Abstract
Context: Gum guggul, a resinous exudate of the plant Commiphora mukul Engl. (Burseraceae), has been found efficacious in the treatment of bone fractures, arthritis, and hyperlipidemic disorders.
Objective: The present study is an effort to explore the anti-bone-resorptive potential of the dried methanol extract of the gummy exudate of C. mukul (MECM) in ovariectomized rat model.
Materials and methods: The animals were randomly divided into five groups of equal size (n = 6). Animals in all the groups were ovariectomized except group 1, which was sham operated. Groups 3, 4 and 5 were treated with Raloxifene, MECM 250 mg/kg and MECM 500 mg/kg, respectively. The 2nd group was fed with vehicle. Assessment: biochemical estimations, viz., alkaline phosphatase (ALP), tartarate resistant acid phosphatase (TRAP), serum calcium (Ca); biomechanical evaluations, and histopathological examinations.
Results: The LD50 of MECM was found to be > 2500 mg/kg orally. A significant elevation was observed in the ALP, TRAP, Ca and cholesterol levels in the 2nd group with a significant reduction in biomechnical strength. Groups 3, 4 and 5, showed a significant reduction in TRAP and ALP levels (p < 0.001). The Ca levels were normalized in the groups 4 and 5, while cholesterol levels dropped in group 5. The bone strength, however, was normalized in all the groups (p < 0.001) along with the histopathology.
Discussion and conclusion: Findings suggested a significant gain in bone strength and nearly complete restoration of bone microarchitecture along with lowered levels of TRAP indicating the anti-bone resorptive potential of the extract.
Introduction
Osteoporosis is a disease characterized by low bone mass and microarchitectural deterioration of bone tissue, leading to bone fragility and an increased susceptibility to fractures (CitationEpstein, 2006). It results from a negative balance between the bone-forming activity of osteoblasts and the resorption activity of osteoclasts (CitationNovack, 2007). It mainly affects post-menopausal women, elderly men and results in hip and vertebral fractures (CitationFrost, 1992; CitationCooper et al., 1992). Hormone replacement therapy (HRT) is an established method in the prevention of fractures and bone loss in post-menopausal women; however, it is associated with an increased risk of breast cancer (CitationCanfell et al., 2008), stroke and heart attacks (CitationBotto et al., 2011), which are generally called estrogen-like side effects (CitationCanderelli et al., 2007). Sodium alendronate, a bisphosphonate, is at present, one of the most popular medicines prescribed to treat osteoporosis. However, gastrointestinal intolerance to bisphosphonates has been reported in some patients (CitationSzejnfeld, 2000). Osteonecrosis of the jaws (CitationSomerman & McCauley, 2006) is another common adverse effect that has been associated with some of the bisphosphonates. Selective Estrogen Receptor Modulators (SERM), such as raloxifene, have also been reported to react adversely. Hot flushes and thromboembolic disorders are the most frequently reported side effects of raloxifene (CitationEttinger et al., 1999).
Therefore, the compliance to HRT, bisphosphonates and SERMs is decreasing and health providers and seekers are looking for alternative therapies (CitationWuttke et al., 2008). Recent research work provides an insight about the potential of alternative strategies sourced from some medicinal plants for the management of osteoporosis as well as to avoid the increased evidence of fractures (CitationPenolazzi et al., 2008). There are number of crude drugs in traditional Chinese medicine that are used to strengthen bones and muscles, and for treatment of the bone injuries and bone-related diseases. Many Indian medicinal plants have also been attributed with anti-arthritic and fracture-healing activities, for instance, Cissus quadrangularis Linn. (Vitaceae), Lepidium meyenii Walp. (Brassicaceae), Trifolium pratense Linn. (Fabaceae), Actaea racemosa (Nutt.) L. (Ranunculaceae), Glycine max (L.) Merr. (Fabaceae), etc. These plants have been used extensively in the Indian system of medicine for treatment of bone and joint disorders (CitationShirwaikar et al., 2010).
Commiphora mukul (Arn.) Bhandari. (Bursaraceae) is a flowering shrub or small tree (2–4 m in height) found in northern Africa, central Asia and is very commonly available in northern India. It exudes a resinous substance which is known as gum guggul (guggulipid). It has been a key component in many remedies of the ancient Indian system of medicine, viz., Ayurveda, Siddha, Unani, and other local health traditions for nearly 3,000 years. It has also found to be efficacious in the treatment of bone fractures, arthritis, inflammation (CitationKimura, et al., 2001), obesity, cardiovascular diseases and hyperlipidemic disorders (CitationUrizar & Moore, 2003; CitationWang et al., 2004). According to some studies it helps in the mineralization of bones in arthritis (CitationKhanna, et al., 2007; CitationSharma & Sharma 1977).
Guggulipid has a long history of use in Ayurveda. The Atharvaveda is the earliest reference for its medicinal and therapeutic properties (CitationSatyavati, 1988). Detailed descriptions regarding its actions, uses and indications as well as the varieties have been described in numerous Ayurvedic treatises including Charaka Samhita (1000 BC), Sushruta Samhita (600 BC) and Vagbhatta (7th century AD). Sushruta described the drug as, responsible for reducing fat and indicated for healing bone fracture, arthritis, and inflammation (Sushruta, 1909). Traditionally, guggul is given in the form of Yog, wherein guggul is mixed with other drugs along with castor oil or Indian clarified butter. The Yog can also be prepared by cooking the guggul with water and powder of other herbal drugs (CitationMishra, 2007).
The present work was therefore, undertaken to evaluate the anti-bone-resorptive property of the methanol extract of C. mukul (guggul).
Materials and methods
The dried and purified exudate of C. mukul (Shuddha guggul) was collected from Alvas Pharmacy, Mizar, District, Udupi (Karnataka), India, in the month of September 2009. The drug was authenticated by Dr. Gopalkrishna Bhat, Professor of Botany, Poorna Prajna College, Udupi (Karnataka), India. A voucher specimen no. PP-571 has been deposited in the Department of Pharmacognosy, Manipal College of Pharmaceutical Sciences, Manipal.
Preparation of extract
The dried shuddha guggul was coarsely powdered and cleaned (2.5 kg) and exhaustively extracted with methanol using a Soxhlet apparatus at a temperature not exceeding 60°C. The total methanol extract was concentrated in vacuo to a syrupy consistency and then freeze-dried (yield 10.8%).
Standardization of the extract
Animals
Female Wistar albino rats weighing about 150–170 g, in the age group of about 90 days, were acclimatized to the experimental room at temperature 23 ± 2°C, controlled humidity conditions (50–55%) and 12 h light/dark cycle. Animals were caged with a maximum of two animals each in a polypropylene cage and were fed with standard food pellets (Hindustan Lever Ltd., India) and water ad libitum. The study was conducted after obtaining ethical committee clearance from the Institutional Animal Ethics Committee of K.M.C., Manipal No. IAEC/KMC/71/2009–2010.
Acute toxicity studies (OECD guidelines 425 adoption)
Healthy, Wistar Albino rats of either sex (20) were randomly divided into two groups of equal size. Animals of both the groups were fasted overnight before the test. The first group was given 2500 mg/kg body weight of the freshly prepared MECM, while the other group was given an equivolume of saline. The animals were observed immediately and then after 30 min, 1, 2, 4, 6 h and thereafter daily for 14 days. At the end of the fourteenth day the animals were sacrificed under ether anesthesia and dissected for examination of vital organs.
Antiosteoporotic activity
Experimental animals were divided randomly into five groups of six animals each. Group 1 was sham operated and served as basal control. All the other groups were ovariectomized and received treatment for 3 months starting from the fifteenth day of ovariectomy. Group 2 received vehicle and served as ovariectomized control (OVX). Group 3 was orally administered with Raloxifen (5.4 mg/kg, Dr. Reddy’s Laboratory, Hyderabad, India). Groups 4 and 5 were orally fed with emulsion of MECM in 2% acacia at two different doses levels, 250 and 500 mg/kg body weight, respectively. At the end of the treatment, blood samples from all the groups were collected from tail vein. The blood was immediately frozen until analysis. The clotted blood was centrifuged at 2000 to 3000 rpm to separate the serum and the serum was subjected for the assessment of biochemical parameters. The animals were then sacrificed under ether anesthesia and bones were isolated for biomechanical testing and to study histopathological changes.
Evaluation parameters
Biomechanical parameters
The freshly isolated bones (right femur, tibia and fourth lumbar vertebra) were tested for their biomechanical strength by using calibrated digital tablet hardness tester (Electrolab tablet hardness tester EH-01P, Mumbai, India) after appropriate modifications.
Three point bending of tibia
A supporter with two loading points, 13 mm apart from each other, was used on the stage of the tablet hardness testing machine. Lateral surface of the tibia at tibio-fibular junction was placed upon the first point and proximal tibia upon the other. A rounded press head compressed the middle of the tibial shaft until fracture occurred (CitationPeng et al., 1994).
Compression of IV lumbar vertebra
The fourth lumbar vertebra was located and isolated. The fresh vertebra was placed on the flat metallic stage and compressed until it was fractured. The reading was recorded in Newtons (CitationOge et al., 2002).
Load testing of femoral neck
The head of femur was loaded with a force parallel to the shaft of the femur until fracture. A metallic clamp with flat surface was used to fix the proximal part of the femur perpendicularly. A concave compressing head (2.5 mm) was used for loading the head with a force that was required to break the head (CitationPeng et al., 1994).
Biochemical parameters
Serum calcium
The serum calcium (Ca) levels were estimated by Cobas C111 autoanalyser using standardized diagnostic reagent kit (Hitech Biomedicals, Mumbai, India), colorimetrically (CitationBurtis & Ashwood, 1986).
Serum alkaline phosphatase
In vitro determination of ALP was carried out by Cobas C111 autoanalyser using standardized diagnostic reagent kit (Hitech Biomedicals, Mumbai, India). ALP from serum converts phenyl phosphate to inorganic phosphate and phenol at pH 10. Phenol so formed reacts in alkaline medium with 4-aminoantipyrine in presence of oxidizing agent potassium-ferricyanide and forms an orange red colored complex, which was measured colorimetrically. The color intensity is proportional to enzyme activity (CitationBurtis & Ashwood, 1986).
Tartrate resistant acid phosphatase
The test was carried out by using Accucare® diagnostic reagent kit (Lab-Care Diagnostic (India) Pvt. Ltd, Mumbai) for the in vitro determination of Tartrate resistant acid phosphatase (TRAP). In acid environment (pH 4.8), α-naphthyl phosphate is hydrolyzed by acid phosphatase to produce α-napthol and phosphate. α-Napthol reacts with diazo-2-chloro-5-toluene (Fast Red TR Salt) forming an azo dye compound which absorbs maximally at 405 nm and is directly proportional to total acid phosphatase activity, which can be measured colorimetrically. When the activity is measured in the presence of tartarate, the prostatic activity is inhibited. Since tartrate inhibits the prostatic fraction of the enzyme, the difference in acid phosphatase activity without and with tartrate represents the activity of prostatic fraction (CitationVarley, 1980).
Serum cholesterol
The serum cholesterol level was estimated by Cobas C111 autoanalyser using standardized diagnostic reagent kit (Hitech Biomedicals, Mumbai, India). In this, the cholesterol esters are hydrolyzed to free cholesterol by the enzyme cholesterol ester hydrolase. The free cholesterol produced is oxidized by the enzyme cholesterol oxidase to cholest-4-en-3-one with the simultaneous production of hydrogen peroxide, which oxidatively couples with 4-aminoantipyrine and phenol in the presence of peroxidase to yield a chromogen with maximum absorption at 500 nm (CitationAllain et al., 1974).
Histopathology
Sections of decalcified femurs fixed in 10% neutral buffered formalin and embedded in paraffin wax were taken using a microtome (Leica RM 2245, Germany) and stained with haematoxyline and eosin. The sections were mounted and observed under a microscope for histopathological changes.
Statistical analysis
The data were analyzed using one-way ANOVA with Tukey’s post hoc test, performed using Graph Pad Prism version 5.00 for Windows, Graph Pad Software, San Diego California USA. p < 0.05 was considered significant.
Results
Oral administration of freshly prepared methanol extract of C. mukul (2500 mg/kg body weight) caused neither mortality nor any signs of clinical abnormality in the treated group. At necropsy, no gross pathological observation could be made in the target organs. The LD50 of C. mukul was thus calculated to be more than 2500 mg/kg body weight.
In the biomechanical tests three point bending (p < 0.001), compression of lumbar vertebra and load testing of femoral head showed significant loss in the bone strength of second group as compared to the sham (first) group. Groups 3–5 showed significant gain in the biomechanical strength as compared to the OVX (second) group ().
Table 1. The effect of methanol extract of Commiphora mukulon biomechanical parameters in ovariectomized rats after 3 months (n = 6/group).
A significant rise in ALP TRAP, Ca and total serum cholesterol (T-chol) levels (p < 0.001) at the end of 3 months following ovariectomy was observed in the second group as compared to the normal control group (1). Groups 3, 4 and 5, showed a significant decrease in the elevated TRAP and ALP levels (p < 0.001) compared to group 2 (). The ALP levels however, remained significantly elevated in all the treated groups when compared to group 1 (p < 0.05).
Table 2. Effect of methanol extract of C. mukulon biochemical parameters in ovariectomized rats after 3 monhs (n = 6/group).
Groups 4 and 5 showed a significant drop in the Ca levels (p < 0.05) when compared to the 2nd group. The T-Chol levels were dropped significantly in the groups 3 (p < 0.001) and 5 (p < 0.05) when compared to the OVX group (2), while group 4 showed no statistically significant change in the cholesterol levels.
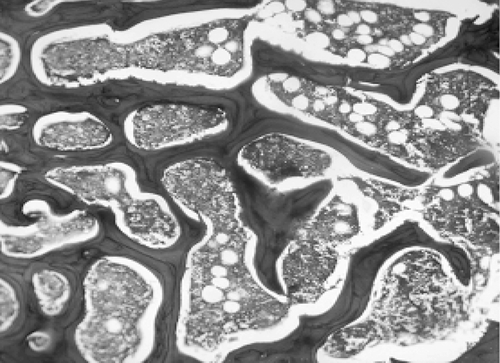
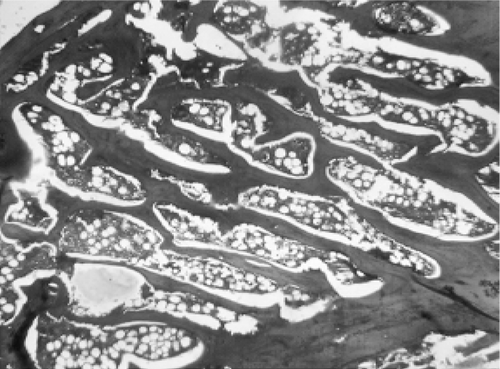
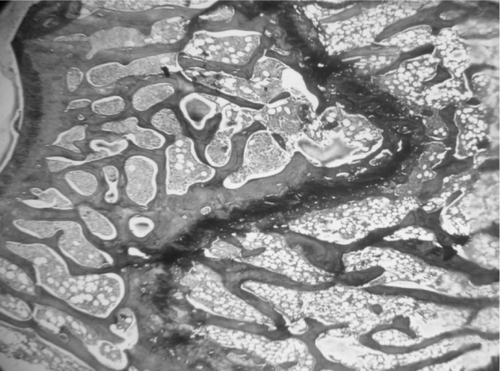
Microscopic examination of the section of femurs of all groups stained with H & E, showed confirmatory results supporting biomechanical and biochemical findings (). The section of group 2 revealed marked disruptive and lytic changes with osteodystrophy (). Histological sections of groups 3, 4 and 5 showed a significant restoration of lytic and disruptive changes (, , ).
Plate 1. Photomicrograph of femur section of group 1 (sham group) showing normal, dense and uniform trabaculae (H&E, 50×).
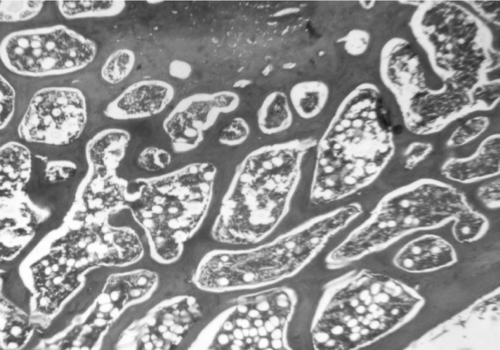
Plate 2. Photomicrograph of femur section of group 2 (ovariectomized) showing marked disruptive and lytic changes (H&E, 50×).
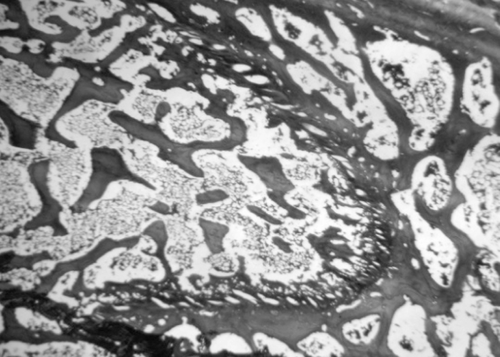
Plate 3. Photomicrograph of femur section of group 3 (raloxifen treated) showing restoration of normal architecture (H&E, 50×).
Discussion
The present work is an effort to study the effect of MECM on the bone resorption as a possible mechanism of bone mineralization using overiectomized rat model. The end results were assessed on the basis of biomechanical parameters that include, three point bending of tibia, compression of IV lumbar vertebra and load testing of femoral head, the direct measures of bone strength (CitationPeng et al., 1994; CitationOge et al., 2002); and biochemical markers like serum alkaline phosphatase, a marker for bone formation (CitationEastell et al., 2001), serum calcium level, an indicator of mineral homeostasis (CitationHeaney et al., 1977; CitationRiis et al., 1987) and TRAP, a marker for bone resorption (CitationHalleen et al., 2001).
In our study, a significant increase in ALP, TRAP and calcium levels was observed in the OVX group (2) suggesting high bone formation and an equivalent increase in bone resorption indicating increased bone turnover. The increase in the bone resorption is also evident from the significantly increased levels of serum Ca. Calcium levels are generally maintained by a negative feedback mechanism, however, in our study the mineral homeostasis appears to be disturbed. The biomechanical testing of ovariectomized rat bones in the second group showed significant loss in their strength. Further, the histopathological examination of the sections of femurs of ovariectomized rats clearly suggested a marked disruption in the trabecular bone architecture which extended up to the cortical bone. All these estimations and observations therefore indicated a negative bone balance, i.e., the loss of bone minerals, in the second group animals following ovriectomy, which simulated osteopenia or osteoporosis due to estrogen depletion.
Treatment with MECM at two different dose levels (250 and 500 mg/kg) from the 15th day of ovariectomy (groups 4 and 5) triggered a significant and dose-dependent reduction in TRAP levels (both groups at p < 0.001), suggesting a significant reduction in bone resorption when compared to the OVX group.
The ALP levels were dropped significantly in the MECM treated groups when compared to OVX (control).However, when compared to group 1 (Sham, the normal control), the ALP levels remained significantly elevated suggesting higher osteoblastic activity. Increased osteoblastic activity with significant osteoclastic inhibition is known to eventually result in positive bone balance (CitationCroucher, 2005). Normalization of serum Ca (p < 0.05) levels when compared to second group explains the slowdown in the bone resorption and uptake of calcium by the osteoblastic function.
The biomechanical testing of bones of these groups also showed significant gain in bone strength as compared to OVX (group 1) in all parameters tested (p < 0.001). The results were comparable to or slightly higher than sham and definitely better than the raloxifene treated group (). Histopathological examination of the femurs of extract treated groups (4 and 5) revealed nearly complete ossification, mineralization, and calcified cartilaginous deposits, which indicates marked restorative action of the extracts.
Our findings, as discussed above, suggested a definite bone mineralization, i.e., a positive bone balance (in both the groups) by a significant inhibition of bone resorption and thereby bone loss. The significant anti-bone resorptive potential of the methanol extract of C. mukul must be due to the suppression of osteoclastogenesis by guggulsterones via suppression of NF-κB activation, as suggested by CitationIchikawa and Aggarwal (2006).
Post-menopausal women are at a high risk for cardiovascular diseases due to higher cholesterol levels (CitationRiggs et al., 2002). Although debated for a long time (CitationTracy, 1966; CitationBarrett-Connor, 1996; CitationTunstall-Pedoe, 1998), menopause is thought to be a determinant of high cholesterol levels (CitationPalmer et al., 1992; Citationvan der Schouw et al., 1996; CitationJacobsen et al., 1997). The accumulation of cholesterol in post-menopausal women is attributed to the deprivation of estrogen as well as to the lipid profile changes during perimenopause (CitationAgrinier et al., 2010). Oxidative stress has also been implicated in hyperchloesteremia. Oxidative stress has been implicated as one of the leading causes for higher cholesterol and cardiovascular risk. CitationLean et al. (2003) demonstrated a significant loss in thiol antioxidant enzymes leading to compromised defence against oxidative stress. It can be therefore, hypothesized that estrogen depletion following ovariectomy increases serum cholesterol. Guggulsterones have been reported to reduce cholesterol levels and have been used to treat obesity (CitationUrizar et al., 2002). However, their effect in the post-menopausal women is unknown. Hence, the effect of MECM on cholesterol in the ovriectomized rat was investigated.
A significant increase in cholesterol level was observed following ovariectomy in group 2 animals. The drug-treated (raloxifen, MECM 250 and 500 mg/kg) groups revealed moderation in the cholesterol levels. However, the reduction was not as significant as the raloxifen treated group. At a higher dose, although, MECM showed a statistically significant reduction in the cholesterol levels, it does not match raloxifen.
A study carried out by CitationGarrett et al. (1990), suggests treatment of post-menopausal women with estrogen augments oxidative defense in tissues and bones causing reduction in bone loss. This implies the loss or depletion of estrogen lowers the body defense against oxidative assault which results in increased levels of reactive oxygen species (ROS). Thus, antioxidants can prove to be effective antiosteoporotic agents. Antioxidant activity of C. mukul has been reported by many authors (CitationChander et al., 2002; CitationSudarshana & Deepa, 2008), which could be another mechanism by which the drug suppresses osteoclastic activity.
Conclusion
In conclusion, this study displayed a definite role of the methanol extract of C. mukul in inhibition of bone loss by suppressing osteoclastic activity either, by suppression of NF-κB activation or by other antioxidant mechanisms; thereby promoting bone mineralization in osteopenic rats induced by ovariectomy. Although guggul is a well-known drug for the treatment of hypercholestremia, its methanol extract did not show potential anti-cholesteremic activity in the overiectomized rats. However, the antiosteoclastic potential of the drug is promising and therefore further investigations may be performed.
Acknowledgements
The authors thank the Manipal University, Manipal, India for providing facilities to carry out this work.
Declaration of interest
The authors have declared that there is no conflict of interest.
References
- Agrinier N, Cournot M, Dallongeville J, Arveiler D, Ducimetière P, Ruidavets JB, Ferrières J. (2010). Menopause and modifiable coronary heart disease risk factors: A population based study. Maturitas, 65, 237–243.
- Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. (1974). Enzymatic determination of total serum cholesterol. Clin Chem, 20, 470–475.
- Barrett-Connor E. (1996). The menopause, hormone replacement, and cardiovascular disease: The epidemiologic evidence. Maturitas, 23, 227–234.
- Botto N, Maffei S, Manfredi S, Colombo MG, Mazzone AM, Andreassi MG. (2011). Prothrombotic mutations, family history and the risk of thrombosis in postmenopausal women: Implications for hormone replacement therapy. Climacteric, 14, 25–30.
- Burtis CA, Ashwood ER. (1986). Tietz Text Book of Clinical Biochemisry, 2nd ed., vol. 833. Saunders: London, pp. 1890–1891.
- Canderelli R, Leccesse LA, Miller NL, Unruh Davidson J. (2007). Benefits of hormone replacement therapy in postmenopausal women. J Am Acad Nurse Pract, 19, 635–641.
- Canfell K, Banks E, Moa AM, Beral V. (2008). Decrease in breast cancer incidence following a rapid fall in use of hormone replacement therapy in Australia. Med J Aust, 188, 641–644.
- Chander R, Khanna AK, Pratap R. (2002). The antioxidant activity of guggulsterone: The active principle from gugglulipid of Commiphora Mukul. J Med Aromat Plant Sci, 24, 370–374.
- Cooper C, Campion G, Melton LJ III. (1992). Hip fractures in the elderly: a world-wide projection. Osteoporosis Int, 2, 285–289.
- Croucher PI. (2005). Osteoblastic metastases. In: Jasmin C, Coleman RE, Coia LR, Capanna R, Saillant G. (Eds.), Textbook of Bone Metastases. England: John Wiley and Sons Ltd., pp. 42–44.
- Eastell R, Baumann M, Wieczorek L. (2001). Bone Markers: Biochemical and Clinical Perspectives. London: Martin Dunitz Ltd.,
- Epstein S. (2006). Update of current therapeutic options for the treatment of postmenopausal osteoporosis. Clin Ther, 28, 151–173.
- Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, Christiansen C, Delmas PD, Zanchetta JR, Stakkestad J, Glüer CC, Krueger K, Cohen FJ, Eckert S, Ensrud KE, Avioli LV, Lips P, Cummings SR. (1999). Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: Results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA, 282, 637–645.
- Frost HM. (1992). The role of changes in mechanical usage set points in the pathogenesis of osteoporosis. J Bone Miner Res, 7, 253–261.
- Garrett IR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR. (1990). Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest, 85, 632–639.
- Halleen JM, Alatalo SL, Janckila AJ, Woitge HW, Seibel MJ, Väänänen HK. (2001). Serum tartrate-resistant acid phosphatase 5b is a specific and sensitive marker of bone resorption. Clin Chem, 47, 597–600.
- Heaney RP, Recker RR, Saville PD. (1977). Calcium balance and calcium requirements in middle-aged women. Am J Clin Nutr, 30, 1603–1611.
- Ichikawa H, Aggarwal BB. (2006). Guggulsterone inhibits osteoclastogenesis induced by receptor activator of nuclear factor-κB ligand and by tumor cells by suppressing nuclear factor-κB activation. Clin Cancer Res, 12, 662–668.
- Jacobsen BK, Nilssen S, Heuch I, Kvåle G. (1997). Does age at natural menopause affect mortality from ischemic heart disease? J Clin Epidemiol, 50, 475–479.
- Khanna D, Sethi G, Ahn KS, Pandey MK, Kunnumakkara AB, Sung B, Aggarwal A, Aggarwal BB. (2007). Natural products as a gold mine for arthritis treatment. Curr Opin Pharmacol, 7, 344–351.
- Kimura I, Yoshikawa M, Kobayashi S, Sugihara Y, Suzuki M, Oominami H, Murakami T, Matsuda H, Doiphode VV. (2001). New triterpenes, myrrhanol A and myrrhanone A, from guggul-gum resins, and their potent anti-inflammatory effect on adjuvant-induced air-pouch granuloma of mice. Bioorg Med Chem Lett, 11, 985–989.
- Lean JM, Davies JT, Fuller K, Jagger CJ, Kirstein B, Partington GA, Urry ZL, Chambers TJ. (2003). A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J Clin Invest, 112, 915–923.
- Mishra S. (2007). Commentary on Bhaisajya Ratnavali. Ed. 1. Varanasi: Choukhamba, Surbharti Prakashan.
- Novack DV. (2007). Estrogen and bone: Osteoclasts take center stage. Cell Metab, 6, 254–256.
- Oge A, Bayraktar F, Sevin G, Uyulgan B, Yilmaz C, Kabalak T. (2002). A comparative study of raloxifen and estrogen on bone strength and cholesterol levels in ovariectomized rats. British Endocrine Societies Joint Meeting 2002, Endocrine Abstracts, Endocrine Abstracts Harrogate, UK, p. 3 P10.
- Palmer JR, Rosenberg L, Shapiro S. (1992). Reproductive factors and risk of myocardial infarction. Am J Epidemiol, 136, 408–416.
- Peng Z, Tuukkanen J, Zhang H, Jämsä T, Väänänen HK. (1994). The mechanical strength of bone in different rat models of experimental osteoporosis. Bone, 15, 523–532.
- Penolazzi L, Lampronti I, Borgatti M, Khan MT, Zennaro M, Piva R, Gambari R. (2008). Induction of apoptosis of human primary osteoclasts treated with extracts from the medicinal plant Emblica officinalis. BMC Complement Altern Med, 8, 59.
- Riggs BL, Khosla S, Melton LJ 3rd. (2002). Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev, 23, 279–302.
- Riis B, Thomsen K, Christiansen C. (1987). Does calcium supplementation prevent postmenopausal bone loss? A double-blind, controlled clinical study. N Engl J Med, 316, 173–177.
- Satyavati GV. (1988). Gum guggul (Commiphora mukul)–the success story of an ancient insight leading to a modern discovery. Indian J Med Res, 87, 327–335.
- Sharma JN, Sharma JN. (1977). Comparison of the anti-inflammatory activity of Commiphora mukul (an indigenous drug) with those of phenylbutazone and ibuprofen in experimental arthritis induced by mycobacterial adjuvant. Arzneimittelforschung, 27, 1455–1457.
- Shirwaikar A, Khan S, Kamariya YH, Patel BD, Gajera FP. (2010). Medicinal plants for the management of post menopausal osteoporosis: A review. The Open Bone Journal, 2, 1–13.
- Somerman MJ, McCauley LK. (2006). Bisphosphonates: Sacrificing the jaw to save the skeleton. Bonekey Osteovision, 3, 12–8.
- Sudarshana V, Deepa. (2008). Antioxidant studies on the ethanolic extract of Commiphora spp. Afr J Biotechnol, 8, 1630–1636.
- Sushruta. (1907). Dosha-Dhatu-Mala-Kshaya-Vriddhi-Vijnaniya-madhyayam. In Bhishagratna KKL, ed. An English Translation of The Sushrutha Samhita Vol I Suthrasthanam. Culcutta: Wilkins Press, 120–139.
- Szejnfeld VL. (2000). Manifestacoes clinicas. Osteoporose: Diagnostico e tratamento. Sarvier: Sao Paulo.
- Tracy RE. (1966). Sex difference in coronary disease: Two opposing views. J Chronic Dis, 19, 1245–1251.
- Tunstall-Pedoe H. (1998). Myth and paradox of coronary risk and the menopause. Lancet, 351, 1425–1427.
- Urizar NL, Liverman AB, Dodds DT, Silva FV, Ordentlich P, Yan Y, Gonzalez FJ, Heyman RA, Mangelsdorf DJ, Moore DD. (2002). A natural product that lowers cholesterol as an antagonist ligand for FXR. Science, 296, 1703–1706.
- Urizar NL, Moore DD. (2003). GUGULIPID: A natural cholesterol-lowering agent. Annu Rev Nutr, 23, 303–313.
- van der Schouw YT, van der Graaf Y, Steyerberg EW, Eijkemans JC, Banga JD. (1996). Age at menopause as a risk factor for cardiovascular mortality. Lancet, 347, 714–718.
- Varley H. (1980). Practical Biochemistry, Vol. 1. London: William Heinemann Medical Books Ltd, p. 913.
- Wang X, Greilberger J, Ledinski G, Kager G, Paigen B, Jürgens G. (2004). The hypolipidemic natural product Commiphora mukul and its component guggulsterone inhibit oxidative modification of LDL. Atherosclerosis, 172, 239–246.
- Wuttke W, Jarry H, Becker T, Schultens A, Christoffel V, Gorkow C, Seidlová-Wuttke D. (2008). Phytoestrogens: Endocrine disrupters or replacement for hormone replacement therapy? Maturitas, 61, 159–170.