimum and efficient extraction method. All extraction methods have been set up to facilitate release of intra-cellular compounds into extraction medium. The more the violation and cell wall is broken, the higher efficiency of extraction method will be achieved. Many extraction methods have been used for the extraction of plant metabolites. Classical maceration (CitationEntezari et al., 2009; CitationStanisavljevi et al., 2009), supercritical fluid extraction (CitationBenelli et al., 2010), ultrasound-assisted extraction (CitationHromadkova et al., 2008; CitationStanisavljevi et al., 2009) and hydrodistillation (CitationBajpai et al., 2009) can be mentioned.
Ultrasound-assisted extraction is a new method of high efficacy in extracting plant bioactives. This technique enhances mass transfer rates by cavitation forces that generate high pressure and consequently cause plant tissues to rupture. This procedure improves the release of intracellular components into extraction solvent which promotes extraction efficiency (CitationCorrales et al., 2008).
Different extraction methods have different efficacy in extracting bioactive materials of plant cells and tissues. Depending on this, the antioxidant and antimicrobial effects of the extracts obtained by these different methods would vary. This is because these effects are proportional to amounts of intra-cellular compounds released in the extraction procedure.
The present study aimed to investigate antioxidant and antimicrobial effects of extracts obtained from two Achillea species namely A. biebersteinii Afan and A. wilhelmsii C. Koch. In addition, the impact of extraction method and pH on these biological activities was evaluated.
Materials and methods
Plant materials
Aerial parts of A. biebersteinii were collected from farms of Astan Qods e Razavi in Toroq, Mashhad in Northeast of Iran and the aerial parts of A. wilhelmsii were collected from Gonabad in West of Iran in May 2010. Specimens were identified by M. R. Joharchi, Ferdowsi University of Mashhad Herbarium Iran, using Flora Iranica (CitationRechinger, 1963) and voucher specimens were deposited at the Herbarium of the Department of Botany in the cited institute [FUMH–Voucher no. ED 22004 (A. biebersteinii) and ED 42232 (A. wilhelmsii)]. The plants were dried at room temperature in a shaded place under air flow and powdered using an electrical mill. The powder was classified by particle size distribution with sieve (no:.100) (CitationLachman et al., 1986). The particle size was less than 149 μ. The powder was stored in a dark, dry and cool place.
Chemicals
Folin-Ciocalteu reagent, butylated hydroxyanisole (BHA, high-performance liquid chromatography (HPLC) grade), sodium carbonate and methanol (HPLC grade) were purchased from Merck, Germany, and 2,2-diphenyl-1-picrylhydrazil (DPPH) from Sigma-Aldrich, USA.
Extraction of plant materials
Maceration and ultrasound-assisted extraction methods were used to obtain leaf and flower extracts. For both methods, 80% methanol was used as solvent.
Maceration extraction
One hundred mL of 80% methanol as extraction solvent was added to 5 g of powdered sample (in a 1:20 w/v ratio) through two 24 h phases using a shaker set at room temperature. After the first phase, the mixture was filtered by Whatman filter paper No.1 and stored in a refrigerator. The part of the mixture remaining on the paper was dried, scratched, weighed and again mixed with methanol (1:20 w/v) and placed on the shaker for 24 h to get thoroughly mixed. After the second 24 h phase, filtration was again performed. The two extracts from the first and second phases were blended. Methanol removal and extract concentration were then performed at 35°C using rotary condenser. Final removal of the solvent was accomplished in vacuum oven under temperature of 35°C and partial vacuum of 60 cm Hg until achieving fixed weight. The dried sample was scraped and stored in dessicator until the time of experiments.
Ultrasound-assisted extraction
Ultrasound-assisted extraction was applied by means of a high intensity ultrasound probe system of 200 W and 24 kHz (model UP 200H, Dr.Hielscher GmbH, Germany) with a horn fitted of microtip: 2 mm (S2) which was immersed in a water bath in which a precipitate glass with the sample (1 g) was placed (internal dimensions: 280:195:135 mm). Acoustic power and intensity of ultrasonic vibrations were 0.171402 W and 21.8346 W/cm2, respectively. Ultrasonic intensity was determined calorimetrically by measuring the time–temperature increase of the solvent under adiabatic conditions (CitationMargulis et al., 2003). The extractions were performed at 35°C (ensured by a temperature controller coupled to the ultrasonic bath) and three different pHs: 5.7, 6.3 and 6.9. After extraction the insoluble part was removed by refrigerated centrifuge (model 2-16 KC, Sigma, Laborzentrigugen GmbH, Germany). Rotary evaporator (Heidolph Laborota 4001 efficient, Germany) was used to remove the solvent after extraction. Soluble part was dried by means of a vacuum oven (Labtech, Korea). A digital scale (HR-200, A&D, Germany) with 0.1 mg accuracy was used for measurement.
Total phenolic determination
Total amount of phenolics in both classic and ultrasound methods was determined according to the Folin-Ciocalteau method (CitationHayouni et al., 2007). To 100 µL of extract having concentration of 2000 ppm in a test tube, 500 µL of diluted solution of Folin-Ciocalteau reagent (1:10 ratio) was added. One min later, 1.5 mL of 20% sodium carbonate was added and stirred. The mixture contained in the test tube was kept for 2 h in darkness and room temperature. Afterwards, absorption of the solution was determined at 760 nm using a Shimadzu UV-Vis spectrophotometer. Distilled water was used as control. Total amount of phenolics was expressed as gallic acid equivalent defined as mg gallic acid per g dried extract. Analyses were performed in triplicate.
Total flavonoid determination
Total flavonoid content was measured using an aluminium chloride (AlCl3)-based colorimetric assay (CitationZhishen et al., 1999). Briefly, plant extracts and catechin (used as the standard compound), were transferred into 10 mL volumetric flasks and the volume was set to 10 mL by distilled water. At zero time, 0.3 mL of sodium nitrite (5% w/v) was added to the flask. After 5 min, 0.6 mL of AlCl3 (10% w/v) was added. Afterwards, at the 6th min, 2 mL of NaOH (1 m) was also added to the mixture followed by 2.1 mL of distilled water. Finally, absorbance was read at 510 nm. Total flavonoid content was expressed as mg catechin equivalents per g dried extract. Analyses were performed in triplicate.
Antioxidant activity (DPPH radical scavenging activity)
Antioxidant activity was determined using 2,2-diphenyl-1-picrylhydrazyl (DPHH) free radical scavenging assay (CitationSiger et al., 2008). To volumes of methanol extract between 100–1000 µL with the concentration of 200 ppm (0.020 g/100 mL) in the test tube, 80% methanol was added to get total volume of 4 mL. Then, 1000 µL of DPPH with concentration of 120 ppm was added and stirred for 30 s. The resultant solution was kept in darkness for 115 min and then absorption was determined at 517 nm by a Shimadzu spectrophotometer. Absorption rate of control sample containing 4 mL methanol and 1 mL DPPH (120 ppm) was also determined after 115 min. Antioxidant activity was calculated according the following formula:
Results were reported as IC50 values (concentration of sample required to scavenge 50% of free radicals). BHA was used as control.
Reducing power assay
Evaluation of the reducing power was carried out based on the method of CitationYildirim et al. (2001). Briefly, different concentrations (50–200 μg/mL) of extracts and BHA (used as the standard compound) were prepared in 1 mL of methanol and then mixed with 2.5 mL of phosphate buffer (0.2 M, pH = 6.6) and 2.5 mL of potassium ferricyanide (10 g/L). Following incubation (50°C; 30 min), 2.5 mL of 100 g/L trichloroacetic acid solution was added to the mixture and then centrifuged at 3000 rpm for 10 min. Afterwards, 2.5 mL of the upper layer was mixed with distilled water (2.5 mL) and 1 g/L FeCl3 (0.5 mL) and the absorbance was read at 700 nm. Analyses were performed in triplicate.
Minimum inhibitory concentration testing
Eight microorganisms including Escherichia coli (PTCC 1399; Persian Type Culture Collection), Escherichia coli 0157 (PTCC 5011), Listeria monocytogenes (PTCC 1165), Salmonella typhi (PTCC 1609) and Pseudomonas aeruginosa (PTCC 1707), as Gram-negative bacteria, Bacillus cereus (PTCC 1247) and Staphylococcus aureus (PTCC 1431), as Gram-positive bacteria and Aspergilus niger (PTCC 5011) and Candida albicans (PTCC 5027) as fungal strains were used. Microorganisms were purchased from PTCC, Iranian Research Organization for Science and Technology, Iran.
The antimicrobial screening tests were performed using 24 h growth culture of bacteria at 37°C and 48 h at 25°C for C. albicans on tryptone soy agar (Himedia, India) which was adjusted to approximately 106 CFU/mL with tryptone soy broth (Himedia, India). For each desired concentration to be tested, a separate solution was prepared by dissolving the dried extract in a few milliliters of methanol and then increasing the volume by Muller Hinton broth until achieving each desired concentration. Each well of 96-well plates were filled with 200 µL of each extract and 20 µL of 106 cell suspension. Culture media alone (to show the sterility of media), gentamicin (5 µg/mL) (as positive control for bacteria) and ketoconazole (250 µg/mL) (as positive control for fungi) were also used. The plates were incubated for 24 h at 37°C for bacteria and 48 h at 25°C for C. albicans.
The growth or no growth of the microorganisms was assessed by methylthiazol tetrazolium (MTT) assay. The amount of 100 µL MTT (5 mg/mL, Sigma) was added to each well and plates were incubated again for about 1 h at 37°C. Minimum inhibitory concentration (MIC) was defined as the lowest concentration of the extract which was able to inhibit the red dye formation.
To determine the MIC of the extracts against A. niger, the poisoned food technique was used with some modifications (CitationPatra et al., 2002). The required quantity of extract was added to sabouraud dextrose agar medium (SDA, Himedia, India). Mycelia discs of 5 mm diameter of A. niger was cut out from the periphery of seven day old culture and aseptically inoculated upside down on the agar surface of the culture medium. Plates were incubated at 25°C and the observations were recorded on the seventh day. Percentage of mycelia growth inhibition (MGI) was calculated using the following formula:
where dc = fungal colony diameter in control (medium without extract), dt = fungal colony diameter in plates containing different concentrations of extract.
The MICs were ascertained by the method of CitationGarber and Huston (1959). This was done by reinoculation of the inhibited fungal discs at MICs on SDA medium. Observations were recorded after 7 days of inoculation. Fungal growth on the seventh day indicated a fungistatic nature, while the absence of fungal growth denoted fungicidal action of the extract.
Statistical analysis
All experiments were run in triplicate. Analyses were performed using statistical software package SPSS (version 11.5). Group comparisons were made using one-way analysis of variance or independent samples t-test. A two-sided p-value of <0.05 was considered statistically significant.
Results
Effect of extraction method on total phenolic content
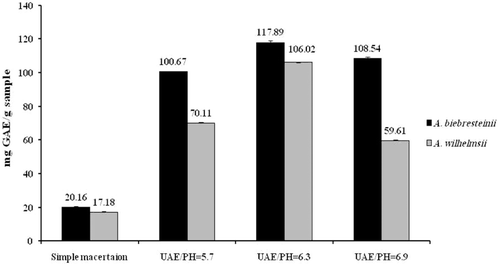
Overall, the extraction of phenolic compounds was significantly higher in the ultrasound compared to the classical maceration method and this was true at all three assessed pHs for both plants (p < 0.001). With respect to different pHs in the ultrasound method, total phenolic content was significantly higher at pH = 6.3 compared to both pHs of 5.7 and 6.9 in both species (p < 0.001). Total phenolics were also higher at pH = 6.9 compared to pH = 5.7 in both tested species (p < 0.001) ().
Effect of plant species on total phenolic content
Total phenolics were found to be higher in the extracts obtained from A. biebersteinii compared to A. wilhelmsii and this was true for both maceration (p = 0.003) and ultrasound (at all assessed pHs) methods (p < 0.001) ().
Effect of extraction method on total flavonoid content
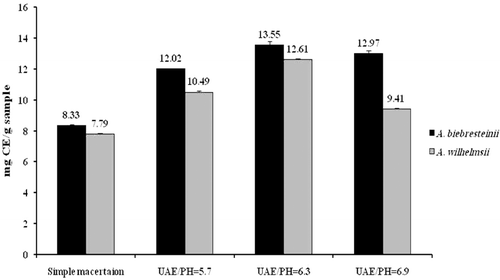
Overall, the extraction of flavonoid compounds was significantly higher in the ultrasound compared to the classical maceration method and this was true at all three assessed pHs for both plants (p < 0.001). With respect to different pHs in the ultrasound method, total flavonoid content was significantly higher at pH = 6.3 compared to both pHs of 5.7 and 6.9 in both A. biebersteinii (p < 0.001 and p = 0.009 for pH = 5.7 and pH = 6.9, respectively) and A. wilhelmsii (p < 0.001). Total flavonoids were higher at pH = 6.9 compared to pH = 5.7 in both tested species (p < 0.001) ().
Effect of plant species on total flavonoid content
Total flavonoids were found to be higher in A. biebersteinii compared to A. wilhelmsii and this was true for both maceration (p = 0.01) and ultrasound-assisted extracts at pH = 5.7 (p < 0.001), pH = 6.3 (p = 0.02) and pH = 6.9 (p < 0.001) ().
Effect of extraction method on radical scavenging activity
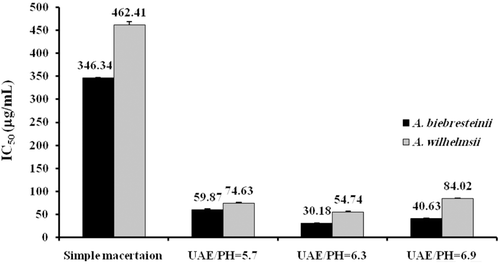
In line with the results of phenolic and flavonoid assays, ultrasound-assisted extraction was associated with higher radical scavenging activity at all three assessed pHs, compared to the classical maceration extraction for both evaluated species (p < 0.001). With respect to the effect of pH, the highest radical scavenging activity was observed at pH = 6.3 which was significantly higher than activities at pHs of 5.7 and 6.9 for both species (p < 0.001). The activity of extract at pH = 5.7 was found to be lower than that at pH = 6.9 in both A. biebersteinii (p < 0.001) and A. wilhelmsii (p = 0.035) ().
Effect of plant species on radical scavenging activity
Significantly higher radical scavenging activity in DPPH test was observed from methanol extract of A. biebersteinii compared to A. wilhelmsii, irrespective of applied extraction method and pH (p < 0.001). In comparison with BHA as the positive control, ultrasound extracts of A. biebersteinii and A. wilhelmsii were found to be significantly more potent at all assessed pHs (p < 0.001). In contrast, BHA had significantly higher activity compared to the maceration extracts of both species (p < 0.001) ().
Effect of extraction method on reducing power
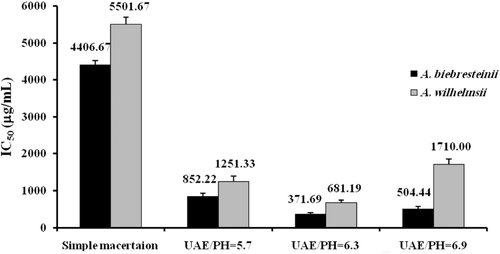
Overall, ultrasound-assisted extraction was associated with a significantly higher reducing capacity at all assessed pHs and in both tested plant species (p < 0.001). As for the effect of pH, the reducing power was found to be significantly lower at pH = 5.7 compared to pHs of 6.3 and 6.9 in both A. biebersteinii (p < 0.001 and p = 0.003 for comparison with pH = 6.3 and pH = 6.9, respectively) and A. wilhelmsii (p = 0.006 and p = 0.021). The IC50 values at pH = 6.3 were lower than those at pH = 6.9 in both species. However, unlike A. wilhelmsii (p < 0.001), this difference did not reach statistical significance in A. biebersteinii (p > 0.05) ().
Effect of plant species on reducing power
In consistence with previous findings, ferric reducing capacity was found to be significantly higher in classical maceration (p = 0.001) as well as ultrasound-assisted (p = 0.014, 0.001 and p < 0.001 at pH = 5.7. 6.3 and 6.9, respectively) extracts of A. biebersteinii compared to those of A. wilhelmsii. The reducing activity of all tested extracts from both species was less than ascorbic acid which was used as the standard compound ().
Antimicrobial activity
MICs of extracts of A. biebersteinii and A. wilhelmsii on all tested bacteria and yeast are reported in . The MIC values against the bacteria were from 0.6 mg/mL to > 40 mg/mL.
Table 1. Minimum inhibitory concentration (mg/mL) of A. biebersteinii and A. wilhelmsii extracts against different bacterial and fungal strains.
As shown in , different extracts of A. biebersteinii were more active against all of the tested microorganisms than A. wilhelmsii. Sensitivities of Gram-positive bacteria were higher for both Achillea extracts than the Gram-negative bacteria. There was not any observable MGI for different extracts against A. niger, as even at highest concentration of the extract, dc was equal to dt.
Discussion
The main issues that were investigated by this research were antioxidant and antimicrobial properties of two endemic Achillea species namely A. biebersteinii and A. wilhelmsii, and the impact of extraction method (classical maceration vs. ultrasound-assisted) and pH on these biological effects. The results of DPPH radical scavenging activity and ferric reducing power tests consistently indicated that ultrasound extracts were of higher activity compared to classical maceration extracts in both tested plant species. As for the activity at different pHs, radical scavenging activity decreased in both species in the following order: 6.3 > 6.9 > 5.7. Since pH = 6.3 is near to the natural pH of the plant, higher activity at this pH could be attributed to the preservation of structure and activity of plant’s active phytochemicals as confirmed by the results of total phenolic and flavonoid assays. The same reason could be stated for the higher activity at pH = 6.9 vs. pH = 5.7 as destruction of phytochemicals is lower in the near-neutral pH compared to acidic pH. Interestingly, the present findings from DPPH radical scavenging and reducing power assays were all in line with the findings of phenolic and flavonoid assays and further confirm the significant impact of phenolic and flavonoid compounds in the total antioxidant activity of a given plant. These phytochemicals have been repeatedly reported for their antioxidant properties and are present in most plant species including those of genus Achillea (CitationStojanovic et al., 2005; CitationTrifunovic et al., 2006; CitationTuberoso et al., 2009). Scavenging of free radicals such as reactive oxygen species by these phytochemicals could lead to the prevention of LDL-oxidation, DNA and membrane damage, and other sequeale of oxidative stress such as aging, mutagenesis and carcinogenesis (CitationRice-Evans et al., 1997; CitationHeim et al., 2002).
Ultrasound-assisted extraction has attracted considerable attention due to its positive impacts on extraction efficiency, extraction time, solvent consumption and particle size (CitationMason et al., 1996; CitationSalisová et al., 1997; CitationVinatoru et al., 1997; CitationValachovic et al., 2001). As mentioned before, these positive improvements by ultrasound waves are mainly attributed to the acoustic cavitation phenomonen. To date, this method has been successfully employed for the extraction of a variety of herbal products and phytochemicals that are of industrial as well as medicinal importance such as pectin, xylans, soybean protein, phenolics, flavonoids, antioxidants, polysaccharides, hydrocarbons, fatty acid esters, etc. (CitationPanchev et al., 1988; CitationHromadkova et al., 1999).
There have been a number of previous reports on the antioxidant activity of Achillea species (CitationTrouillas et al., 2003; CitationCandan et al., 2003; CitationOzgen et al., 2004; CitationSökmen et al., 2004; CitationKundakovic et al., 2005a,Citationb; CitationConforti et al., 2005; CitationBaris et al., 2006, Citation2011; CitationVitalini et al., 2006; CitationWojdylo et al., 2007; CitationArdestani et al., 2007; CitationKizil et al., 2010) (). In two investigations conducted by CitationKundaković et al. (2005a) ethyl acetate and buthanolic extracts of A. alexandri-regis were found to possess hydroxyl radical scavenging activity (CitationKundakovic et al., 2005a), and methanol extract was reported to be active in DPPH radical scavenging test (CitationKundakovic et al., 2005b). In another study, infusions of 15 Achillea species (including A. biebersteinii) from Turkey were evaluated for their antioxidant protection against H2O2-induced oxidative damage. The results indicated that all of the tested species were effective and could positively modulate the activity of antioxidant enzymes (catalase, superoxide dismutase and glutathione peroxidase), increase glutathione and decrease lipid peroxide levels (CitationKonyalioglu and Karamenderes 2005). Methanol extracts from the aerial parts of A. distans and A. moschata were also reported to possess antioxidant capacity in DPPH as well LDL-oxidation assays (CitationSökmen et al., 2004). With respect to A. ligustica, the methanol extract and phenolic fraction from the flowered parts of A. ligustica were reported to exert radical scavenging activity on DPPH (CitationConforti et al., 2005). In another study, ethanol extracts from the flowered tops of A. ligustica were found effective antioxidants against cellular oxidative stress as well as in DPPH and linoleic acid autoxidation assays (CitationTuberoso et al., 2009).
Table 2. Studies on the antioxidant effects of Achillea species.
As for the A. biebersteinii, apart from the study of CitationKonyalioglu and Karamenderes, (2005), there has been some other reports showing the antioxidant activity of: a) polar subfraction of the methanol extract in the DPPH, inhibition of superoxide and hydroxyl radicals, and inhibition of the lipid peroxidation assays (CitationVitalini et al., 2006); b) ethanolic extract in lipid peroxidation, protein oxidation, DNA damage, DPPH, metal chelating, reducing power and deoxyribose assays (CitationKizil et al., 2010; CitationBaris et al., 2011); c) methanol extract in DPPH and linoleic acid oxidation assays (CitationBaris et al., 2006); and d) water extract in lipid peroxidation, DPPH free radical scavenging and reducing power assays (CitationOzgen et al. 2004). Nevertheless, studies on the antioxidant activity of A. wilhelmsii have been much fewer with only one report by CitationOzgen et al., (2004). In addition, no previous investigation has been performed on the biological activities of the ultrasound-assisted extracts from Achillea spp. and their comparison with simple maceration extracts.
In the present study, antibacterial and antifungal activities of Achillea extracts were also investigated. Gram-positive bacterial strains were found to be more susceptible to Achillea extracts. In addition, similar to the findings of antioxidant activity, A. biebersteinii was found to possess stronger antibacterial activity on tested strains. With respect to the antimicrobial properties of phenolics and flavonoids (CitationCushnie et al., 2005), this observation could be partly attributed to the higher phenol and flavonoid content of this species. Data regarding antibacterial and/or antifungal activities of Achillea species are scant with only one previous report by CitationBaris et al. (2006) on A. biebersteinii. In this latter study, weak antibacterial and strong antifungal activity was reported form A. biebersteinii essential oil while the extract did not exert any antimicrobial activity.
Conclusion
Findings of the present study suggest that methanol extracts of A. biebersteinii and A. wilhelmsii possess antioxidant activity, being higher in the former species. Furthermore, current results further confirm the application of high-intensity ultrasound to increase the extraction of phenolics and flavonoids and hence antioxidant effects. Aside from the extraction method, observed antioxidant effects were dependent on the pH of extract and the highest activity was observed at pH = 6.3. These findings might serve as a base for the design of future studies on the optimization of extraction from Achillea species. Finally, further studies are warranted to confirm these results using additional antioxidant activity assays and also to elucidate the effect of extraction method, pH and species on other biological activities of these plants.
Declaration of interest
The authors declare no conflicts of interest.
References
- Ardestani A, Yazdanparast R. (2007). Antioxidant and free radical scavenging potential of Achillea santolina extracts. Food Chem, 104, 21–29.
- Bajpai VK, Al-Reza SM, Choi UK, Lee JH, Kang SC. (2009). Chemical composition, antibacterial and antioxidant activities of leaf essential oil and extracts of Metasequioa glyptostroboides Miki ex Hu. Food Chem Toxicol, 47, 1876–1883.
- Baris D, Kizil M, Aytekin C, Kizil G, Yavuz M, Ceken B. (2011). In vitro antimicrobial and antioxidant activity of ethanol extract of three Hypericum and three Achillea species from Turkey. Int J Food Prop, 14, 339–355.
- Baris O, Gulluce M, Sahin F, Ozer H, Kilic H, Ozkan H, Sokmen M, Ozbek T. (2006). Biological activities of the essential oil and methanol extract of Achillea biebersteinii Afan. (Asteraceae). Turk J Biol, 30, 65–73.
- Benelli P, Riehl CAS, Smania A Jr, Samania EFA, Ferreiera SRS. (2010). Bioactive extracts of orange (Citrus sinensis L. Osbeck) pomace obtained by SFE and low pressure techniques mathematical modeling and extract composition. J Supercrit Fluid, 55, 132–141.
- Bozin B, Mimica-Dukic N, Bogavac M, Suvajdzic L, Simin N, Samojlik I, Couladis M. (2008). Chemical composition, antioxidant and antibacterial properties of Achillea collina Becker ex Heimerl s.l. and A. pannonica Scheele essential oils. Molecules, 13, 2058–2068.
- Candan F, Unlu M, Tepe B, Daferera D, Polissiou M, Sökmen A, Akpulat HA. (2003). Antioxidant and antimicrobial activity of the essential oil and methanol extracts of Achillea millefolium subsp. millefolium Afan. (Asteraceae). J Ethnopharmacol, 87, 215–220.
- Conforti F, Loizzo MR, Statti GA, Menichini F. (2005). Comparative radical scavenging and antidiabetic activities of methanolic extract and fractions from Achillea ligustica ALL. Biol Pharm Bull, 28, 1791–1794.
- Corrales M, Toepfl S, Butz P, Knorr D, Tauscher B. (2008). Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: A comparison. Innovat. Food Sci Emerg Technol, 9, 85–91.
- Cushnie TP, Lamb AJ. (2005). Antimicrobial activity of flavonoids. Int J Antimicrob Agents, 26, 343–356.
- De R, Kundu P, Swarnakar S, Ramamurthy T, Chowdhury A, Nair GB, Mukhopadhyay AK. (2009). Antimicrobial activity of curcumin against Helicobacter pylori isolates from India and during infections in mice. Antimicrob Agents Chemother, 53, 1592–1597.
- Entezari M, Hashemi M, Ashki M, Ebrahimian S, Bayat M, Azizi Saraji AR, Rohani SR. (2009). Studying the effect Echinophora platyloba extract on bacteria (Staphylococus aureus and Pseudomonas aeroginosa) and fungi (Candidia albicans, Aspergilus flavus and Aspergilus niger) in vitro. World J Med Sci, 4, 89–92.
- Garber RH, Houston BR. (1959). An inhibitor of Verticillium alboatrum in cotton seed. Phytopathology, 49, 449–450.
- Hayouni E, Abedrabba M, Bouix M, Hamdi M. (2007). The effects of solvents and extraction method on the phenolic contents and biological activities in vitro of Tunisian Quercus coccifera L. and Juniperus phoenicea L. fruit extracts. Food Chem, 105, 1126–1134.
- Heim KE, Tagliaferro AR, Bobilya DJ. (2002). Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J Nutr Biochem, 13, 572–584.
- Hromádková Z, Kost’álová Z, Ebringerová A. (2008). Comparison of conventional and ultrasound-assisted extraction of phenolics-rich heteroxylans from wheat bran. Ultrason Sonochem, 15, 1062–1068.
- Hromadkova Z, Kovacikova J, Ebringerova A. (1999). Study of the classical and ultrasound-assisted extraction of the corn cob xylan. Industrial Crops and Products, 9, 101–109.
- Kizil M, Kizil G, Yavuz M, Ceken B. (2010). Protective activity of ethanol extract of three Achillea species against lipid peroxidation, protein oxidation and DNA damage in vitro. Acta Aliment Hung, 39, 457–470.
- Konyalioglu S, Karamenderes C. (2005). The protective effects of Achillea L. species native in Turkey against H(2)O(2)-induced oxidative damage in human erythrocytes and leucocytes. J Ethnopharmacol, 102, 221–227.
- Kundakovic T, Mimica Dukic N, Kovacevic N. (2005a). Free radical scavenging activity of Achillea alexandri-regis extracts. Fitoterapia, 76, 574–576.
- Kundakovic T, Stanojkovic T, Juranic Z, Kovacevic N. (2005b). Cytotoxic and antioxidant activity of Achillea alexandri-regis. Pharmazie, 60, 319–320.
- Lachman L, Liberman AH, Kanig JL. (1986). The Theory and Practice of Industrial Pharmacy. Third edition. Section I. Philadelphia PA: Principle of Pharmaceutical Processing. Lea & Febiger. pp. 21–27.
- Margulis MA, Margulis IM. (2003). Calorimetric method for measurement of acoustic power absorbed in a volume of a liquid. Ultrason Sonochem, 10, 343–345.
- Martinia SD, Addario C, Colacevich A, Focardi S, Borghini F, Santucci A, Figura N, Rossi C. (2009). Antimicrobial activity against Helicobacter pylori strains and antioxidant properties of blackberry leaves (Rubus ulmifolius) and isolated compounds. Int J Antimicrob Agents, 34, 50–59.
- Mason TJ, Paniwnyk L, Lorimer JP. (1996). The uses of ultrasound in food technology. Ultrason Sonochem, 74, 511–516.
- Matasyoh JC, Maiyo ZC, Ngure RM, Chepkorir R. (2009). Chemical composition and antimicrobial activity of the essential oil of Coriandrum sativum. Food Chem, 113, 526–529.
- Nedorostova L, Kloucek P, Kokoska L, Stolcova M, Pulkrabek J. (2009). Antimicrobial properties of selected essential oils in vapour phase against food borne bacteria. Food Control, 20, 157–160.
- Ozgen U, Mavi A, Terzi Z, Coskun M, Yildirim A. (2004). Antioxidant activities and total phenolic compounds amount of some Asteraceae species. Turk J Pharm Sci, 1, 203–216.
- Panchev I, Kirchev N, Kratchanov C. (1988). Improving pectin technology. II. Extraction using ultrasonic treatment. Int J Food Sci Technol, 23, 337–341.
- Patra M, Shahi SK, Midgely G, Dlkshit A. (2002). Utilization of essential oil as natural antifungal against nail-infective fungi. Flavour Fragr J, 17, 91–94.
- Rechinger KH. (1963). Flora Iranica. Graz, Austria: Akademische Durck–U. Verlagsanstalt.
- Rice-Evans C, Miller N, Paganga G. (1997). Antioxidant properties of phenolic compounds. Trends Plant Sci, 2, 152–159.
- Salisová M, Toma S, Mason TJ. (1997). Comparison of conventional and ultrasonically assisted extractions of pharmaceutically active compounds from Salvia officinalis. Ultrason Sonochem, 4, 131–134.
- Siger A, Nogala-Kalucka M, Lampart-Szczapa E. (2008). The content and antioxidant activity of phenolic compounds in cold-pressed plant oils. J Food Lipids, 15, 137–149.
- Sökmen A, Sökmen M, Daferera D, Polissiou M, Candan F, Unlü M, Akpulat HA. (2004). The in vitro antioxidant and antimicrobial activities of the essential oil and methanol extracts of Achillea biebersteini Afan. (Asteraceae). Phytother Res, 18, 451–456.
- Stanisavljevi I, Stojievi S, Velickovi D, Veljkovic V, Lazic M. (2009). Antioxidant and antimicrobial activities of Echinacea (Echinacea purpurea L.) extracts obtained by classical and ultrasound extraction. Chinese J Chem Eng, 17, 478–483.
- Stojanovic G, Radulovic N, Hashimoto T, Palic R. (2005). In vitro antimicrobial activity of extracts of four Achillea species: the composition of Achillea clavennae L. (Asteraceae) extract. J Ethnopharmacol, 101, 185–190.
- Tajik H, Shokouhi S, Jalali F, Sobhani A, Shahbazi Y, Soleiman Zadeh M. (2008). In vitro assessment of antimicrobial efficacy of alcoholic extract of Achillea millefolium in comparison with penicillin derivatives. J Anim Vet Adv, 7, 508–511.
- Tajkarimi MM, Ibrahim SA, Cliver DO. (2010). Antimicrobial herb and spice compounds in food. Food Control, 21, 1199–1218.
- Trifunovic S, Vajs V, Juranic Z, Zizak Z, Tesevic V, Macura S, Milosavljevic S. (2006). Cytotoxic constituents of Achillea clavennae from Montenegro. Phytochemistry, 67, 887–893.
- Trouillas P, Calliste C, Allais D, Simon A, Marfak A, Delage C, Duroux J. (2003). Antioxidant, anti-inflammatory and antiproliferative properties of sixteen water plant extracts used in the Limousin countryside as herbal teas. Food Chem, 80, 399–407.
- Tuberoso CI, Montoro P, Piacente S, Corona G, Deiana M, Dessì MA, Pizza C, Cabras P. (2009). Flavonoid characterization and antioxidant activity of hydroalcoholic extracts from Achillea ligustica All. J Pharm Biomed Anal, 50, 440–448.
- Valachovic P, Pechova A, Mason TJ. (2001). Towards the industrial production of medicinal tincture by ultrasound assisted extraction. Ultrason Sonochem, 8, 111–117.
- Vinatoru M, Toma M, Radu O, Filip PI, Lazurca D, Mason TJ. (1997). The use of ultrasound for the extraction of bioactive principles from plant materials. Ultrason Sonochem, 4, 135–139.
- Vitalini S, Grande S, Visioli F, Agradi E, Fico G, Tome F. (2006). Antioxidant activity of wild plants collected in Valsesia, an alpine region of Northern Italy. Phytother Res, 20, 576–580.
- Wojdylo A, Oszmianski J, Czemerys R. (2007). Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem, 105, 940–949.
- Yildirim A, Mavi A, Kara AA. (2001). Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J Agric Food Chem, 49, 4083–4089.
- Zhishen J, Mengcheng T, Jianming W. (1999). The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem, 64, 555–559.