Abstract
Context: Multidrug-resistance is a serious obstacle encountered in leukemia treatment. Recent studies have shown microRNA-21 (miR-21) is overexpressed in several types of cancer and contributes to tumor resistance to chemotherapy. In our previous studies, we found triptolide (TPL) could enhance adriamycin-induced cytotoxicity and apoptosis in K562/A02 cells.
Objective: In the present study, we investigated the mechanism of TPL on the sensitivity of K562/A02 cells to adriamycin.
Materials and methods: Cell viability was assessed by methyl thiazolyl tetrazolium (MTT) assay. Expression of mature miR-21 was determined by SYBER green PCR. The miR-21 mimics and inhibitors were chemically synthesized and transfected into K562 cells or K562/A02 cells. PTEN protein levels was determined by western blots. PTEN promoter activity was measured by luciferase assays.
Results: TPL (5 nmol/L) increased the sensitivity of K562/A02 to adriamycin. When adriamycin was combined with 5 nmol/L TPL, the mean apoptotic population of K562/A02 cells was increased from 4.3 to 18.5%, respectively. K562/A02 cells showed a significant reduction in miR-21 and phosphatase and tensin homolog deleted on chromosome ten (PTEN) expressions after TPL treatment. K562/A02 cells that were transfected with the miR-21 inhibitor had a significantly higher PTEN protein level than the control. K562 cells that were pre-treated with PTEN siRNA had increased survival rate compared to the control group.
Discussion and conclusion: Our findings indicated that triptolide modulates the sensitivity of K562/A02 cells to adriamycin by regulating miR-21 expression. Triptolide inhibited miR-21 expression and enhanced PTEN levels in K562/A02 cells.
Keywords::
Introduction
Adriamycin (ADR), a member of anthracycline family, is a chemotherapeutic agent that is used to treat cancer (CitationLambert & Hainaut, 2007). Adriamycin-based chemotherapy was developed as a clinical application for treating leukemia and became one of the most effective anti-cancer drugs (CitationLayke & Lopez, 2006; CitationPerron & Provost, 2008). However, as with other chemotherapy agents, leukemia cells resistance to ADR continues to be a major clinical obstacle for the successful treatment of leukemia, thus limiting its clinical use (CitationLu et al., 2005).
Recently, more and more studies have shown that microRNAs (miRNAs) may play an important role in the chemosensitivity of cancer cells (CitationBlower et al., 2008; CitationXia et al., 2008; CitationZhu et al., 2008). It is reported that the miR-21 gene has been identified as the only miRNA commonly overexpressed in solid tumors (CitationAsangani et al., 2008). Inhibition of miR-21 could decrease cell growth, induce apoptosis, and suppress migration and invasion in many cancer cells (CitationChan et al., 2005; CitationCheng et al., 2005; CitationAsangani et al., 2008; CitationLu et al., 2008). Furthermore, several studies found the association between miR-21 and sensitivity of leukemia cells to chemotherapy drugs (CitationLi et al., 2010a,Citationb).
In our previous studies, we found triptolide (TPL), a diterpenoid triepoxide derived from Tripterygium wilfordii Hook f (TWHf; Lei Gong Teng), could enhance adriamycin-induced cytotoxicity and apoptosis in K562/A02 cells. But the mechanism is not clear. In this work, we show that triptolide could inhibit miR-21 expression in K562/A02 cells. Additional experiments suggest miR-21 contributes resistance to ADR by inhibiting PTEN expression. Our findings indicate that triptolide modulates the sensitivity of K562/A02 cells to adriamycin by regulating miR-21 expression. Triptolide inhibited miR-21 expression and enhanced PTEN levels in K562/A02 cells.
Materials and methods
Cells and chemicals
The human chronic myeloid leukemia cell line K562, and its multidrug-resistant counterpart K562/A02, were purchased from the Shanghai Institute of Cell Biology, China Academy of Sciences. K562 cells were cultured in a complete RPMI-1640 medium at 37°C in a humidified atmosphere of 5% CO2. K562/A02 was cultured in a medium containing 1 μg/ml adriamycin for maintaining the MDR phenotype. The cells were passaged every 2–3 days and cultured for 2 weeks in drug-free medium prior to their use in the experiments. TPL (Sigma, St. Louis, MO) was dissolved in dimethyl sulfoxide as a 100 nmol/l stock and added to cells at the indicated concentrations.
Cell viability assay
Cell viability was assessed by methyl thiazolyl tetrazolium (MTT) assay as reported (CitationKim et al., 2007). Cells were seeded into 96-well plates in RPMI-1640 medium containing 10% FBS. After 24 h, in the absence and presence of TPL, K562/A02 cells were treated with serial dilutions of ADR. Approximately 72 h after ADR treatment, MTT was added to a final concentration of 0.5 mg/mL, and the cells were incubated for 4 h at 37°C. The optical density was read at 570 nm with a microplate spectrophotometer. Each group was conducted in six individual wells and each experiment repeated three times.
Apoptosis assay
Annexin V/FITC and PI apoptosis detection kit (Becton Dickinson) was used to quantitative the phosphatidylserine in apoptotic cells. Briefly, cells (5 × 105 per well) were seeded into 6-well plates and then treated with 3 μg/mL adriamycin and with or without TPL as mentioned above. After 24 h, the cells were harvested and washed twice with ice-cold PBS (pH 7.2). After 5 min of centrifuging at 200 g, Annexin V/FITC and PI double-staining were performed according to manufacturer’s instruction. Cell apoptosis was analyzed on a FACScan flow cytometry (Becton Dickinson). Annexin V-positive, PI-negative cells were scored as apoptotic. Double-stained cells were considered either as necrotic or as late apoptotic. To confirm the apoptotic process, we investigated the enzymatic activation of apoptotic proteins by measuring the cleavage of poly ADP-ribose polymerase (PARP), which is a caspase-3 substrate.
Quantitative real-time PCR of miR-21
Total RNA was extracted from culture cells with TRIzol® (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. Expression of mature miR-21 was determined by SYBER green PCR using U6 as the internal normalizing control as described previously (CitationChen et al., 2005). All SYBER green PCRs were performed in triplicate. The fold-changes for miR-21 expression levels were calculated using ΔCT (where CT is the threshold cycle value) and 2−ΔΔCT.
MiRNA transfection
The miR-21 mimics and inhibitors were chemically synthesized by Genepharma Corporation (Shanghai, China). K562 and K562/A02 cells were seeded in 6-well plates at 4 × 105 cells/well and cultured for 24 h and then infected with 100 pmol the mimics or inhibitors of miR-21 or negative control (NC) RNA as control using Lipofectamine 2000 (Invitrogen), according to the manufacturer’s protocol.
Western blot assay
Protein expression was determined by western blots. Briefly, 5 × 106 cells were harvested, and cell lysates were prepared using a commercial kit according to the manufacturer’s instructions. Equal amounts of proteins were separated by 12% SDS-PAGE and electro-blotted onto a PVDF membrane. The membrane was incubated with primary antibody (1:300 dilution) for 2 h at room temperature and then incubated with secondary antibody (1:5000 dilution) for 2 h at room temperature. Next, the membrane was incubated in a mixture of ECL-plus A and B (40:1) for 5 min at room temperature and scanned immediately on a Typhoon 9400 Variable Mode Imager. Densitometric analysis was performed using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an internal standard.
Luciferase assay
A PTEN 3′UTR luciferase reporter was constructed as described previously. The PTEN 3′UTR was PCR amplified from human genomic DNA with the following primers: foward, 5′-TCGCTCGAGATTTTTTTTTATCAAGAGGG-3′ and reverse, 5′-TCGGCGGCCGCGACAAGAATGAGACTTTAATC-3′. Mutations within the putative miR-21 binding sites were created with a QuikChange site-directed mutagenesis kit. The mutagenic primers were as follows: 5′-ACTTGTGGCAACAGTTTACTTTGCAGTTG-3′ and 5′-CAACTGCAAAGTAAACTGTTGCCACAAGT-3′. K562 cells were plated in 12-well plates at a density of 4 × 104. The following day, cells were cotransfected with 1.2 μg of reporter plasmid and 0.1 μg of Ranilla luciferase plasmid. Luciferase activity was measured using a commercial kit (Promega). Ranilla luciferase activity was measured using a Ranilla luciferase enzyme assay system to normalize the transfection efficiency.
siRNA transfection
SignalSilence® PTEN siRNA Kit from Cell Signaling Technology (CST) was purchased and the transfection was performed according to the manufacturer’s instructions. The cells were prepared for further experiments 48 h after transfection. The transfection efficiency was evaluated by Flow cytometry by calculating the percentage of fluorescein-positive cells. The transfection efficiency was approximately 70%.
Statistical analysis
All experiments were repeated at least three times; data were expressed as mean ± SD and analyzed with the Statistical Package for Social Science (SPSS Release 12.0; SPSS Inc., Chicago, IL). Comparisons were made using ANOVA. P values below 0.05 were considered as statistically significant.
Results
Cytotoxicity of TPL
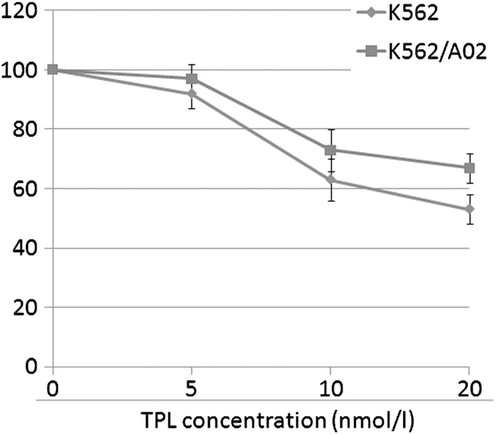
To investigate the effect of TPL on multidrug-resistance, human leukemia cell line K562/ and K562/A02 were used to determine the non-toxic dose of TPL. The cytotoxic effects of TPL on K562/ and K562/A02 cells were measured after a 72 h treatment (). Reversal multidrug agents are required to be kept at appropriate concentrations at which the drugs have no inhibitory or toxic effects on the tested cells. Consequently, a concentration of 5 nm TPL was used to further study the reversal effect of multidrug-resistance.
Reversal of adriamycin resistance in vitro by TPL
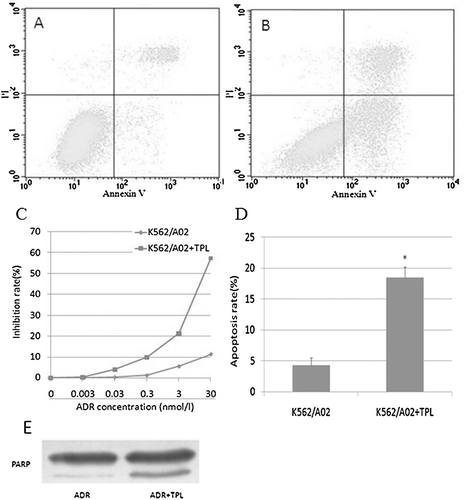
To investigate the effect of TPL on the sensitivity of K562/A02 cells to a chemotherapeutic agent, cells were incubated with 5 nm TPL and a full range of concentrations of adriamycin. Results () showed that 5 nm TPL increased the sensitivity of K562/A02 to adriamycin. Furthermore, the regulation of TPL on the cytotoxicity of adriamycin toward K562/A02 cells was also evaluated by quantification of apoptotic cells. As shown in , when adriamycin treatment alone, the mean apoptotic population of K562/A02 cells was 4.3%. When adriamycin was combined with 5 nm TPL, the mean apoptotic population of K562/A02 cells was increased from 4.3 to 18.5%, respectively (). The apoptosis rates were summarized in . The cleavage of PARP confirmed the apoptotic process (). The results suggested that the increased cytotoxicity on K562/A02 cells from the combination of TPL with adriamycin was achieved through enhancing the adriamycin-induced apoptosis.
Figure 2. Reversal of adriamycin resistance in vitro by TPL. K562/A02 cells were incubated with 3 μg/ml of adriamycin in the presence of TPL or absence of TPL for 24 h. The rate of apoptosis was measured using flow cytometry. The cells inhibition rate was measured by MTT. A: apoptosis in K562/A02 cells; B: apoptosis in K562/A02 cells treated with TPL; C: inhibition rate; D: apoptosis rate; E: the cleavage of PARP.
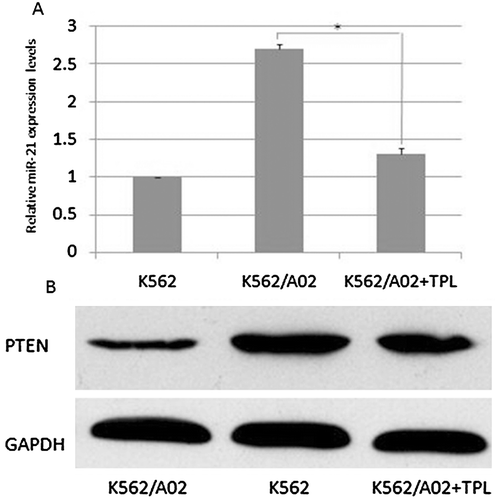
Down-regulation of miR-21 expression and up-regulation PTEN level by TPL
To demonstrate the effect of TPL on miR-21 expression and function, quantitative PCR was performed for miR-21 and U6 snRNA. U6 snRNAs were used as internal controls. The quantitative PCR results showed that the miR-21 expression level was higher in the K562/A02 cells than in the K562 cells, indicating that miR-21 may be associated with adriamycin resistance in K562 cells. As shown in , K562/A02 cells showed a significant reduction in miR-21 expression after TPL treatment. To evaluate effects of TPL on PTEN protein levels in leukemia cells, western blots were used to detect the level of PTEN protein. As shown in , TPL effectively up-regulated PTEN protein levels in K562/A02 cells. This result suggested that TPL can down-regulate miR-21 and up-regulate PTEN expression in K562/A02 cells.
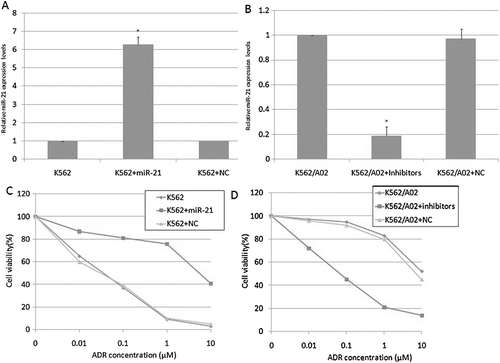
Expression of miR-21 associated with adriamycin resistance in K562 cells
We further investigated the effects of miR-21 on adriamycin cytotoxicity in K562 and K562/A02 cells. The miR-21 levels was increased after infection with miR-21 in K562 cells () and the miR-21 levels was decreased after infection with miR-21 inhibitor in K562/A02 cells (). As is shown in , K562 cells that were infected with miR-21 had a significantly higher survival than the control group (P < 0.05). As is shown in , K562/A02 cells that were infected with the miR-21 inhibitor had a significantly lower survival than the control group (P < 0.05), suggesting that decreasing miR-21 expression alters adriamycin sensitivity in K562/A02 cells. This result suggested that miR-21 contributes to adriamycin resistance in the K562 cells.
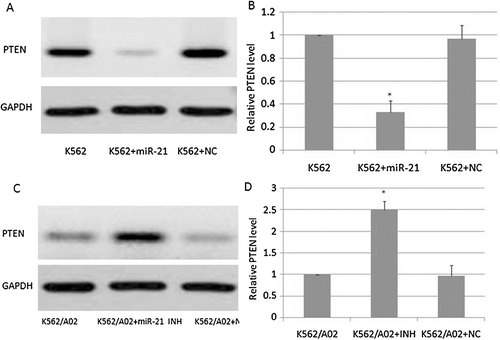
PTEN protein level modulated by miR-21 in K562 cells
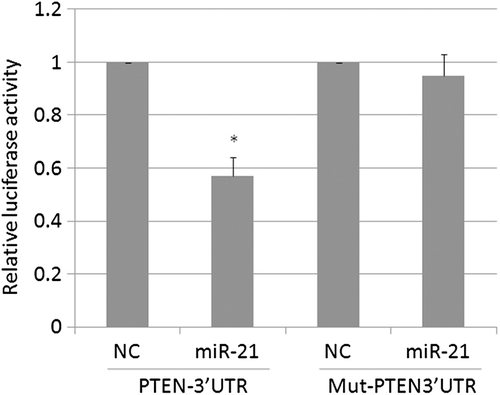
We investigated the effects of miR-21 on PTEN protein level in K562 and K562/A02 cells. As is shown in and , K562/A02 cells that were infected with the miR-21 inhibitor had a significantly higher PTEN protein level than the control group (P < 0.05), suggesting that decreasing miR-21expression alters PTEN expression in K562/A02 cells. As is shown in and , the K562 cells that were infected with miR-21 had a significantly lower PTEN protein level than the control group (P < 0.05). As is shown in , miR-21 markedly decreased the activity of the PTEN-3′UTR reporter in K562 cells (P < 0.05). However, reporter activity was not influenced in K562 cells infected with the PTEN-3′UTR mutant reporter (P > 0.05). This result suggested that PTEN protein level was modulated by miR-21 in K562 cells.
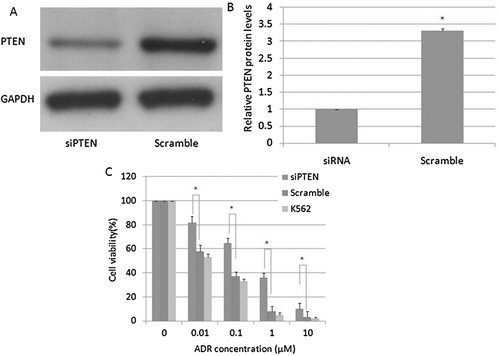
The role of PTEN in K562 adriamycin resistance
To investigate the relationship between PTEN and adriamycin resistance, we transfected PTEN siRNA or a scrambled siRNA into K562 cells, followed by treatment with various doses of adriamycin. PTEN siRNA effectively reduced the PTEN protein level ( and ). Furthermore, K562 cells that were pre-treated with PTEN siRNA had increased survival rate compared to the control group (). This result suggested that, at least in part, decreased PTEN was associated with adriamycin resistance in K562 cells.
Discussion
In this paper, for the first time, we show that TPL can effectively inhibit expression of miR-21 in the leukemia cell line K562. MiR-21 may regulate the survival of leukemia cell lines by targeting PTEN expression and causing subsequent changes in adriamycin sensibility.
Triptolide (TPL) is a diterpenoid triepoxide derived from the herb Tripterygium wilfordii. Recently, TPL has been found to possess the ability to inhibit proliferation and induce apoptosis in cancer cells. Furthermore, TPL has also been reported that TPL and its water-soluble derivative TPL-88 could enhance effect cytotoxicity of some cytokines and conventional anti-cancer drugs (CitationLee et al., 1999; CitationChang et al., 2001; CitationCarter et al., 2006; CitationTang et al., 2007). However, the effect of TPL on drug-resistant cancer cells has not been explored. In our work, we investigated the reversal effect of TPL on drug resistance in K562/A02 cells. Combined-treatmenting cells with TPL, a significant increase of the sensitivity of adriamycin against K562/A02 cells was observed.
Recently, more studies have shown that miRNAs may have an effect on the chemosensitivity of cancer cells (CitationBlower et al., 2008; CitationXia et al., 2008; CitationZhu et al., 2008). It is reported that the miR-21 gene has been identified as the only miRNA commonly overexpressed in solid tumors (CitationAsangani et al., 2008). Inhibition of miR-21 could decrease cell growth, induce apoptosis, and suppress migration and invasion in many cancer cells (CitationChan et al., 2005; CitationCheng et al., 2005; CitationAsangani et al., 2008; CitationLu et al., 2008). Furthermore, several studies found that inhibition of miR-21 could sensitize leukemia cells to chemotherapy drugs (CitationLi et al., 2010a,Citationb). In this work, we also found that TPL markedly decreased the miR-21 expression in K562/A02 cells. Consequently, we investigated the association between miR-21 level and sensibility of adriamycin in K562 cells. K562/A02 cells that were transfected with the miR-21 inhibitor had a significantly lower survival than the control group and K562 cells that were transfected with miR-21 had a significantly higher survival than the control group. The results indicated that TPL modulated drug resistance may through decreasing miR-21 level in K562/A02 cells.
Two studies first verified that PTEN is a direct target of miR-21 and showed that this microRNA plays a role in human cholangiocarcinoma cell lines and human hepatocellular cancer (CitationMeng et al., 2006, Citation2007). Studies have shown that transfection of PTEN in the drug-resistant AML cell line (HL60AR) or ALL cell line (EU21) causes chemosensitivity to anti-cancer agents (CitationZhou et al., 2003; CitationTabellini et al., 2005). In the present work, we found PTEN protein level was negative correlated with miR-21 expression. Additionally, K562 cells that were pre-treated with PTEN siRNA had increased survival rate to the control group. This result suggested that, at least in part, PTEN played a role in adriamycin resistance in K562 cells. The increased expression of miR-21 is known to be associated with inactivation of PTEN, a know tumor suppressor gene, resulting in activation of PI3K/Akt/mTOR signaling pathway (CitationBunney & Katan, 2010). The PI3K/Akt pathway is a pivotal signaling pathway and plays an important role in cell growth, survival, proliferation and tumorigenesis (CitationFranke et al., 2003). PI3K-activated (phosphorylated) Akt promotes cell survival by inhibiting apoptosis through its ability to phosphorylate/inactivate downstream targets of apoptotic machinery, such as pro-apoptotic Bcl2 family member BAD and GSK-3β (CitationFranke & Cantley, 1997). The (PI3K)/Akt pathway is regulated by several critical upstream factors, e.g., tumor suppressor PTEN (CitationCantley & Neel, 1999). In this study, we confirmed that TPL treatment could result in the down-regulation of miR-21, resulting in the up-regulation of PTEN in vivo, suggesting that the enhanced ADR sensitivity is associated with up-regulation of PTEN resulting from the inhibition of miR-21 expression.
Conclusion
Our results show that TPL can reverse drug resistance and enhance the cytotoxicity of adriamycin in K562/A02 cells. Mechanistic studies found TPL modulated sensibility of adriamycin via inhibiting miR-21 expression and increasing PTEN protein levels. This suggests that TPL may be a potential candidate for reversing drug resistance in leukemia chemotherapy.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, Post S, Allgayer H. (2008). MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene, 27, 2128–2136.
- Blower PE, Chung JH, Verducci JS, Lin S, Park JK, Dai Z, Liu CG, Schmittgen TD, Reinhold WC, Croce CM, Weinstein JN, Sadee W. (2008). MicroRNAs modulate the chemosensitivity of tumor cells. Mol Cancer Ther, 7, 1–9.
- Bunney TD, Katan M. (2010). Phosphoinositide signalling in cancer: Beyond PI3K and PTEN. Nat Rev Cancer, 10, 342–352.
- Cantley LC, Neel BG. (1999). New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA, 96, 4240–4245.
- Carter BZ, Mak DH, Schober WD, McQueen T, Harris D, Estrov Z, Evans RL, Andreeff M. (2006). Triptolide induces caspase-dependent cell death mediated via the mitochondrial pathway in leukemic cells. Blood, 108, 630–637.
- Chan JA, Krichevsky AM, Kosik KS. (2005). MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res, 65, 6029–6033.
- Chang WT, Kang JJ, Lee KY, Wei K, Anderson E, Gotmare S, Ross JA, Rosen GD. (2001). Triptolide and chemotherapy cooperate in tumor cell apoptosis. A role for the p53 pathway. J Biol Chem, 276, 2221–2227.
- Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, Lao KQ, Livak KJ, Guegler KJ. (2005). Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res, 33, e179.
- Cheng AM, Byrom MW, Shelton J, Ford LP. (2005). Antisense inhibition of human miRNAs and indications for an involvement of miRNA in cell growth and apoptosis. Nucleic Acids Res, 33, 1290–1297.
- Franke TF, Cantley LC. (1997). Apoptosis. A Bad kinase makes good. Nature, 390, 116–117.
- Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. (2003). PI3K/Akt and apoptosis: size matters. Oncogene, 22, 8983–8998.
- Kim YK, Song YJ, Seo DW, Kang DW, Lee HY, Rhee DK, Han JW, Ahn CM, Lee S, Kim SN. (2007). Reversal of multidrug resistance by 4-chloro-N-(3-((E)-3-(4-hydroxy-3-methoxyphenyl)acryloyl)phenyl)benzamide through the reversible inhibition of P-glycoprotein. Biochem Biophys Res Commun, 355, 136–142.
- Lambert R, Hainaut P. (2007). The multidisciplinary management of gastrointestinal cancer. Epidemiology of oesophagogastric cancer. Best Pract Res Clin Gastroenterol, 21, 921–945.
- Layke JC, Lopez PP. (2006). Esophageal cancer: A review and update. Am Fam Physician, 73, 2187–2194.
- Lee KY, Chang W, Qiu D, Kao PN, Rosen GD. (1999). PG490 (triptolide) cooperates with tumor necrosis factor-alpha to induce apoptosis in tumor cells. J Biol Chem, 274, 13451–13455.
- Li Y, Zhu X, Gu J, Dong D, Yao J, Lin C, Huang K, Fei J. (2010a). Anti-miR-21 oligonucleotide sensitizes leukemic K562 cells to arsenic trioxide by inducing apoptosis. Cancer Sci, 101, 948–954.
- Li Y, Zhu X, Gu J, Hu H, Dong D, Yao J, Lin C, Fei J. (2010b). Anti-miR-21 oligonucleotide enhances chemosensitivity of leukemic HL60 cells to arabinosylcytosine by inducing apoptosis. Hematology, 15, 215–221.
- Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. (2005). MicroRNA expression profiles classify human cancers. Nature, 435, 834–838.
- Lu Z, Liu M, Stribinskis V, Klinge CM, Ramos KS, Colburn NH, Li Y. (2008). MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene, 27, 4373–4379.
- Meng F, Henson R, Lang M, Wehbe H, Maheshwari S, Mendell JT, Jiang J, Schmittgen TD, Patel T. (2006). Involvement of human micro-RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines. Gastroenterology, 130, 2113–2129.
- Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. (2007). MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology, 133, 647–658.
- Perron MP, Provost P. (2008). Protein interactions and complexes in human microRNA biogenesis and function. Front Biosci, 13, 2537–2547.
- Tabellini G, Cappellini A, Tazzari PL, Falà F, Billi AM, Manzoli L, Cocco L, Martelli AM. (2005). Phosphoinositide 3-kinase/Akt involvement in arsenic trioxide resistance of human leukemia cells. J Cell Physiol, 202, 623–634.
- Tang XY, Zhu YQ, Tao WH, Wei B, Lin XL. (2007). Synergistic effect of triptolide combined with 5-fluorouracil on colon carcinoma. Postgrad Med J, 83, 338–343.
- Xia L, Zhang D, Du R, Pan Y, Zhao L, Sun S, Hong L, Liu J, Fan D. (2008). miR-15b and miR-16 modulate multidrug resistance by targeting BCL2 in human gastric cancer cells. Int J Cancer, 123, 372–379.
- Zhou M, Gu L, Findley HW, Jiang R, Woods WG. (2003). PTEN reverses MDM2-mediated chemotherapy resistance by interacting with p53 in acute lymphoblastic leukemia cells. Cancer Res, 63, 6357–6362.
- Zhu H, Wu H, Liu X, Evans BR, Medina DJ, Liu CG, Yang JM. (2008). Role of MicroRNA miR-27a and miR-451 in the regulation of MDR1/P-glycoprotein expression in human cancer cells. Biochem Pharmacol, 76, 582–588.