Abstract
Context: Balanites aegyptiaca (L.) Delile (Zygophyllaceae) is a tropical tree that has many folk uses in various countries. The bark extract is used for the control of the fresh water snails that act as intermediary host of Schistosoma.
Objective: Study the molluscicidal activity and chemical constituents of seed oil and seed glycosides of B. aegyptiaca against Monacha cartusiana and determine the structure-activity relationship.
Materials and methods: Two bioassay methods (residual film application and the leaf dipping technique) were used to evaluate the toxicity effect of the seed oil and glycosides, in concentrations of 1.000, 0.500, 0.250 and 0.125%. The seed oil was analysed by GC/MS. Acid hydrolysis and chromatographic separation were used to study the seed saponins.
Results: The bioassay of B. aegyptiaca against the land snail, M. cartusiana, indicated the activity of the seed oil and the high activity of the seed saponins. The seed glycosides gave 30.0, 53.3, 73.0 and 73.3% mortality for concentrations of 0.125, 0.250, 0.500 and 1.00%, respectively. The LC50 values were 0.335 and 0.256%, respectively. The seed oil was analysed by GC/MS. Acid hydrolysis of the seed saponins gave a mixture of diosgenin, yamogenin and 3,5-spirostadiene.
Discussion and conclusion: To study the structure-activity relationship, a triterpenoidal saponin and a triterpenoidal saponins rich extract (of Zygophyllum coccenum) were proven to be inactive. Thus, the activity is associated with the steroidal, not triterpenoidal saponins. Moreover, a spirostane aglycone without sugar moiety, was found to be inactive and attained the activity by glycosidation.
Introduction
Balanites aegyptiaca (L.) Delile (Zygophyllaceae) is a tropical tree that has many folk uses in various African countries and is largely used as a component of many popular preparations for its abortive, antiseptic, antimalarial, antisyphilitic and antiviral activity (CitationKokwano, 1976; CitationDuke, 1983; CitationChothani & Vaghasiya, 2011). The fruits are commonly used to purge, to remove intestinal parasites and sometimes to treat Schistosomum japonicum (CitationKoko et al., 2000). The bark aqueous extract is traditionally used as antijaundice, while the one of fruit mesocarps is administered as oral hypoglycemic (CitationEl-Saadany et al., 1986; CitationKamel et al., 1991) and seems to be effective against the Fasciola gigantic (CitationKoko et al., 2000). It is used in East Africa as a component of several primitive medicinal remedies (CitationLiu & Nakanishi, 1982; CitationMohamed et al., 1999).
The bark extract is traditionally used for the control of the fresh water snails that act as intermediary host of Bilharzia (CitationKloos & McCullough, 1987) and to water flea that acts as an alternate host of the guinea worm (CitationHall & Walker, 1991).
With an estimated 35,000 species, terrestrial molluscs are one of the most successful and diverse animal groups in land-based ecosystems. These animals have long been of importance to human societies as food, medicine, crop pests, vectors of parasites, and as tools, personal ornamentation and currency in trade (CitationHeller, 2001).
In this article we report the activity of the seed oil and saponins of B. aegyptiaca against the land snail M. cartusiana.
Materials and methods
Instrumentation
NMR
The instruments used in the measurement of NMR are Jeol 500 MHz (at the Central Research Lab., Alexandria University, Alexandria), TMS as internal standard; MS: were recorded on QP-7000 Shimadzu GC/MS (at Faculty of Science, King Abdulaziz University, Jeddah); normal CC: silica gel Merck grain size 0.2–0.063 mm; flash CC: silica gel Merck grain size <0.063 mm; thin layer chromatography (TLC): Merck GF 254 precoated plates 20 × 20 cm on aluminum sheets and preparative TLC: silica gel Merck GF 254 were prepared on 20 × 20 cm glass plates. The compounds on the plate were visualized under 254 and 365 nm UV lamps.
Tested snails
Adult snails of M. cartusiana (~15 month age, 2 g weight) were collected in April 2006 from Egyptian clover fields and gardens near Mansoura City, Dakahlia governorate. The collected snails were transferred in cloth bags and kept in laboratory. Healthy individuals, as evidenced from a regular balanced movement, were kept in glass boxes containing moistened soil (temperature 24°C and humidity ~40%) and fed on fresh lettuce leaves for two weeks for acclimatization (CitationMortada et al., 2005).
Residual film application
The treated surface exposure method of bioassay was used to evaluate the toxicity effect of plant extracts. Serial concentrations (0.125, 0.250, 0.500 and 1.000%) of crude extracts or its fractions were deposited on the bottom of Petri dishes (9.5 cm in diameter) and the covers. After the solvent had evaporated, 10 snails were placed in each dish, covered and kept at room temperature for 24 h. Three replicates were carried out per each concentration, in addition to an untreated check group. Mortality percentages were recorded after 1, 3, 5 and 7 days post treatment and corrected for natural mortality according to Abbott’s formula (CitationAbbott, 1925), then subjected to Probit analysis by Finney’s method (CitationFinney, 1971).
Leaf dipping technique
Similar pieces of green lettuce leaves were dipped in glass jar containing 100 mL of the tested extract, in the same serial concentrations as mentioned above, for 10 s, then left until solution dropping stopped before being offered to the snails. Ten adult individuals were exposed to each treated leaf in disposable plastic boxes (24 × 16 × 10 cm). The procedure was completed as mentioned under the residual film application method.
Plant material
Balanites aegyptiaca (L.) Delile (Balanitaceae) fruits were purchased from the local market in January 2006. The botanical identification was checked by Prof. I. Mashaly, Herbarium of Botany Department, Faculty of Science, Mansoura University, Mansoura, Egypt.
Extraction of the fruits and seeds
Balanites aegyptiaca fruits, 1 kg, were soaked in water/methanol, 1:1, for 48 h. Filtration and evaporation gave the fruit extract, 206 g. The fleshy mesocarp was removed giving the kernels (stones), 540 g, which were broken and the seeds, 122 g, were collected, crushed and extracted by boiled petroleum ether to give 2.5 g extract, fraction B1, followed by boiled ethanol to give 5.8 g extract, fraction B2.
Acid hydrolysis of the saponin fraction
A sample from the ethanol extract (saponins, 100 mL) was mixed with HCl (acid concentration 5%), stirred at room temperature for 30 min and extracted by CHCl3. The chloroform extracts were collected and dried over anhydrous sodium sulfate and evaporated to dryness.
Glycosidation of hecogenin acetate
A mixture of hecogenin acetate (0.924 g, 2 m mole) and anhydrous glucose (0.36 g, 2 m mole) in 5 mL absolute ethanol and 0.5 mL bi-distilled water was heated for 20 min. A pinch of ZnCl2 was then added and the reaction mixture was heated under reflux for about 12 h and filtered (CitationHammouda et al., 1986). The filtrate was evaporated to give the produced glycoside (1.104 g, about 90% yield).
Separation of the fat fraction B1
A sample of B1 was injected in diethyl ether solvent in the GC/MS to give n-nonanal 1 (4.8% at Rt 6.5 min.), (E)-2-decenal 2 (7.1% at Rt 9.2 min), (E,Z)-2,4-decadienal 3 (1.4% at Rt 9.7 min), (E,E)-2,4-decadienal 4 (2.5% at Rt 10.1 min.), palmitic acid 5 (4.1% at Rt 19.2 min), ethyl palmitate 6 (1.6% at Rt 19.6 min), linoleic acid 7 (11.0% at Rt 21.3 min), (E)-9-octadecenoic acid 8 (12.0% at Rt 21.4 min), ethyl 9(E),12(E)-octadecadienoate 9 (4.6% at Rt 21.6 min), ethyl oleate 10 (3.8% at Rt 21.7 min), eicosanoic acid 11 (4.1% at Rt 23.1 min), 13(Z)-docosenoic acid 14 (15.4% at Rt 25.2 min), docosanoic acid 12 (12.3% at Rt 24.8 min) and 13-docosynoic acid 13 (20.2% at Rt 24.9 min).
Results
The seeds of Balanites aegyptiaca were extracted by petroleum ether to give the fat fraction (B1), followed by ethanol to give the glycosidic fraction (B2). The bioassay against the land snail, Monacha cartusiana, indicated the activity of the seed oil, B1, and the high activity of the seed glycosides, B2. The seed glycosides gave 30.0, 53.3, 73.0, and 73.3% mortality for concentrations of 0.125, 0.250, 0.500, and 1.00%, respectively. The LC50 was 0.256% ().
Table 1. Molluscicidal activity and LC50 of the fractions B1 and B2 of the extract of Balanites aegyptiaca, glycyrrhizic acid 18, hecogenin acetate 19 and hecogenin acetate glucoside 20, comparative with neomyl 90% as a reference molluscicide against Monacha cartusiana land snails using leaf dipping technique under laboratory condition.
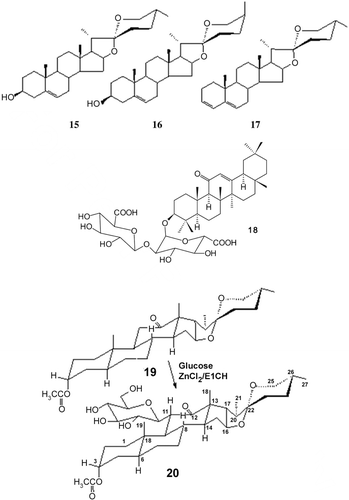
Figure 1. Chemical structures of the aglycones 15, 16, 17, glycyrrhizic acid 18, hecogenin acetate 19 and hecogenin acetate glucoside 20.
The fat fraction (B1) was analysed by GC/MS to afford fourteen compounds 1–14. Several chromatographic separation trials of the glycosidic fraction (B2) didn’t give pure compounds, but the 1H NMR of the separated fractions indicated the presence of saponins, which was supported by a positive Molisch’s test and by the charring on TLC, happened upon spraying with sulfuric acid spray reagent. This was found to be in agreement with the separation of many saponins from Balanites aegyptiaca (CitationDawidar & Fayez, 1969; CitationLiu & Nakanishi, 1982; CitationPettit et al., 1991; CitationHosny et al., 1992; CitationKamel, 1998; CitationFarid et al., 2002; CitationSperoni et al., 2005; CitationStaerk et al., 2006).
Acetylation followed by chromatographic separation also didn’t give pure compounds. Acid hydrolysis gave a mixture of diosgenin 15, yamogenin 16 and 3,5-spirostadiene 17.
Discussion
The molluscicidal activity here is associated with steroidal saponins. To test if the triterpenoidal saponins are also active as molluscicides, we extracted Zygophyllum coccenum, a plant species containing triterpenoidal saponins (CitationElGamal et al., 1995) and tested the methanol extract as a molluscicide. It was found to be inactive. Another confirmation was attained from investigating the molluscicidal activity of a triterpenoidal glycoside, glycyrrhizic acid [3-O-(2-O-β-d-glucopyranuronosyl-α-D-glucopyranuronosyl)-18β-glycyrrhetinic acid] 18 in the form of the monoammonium salt hydrate, which was found to be inactive. Thus, the activity is associated with the steroidal saponins. To test if the aglycone without the sugar moiety is molluscicidal, hecogenin acetate (3α-acetoxyspirostan-12-one) 19 was tested and no activity was produced. Furthermore, glycosidation of 19 revealed an active glycoside 20 (26.6, 63.3, 73.3 and 93.3% mortality for concentrations of 0.125, 0.250, 0.500 and 1.000%, respectively; LC50 0.217%, ).
The structure of the glycosidic product 20 was elucidated from the 1H NMR spectral data where the signals of H-3 (at δ 4.63), H-16 (at δ 4.30), H-26, H-26′ (at δ 3.44, 3.31) and H-11 axial (at δ 2.36) are still present around the same δ values, while the signal of H-11 equatorial (at δ 2.47) has disappeared, indicating the glycosidation position at C-11 in the equatorial bond.
Thus, compound 20 was identified as 11β-d-glucosylhecogenin acetate.
Conclusion
In this article, the seed saponins and the seed oil of B. aegyptiaca were proved to be molluscicidal against the land snail Monacha cartusiana. The LC50 values were found to be 0.256 and 0.335, respectively. It was proven that the molluscicidal activity of saponins requires the presence of steroidal not triterpenoidal aglycone and the sugar moiety is essential.
Declaration of interest
The authors report no conflicts of interest.
Acknowledgment
The authors are indebted to Prof. Dr. I. Mashaly, Botany Department, Faculty of Science, Mansoura University, for the botanical identification.
References
- Abbott WS. (1925). A method of computing the effectiveness of an insecticide. J Econ Entomol, 18, 265–267.
- Chothani DL, Vaghasiya HU. (2011). A review on Balanites aegyptiaca Del (desert date): phytochemical constituents, traditional uses, and pharmacological activity. Pharmacogn Rev, 5, 55–62.
- Dawidar AM, Fayez MBE. (1969). Steroid sapogenins-XIII. The constituents of Balanites aegyptiaca. Phytochemistry, 8, 261–265.
- Duke JA. (1983). Medicinal Plants in the Bible. New York: Trado-Medic Books.
- ElGamal MHA, Shaker KH, Pöllmann K, Seifert K. (1995). Triterpenoid saponins from Zygophyllum species. Phytochemistry, 40, 1233–1236.
- El-Saadany SS, Abdel-Rahim EA, Wasif MM. (1986). Biochemical action of Balanites aegyptiaca fruits as a possible hypoglycemic agent. Food Chem, 19, 307–316
- Farid H, Haslinger E, Kumert O. (2002). New steroidal glycosides from Balanites aegyptiaca. Helv Chim Acta, 85, 1019–1026.
- Finney DJ. (1971). Probit Analysis. 2nd Edition. London: Cambridge Univ Press.
- Hall JB, Walker DH. (1991). Balanites aegyptiaca Del., A monograph banger: University of Wales.
- Hammouda M, Abou-Elzahab MM, Hamama WS, Afsah EM. (1986). Condensation of 3-methyl-1-phenyl-2-pyrazolin-5-one with aldehydosugars. Proc 1st Chem Conf: Fac. Sci., Mansoura Univ, p. 140, Sept. 24–26.
- Heller J. (2001). Life history strategies. In: Barker GM, ed.. The Biology of Terrestrial Molluscs. Oxon, UK: CABI Publishing.
- Hosny M, Khalifa T, Calis I, Wright AD, Sticher O. (1992). Balanitoside, a furostanol glycoside, and 6-methyl diosgenin from Balanites. Phytochemistry, 31, 3565–3569.
- Kamel MS, Ohtani K, Kurokawa T, Assaf MH, el-Shanawany MA, Ali AA, Kasai R, Ishibashi S, Tanaka O. (1991). Studies on Balanites aegyptiaca fruits, an antidiabetic Egyptian folk medicine. Chem Pharm Bull, 39, 1229–1233.
- Kamel MS. (1998). A furostanol saponin from fruits of Balanites aegyptiaca. Phytochemistry, 48, 755–757.
- Kloos H, McCullough FS. (1987). Plants with Recognized Molluscicidal Activity. New York: John Wiley & Sons, p. 45.
- Koko WS, Galal M, Khalid HS. (2000). Fasciolicidal efficacy of Albizia anthelmintica and Balanites aegyptiaca compared with albendazole. J Ethnopharmacol, 71, 247–252.
- Kokwano JO. (1976). Medicinal plants of East Africa: East Africa Literature Bureau. Dar el Salaam, Kampala, Nairobi, Chapter 34.
- Liu H, Nakanishi K. (1982). The structures of balanitins, potent molluscicides isolated from Balanites aegyptica. Tetrahedron, 38, 513–519.
- Mohamed AH, Eltahir KE, Ali MB, Galal M, Ayeed IA, Adam SI, Hamid OA. (1999). Some pharmacological and toxicological studies on Balanites aegyptiaca bark. Phytother Res, 13, 439–441.
- Mortada MM, Soliman MA, Khidr FK. (2005). Molluscicidal activity of certain compounds against Monacha cartusiana lans snails under laboratory and field conditions. J Agric Sci Mansoura Univ, 30, 8147–8151.
- Pettit GR, Doubek DL, Herald DL, Numata A, Takahasi C, Fujiki R, Miyamoto T. (1991). Isolation and structure of cytostatic steroidal saponins from the African medicinal plant Balanites aegyptica. J Nat Prod, 54, 1491–1502.
- Speroni E, Cervellati R, Innocenti G, Costa S, Guerra MC, Dall’ Acqua S, Govoni P. (2005). Anti-inflammatory, anti-nociceptive and antioxidant activities of Balanites aegyptiaca (L.) Delile. J Ethnopharmacol, 98, 117–125.
- Staerk D, Chapagain BP, Lindin T, Wiesman Z, Jaroszewski JW. (2006). Structural analysis of complex saponins of Balanites aegyptiaca by 800 MHz 1H NMR spectroscopy. Magn Reson Chem, 44, 923–928.