Abstract
Context: Rheumatoid arthritis (RA) is a chronic inflammatory disease caused by inflammation of the synovial membrane, leading to articular cartilage destruction. Shark cartilage polysaccharide (SCP) is a biodegradable, biocompatible, nontoxic, non-immunogenic and non-inflammatory polysaccharide that may be used in treating RA.
Objective: The anti-RA activities of SCP given orally to rats are investigated here for the first time.
Materials and methods: SCP treatment group were administered with SCP-1, SCP-2 in the dosage of 9 mg/day for 24 days. The effect of SCP-1 and SCP-2 on the body weight, paw swelling, morphological changes of ankle and IL-6, IL-12 secretion in RA rats are examined.
Results: On day 24, there were no obvious differences in BMI between positive, SCP-1 and SCP-2 group. The swelling degree of SCP-1 and SCP-2 group was less serious than model group. X-ray revealed that SCP-1, SCP-2 group owned normal joint alignment and more smooth and tidy articular surface. The SCP-1 and SCP-2 have inhibitory effect on IL-6 (430.3 ± 25.6 pg/mL, 439.1 ± 35.9 pg/mL) and IL-12 (63.9 ± 20.1 pg/mL, 64.9 ± 14.1 pg/mL) secretion, which showed significant differences with model group (612 ± 72.3 pg/mL, 230.1 ± 29.2 pg/mL), but still higher than normal group (361.8 ± 47.1 pg/mL, 34.2 ± 15.1 pg/mL) and lower than positive group (418.1 ± 42.5 pg/mL, 90.2 ± 17.8 pg/mL). Especially, when the concentration of SCP was 125 μg/mL, the contents of IL-6 (431.1 ± 43.3 pg/mL, 401.7 ± 55.7 pg/mL) and IL-12 (63.2 ± 12.3 pg/mL, 52.3 ± 8.7 pg/mL) were lowest.
Discussion and conclusion: These findings demonstrate that SCP have excellent anti-RA activities and thus have great potential as a drug for treating RA diseases.
Introduction
Rheumatoid arthritis (RA) is a common systemic autoimmune disease, which is characterized by chronic polyarthritis symptom (CitationWalsmith et al., 2002). According to the RA pathogenesis studies, anti-inflammatory therapy has become one important treatment (CitationWilson et al., 2000, 2003). Cytokines are extracellular protein/peptide messengers that play a critical role in cell-to-cell communication in RA. They are the primary mediators by which communication occurs between leukocytes such as lymphocytes and macrophages, and between other immune and non-immune cells and, thus, contribute significantly to regulating and controlling immune responses (CitationRomagnani, 2004; CitationMerly et al., 2007). In the pathogenesis of RA, IL-6 (CitationMcNiff et al., 1995; CitationJikko et al., 1998; CitationIshihara & Hirano, 2002; CitationNishimoto & Kishimoto, 2004; CitationWong et al., 2006) and IL-12 are two very important inflammatory mediators. IL-6 and IL-12 can stimulate the release of inflammatory cytokines and attract inflammatory cells into the joint cavity, which result in aggravating the pathogenesis of RA (CitationTaylor, 2003; CitationMaruotti et al., 2006). It is reported that glycosaminoglycans (GAGs) such as shark cartilage extract has anti-inflammatory effects and fibrinolytic activities (CitationChen et al., 2000; CitationRatel et al., 2005), the effects of shark cartilage extract on the attachment and spreading properties and the focal adhesion structure of cultured bovine pulmonary artery endothelial cells were examined, treatment with cartilage extract resulted in cell detachment from the substratum, and the inhibitory effects of cartilage extract on cell attachment and spreading are mediated by modification of the organization of focal adhesion proteins.
Shark cartilage contains proteins, polysaccharides, fat, minerals, calcium, phosphorus and moisture (CitationPearson W et al., 2007). Shark cartilage polysaccharides (SCPs) are composed of GAGs including keratan sulfate (KS), chondroitin sulfate A (CSA), chondroitin sulfate C (CSC) and hyaluronic acid (HA). The major disaccharides of CSA and CSC are glucuronic acid and sulfated N-acetylgalactosamine (GalNAc), which are connected by β1-3 glucosidic bond, and the disaccharide units are connected by β1-4 glucosidic bond to each other, forming the liner chain polysaccharides. Shark cartilage is a promising source of CS (CitationPing Wang & Junmin Tang, 2009). CS is an anionic linear polysaccharide which consists of alternating disaccharide units of glucuronic acid (GlcUA) and GalNAc. Over the past few years, through many scientific studies about the use of GAGs and chondroitin by millions of people, experiments have proved that these substances orally can help people with joint problems, most significantly people with osteoarthritis. These polysaccharides can relieve joint pain, and can actually help rebuild cartilage and slow the progression of osteoarthritis. Other than its antineuralgia, antihemicrania and antitumor activities, SCPs is speculated to have anti-rheumatoid arthritis activity.
The purpose of the research conducted in our lab was to evaluate the immuno-effectiveness and bioactivity of commercial shark cartilage that is available as a RA therapy agent. In this study, the peritoneal macrophages were extracted from RA rat, the secretion of IL-6 and IL-12 from peritoneal macrophages culture supernatant was researched in SCP-treated group and model group, and the influence of different concentrations of SCP on the secretion of IL-6 and IL-12 were further studied.
Materials and methods
Materials
Shark cartilage polysaccharide SCP-1 (molecular weight: 60 kDa, determined by HPLC after enzymatic degradation of SCP-2) and SCP-2 (molecular weight: 100 kDa, determined by HPLC, C4384) were purchased from Sigma, sulfur ≥ 4%, 6-sulfate:4-sulfate ratio by HPLC ≥ 0.33:1. Freund’s complete adjuvant (CFA), Sigma, F5881. Methotrexate (MTX), Sigma, M4010. Calf serum, Hangzhou, Four Seasons Green Engineering Co., Ltd. PBS, Gibco Co, USA. Trypan blue, Sigma. RPMI-1640, GIBCO, USA. Trypsin, Amresco, USA. EDTA, Shanghai Biotechnology Co., Ltd. LPS, Sigma. Rat-IL-6 ELISA KIT, Bender Medsystems, USA. Rat-IL-12 ELISA KIT, Biosource Medsystems, USA. LG-10 centrifuge, Beijing Medical Centrifuge Factory. Inverted optical microscope, LEICA, Germany. CO2-incubator, HERAEUS, Germany. Multiskan-MK3 microplate reader Leibo, Thermosystems, Canada. Ultra-low temperature refrigerator, SANYO, Japan.
Laboratory animal and animal grouping
Wistar rats (male), 8–10 weeks, weighing 120–180 g, were supplied by the Experimental Animal Center of Shandong Province, and carried out in accordance to the ‘‘NIH Guidelines for the Care and Use of Laboratory Animals’’. Male Wistar rats (25) were randomly divided into five groups (n = 5), which were: A. model group, B. positive control group, C. normal control group, D. SCP-1 treatment group, E. SCP-2 treatment group. The mice were maintained in a specific pathogen-free environment that was temperature controlled (23 ± 2°C) and humidity controlled (60 ± 10%), under a 12 h light/dark cycle.
Rheumatoid arthritis model in rats
After a few days feeding, each rat was injected with 0.1 mL complete Freund’s adjuvant (CFA) (10 mg/mL) in the right rear paw subcutaneous except the normal group. In the same day, SCP-1 treatment group and SCP-2 treatment group were administered with SCP-1, SCP-2 in the dosage of 9 mg/day for 24 days. Positive group was administered once a week with methotrexate (MTX) 0.55 mg/kg for 24 days. Normal group was given 2 mL normal saline each day, for 24 days.
The changes of body weight and paw swelling index
The weight and the paw swelling index of rat was measured on day 1, 3, 6, 12, 18 and 24 after administration (CitationLiu M et al., 2011). The thickness of paw was measured before and after proinflammatory and paw swelling index was calculated by the following formula:
Paw swelling index = (thickness after proinflammatory − thickness before proinflammatory)/thickness before proinflammatory × 100%.
X-ray evaluation
X-ray photograph of the hind legs were taken after the rats were killed (60 kV, 15 s). The alteration of ossicular skeleton by X-ray method could assess the degree of arthritis.
Preparation of the peritoneal macrophage suspension
Rats from different groups were killed by cervical dislocation. Then, the rats were dissected to expose the peritoneal wall and 75% alcohol was used to scrub peritoneal wall. Ice-cold PBS (20 mL) was injected into the abdominal cavity and abdomen was gently pressed (about 2 min). Peritoneal exudate cells were harvested by flushing peritoneal cavities with ice-cold PBS. Cells were plated in a 24-well plate at an initial density of 1 × 106 cells/mL in a 500 mL volume of RPMI 1640, supplemented with 10% FBS. Two hours later, the wells were washed to remove all nonadherent cells and 500 mL of fresh medium was added together with LPS (1.0 mg/mL/well).
Determination of cytokines
After a 48-h incubation, the supernatants from 1 × 106 peritoneal macrophage were collected and IL-6 ELISA was performed using a standard kit from Sigma. The same kind of test was used for IL-12 measurement.
Statistical analysis
All data were expressed as mean ± SEM. Significant differences among groups were analyzed by one-way analysis of variance (ANOVA). Differences were considered statistically significant at p < 0.05.
Results
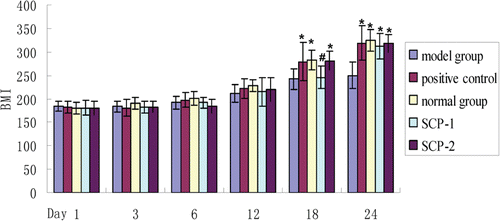
Body weight and paw swelling index changes
The weight of rat was measured on days 1, 3, 6, 12, 18, and 24 after administration. Measurement results were shown in . From , it was found that the body weight of rats from different groups gradually increased in 24 days. However, the body weight of rats in model group grew slowly, and this trend continued to day 24. There were no significant differences between each group from day 1 to day 12. But there were obvious differences in body weight between model group and normal group, and the differences increased significantly until day 24, which indicated that the RA model was successfully established. The body weight of rats from SCP-1 and SCP-2 group did not significantly higher than that of model group until on day 18 (p < 0.05), but there were no obvious differences in body weight between positive group and SCP-2 group, which indicated that SCP-2 has similar therapeutic effect compared with positive control. On day 24, there were no obvious differences in body weight between positive group, SCP-1 group and SCP-2 group, which indicated that both SCP-1 and SCP-2 have similar therapeutic effect in RA therapy.
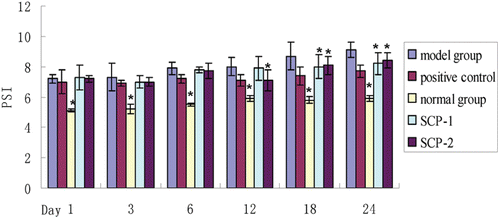
Changes in rat paw swelling index
The paw swelling index of rat was measured on days 1, 3, 6, 12, 18 and 24 after administration. From , it can be concluded that at first few days, all groups except normal group, the joints swelling of entire foot and ankle of the right hind leg was apparent, which was significantly higher than that of normal group. MTX (positive control) has the most benefit effect on paw swelling index, and the paw swelling index of positive control was closely to model group. The rats paw swelling reached to the peak on day 21 and day 24. There were no significant differences between the model group and treatment groups until day 18. Besides, the swelling degree of rats from SCP-1 and SCP-2 group were less serious than that of model group on day 18 and day 24 (p < 0.05). However, there were no significant differences between the two treatment groups.
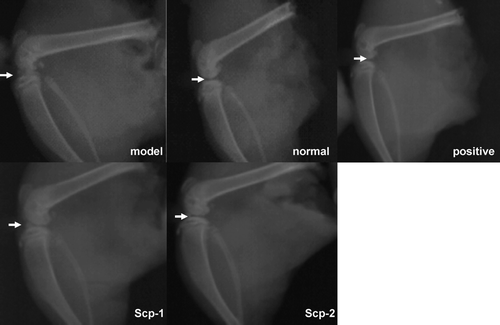
X-ray evaluation
Rats of model group had significantly narrow knee gap, irregular joint edges and low-density areas along the joint edge caused by multiple erosions (). Compared with the normal group, it could be seen the rheumatoid arthritis model was successfully established. X-ray revealed that rats of SCP-1 and SCP-2 groups had normal joint alignment and more smooth and well organized articular surfaces. In addition, a narrow gap was not obvious and knee lesions were less serious than that of the model group.
Figure 3. X-ray evaluation of all the groups. Model group: joint space narrowing, joint edges irregular, not sharpness, patella forward displacement, low-density area of joint edge caused by multiple erosion. Positive control: Normal joint alignment, articular surface smooth, gap narrow not obvious, small amount of proliferation of the articular surface. Normal control: normal joint alignment, articular surface smooth, normal bone structure. SCP-1: normal joint alignment, articular surface smooth, gap narrow not obvious, small amount of proliferation of the articular surface. SCP-2: normal joint alignment, articular surface smooth, gap narrow not obvious.
The determination of IL-6 and IL-12
The concentration of rat IL-6 and IL-12 was determined at 450 nm, and the standard curves were fit by Origin Pro7.0. The regression equation of IL-6 and IL-12 were as follows, y = 0.0015x + 0.0689, R2 = 0.9975, y = 0.0032x + 0.1646, R2 = 0.9927.
The contents of IL-6 and IL-12 from rat peritoneal macrophages of different experimental groups are shown in . The results showed the content of IL-6 from peritoneal macrophage culture supernatant of the model group was 611.93 ± 72.3 pg/mL, which was higher than that of the normal group (361.84 ± 47.1 pg/mL) (p <0.05). The content of IL-6 in the positive group was 418.11 ± 42.5 pg/mL, which was significantly lower than that of the model group. In two treatment groups the rat IL-6 levels were also lower than that of the model group but still higher than that of the positive group. It can be seen from that two SCPs have an inhibitory effect on IL-6 secretion, which showed significant differences with the model group and the normal control (p < 0.05), but showed no significant differences with the positive group. There was no significant difference between the treatment groups.
Figure 4. Content of rat IL-6 and IL-12 in all the groups (*: vs model group, p < 0.05, #: vs normal group, p < 0.05).
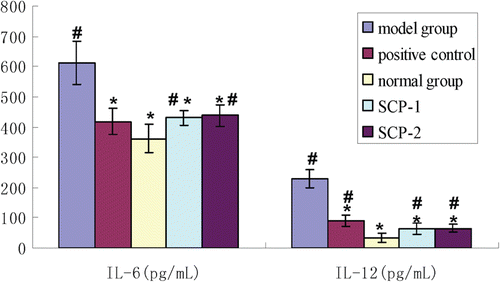
The content of IL-12 from peritoneal macrophage supernatants of the model group was 230.13 ± 29.2 pg/mL, which was significant higher than that of the normal group (34.19 ± 15.1 pg/mL) (p <0.05).The content of IL-12 in the positive group was only 90.19 ± 17.78 pg/mL. Similarly, the rat IL-12 levels in two treatment groups were also lower than that of the model group and the positive group but still higher than that of the normal group. Two SCPs have an inhibitory effect on IL-12 secretion, which showed significant differences with the positive group and the normal control (p < 0.05) but showed no significant difference with the model group. There was no significant difference between the treatment groups.
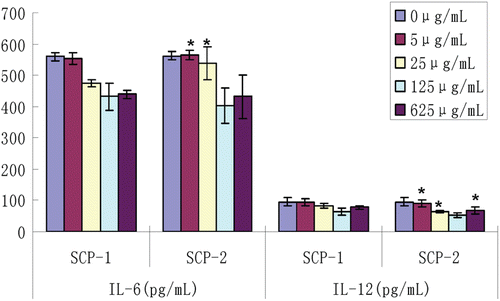
The effect of different concentrations of SCPs on the contents of IL-6 and IL-12
It can be seen that as the concentration of SCP increased, IL-6 and IL-12 secreted by macrophages decreased (). When the concentration of SCPs was 125 μg/mL, the contents of IL-6 and IL-12 were lowest, and there were no significant differences between the SCP-1 and SCP-2 groups (p > 0.05). On the contrary, SCP-2 showed a better therapeutic effect than SCP-1 (p < 0.05) in other concentrations (0, 5, 25, and 625 μg/mL).
Discussion
In this experiment, the RA model was successfully established and the weight of the rats and paw swelling during the experiment were observed. In addition, IL-6 and IL-12 levels of culture supernatants of peritoneal macrophages were determined by ELISA after SCPs administration (CitationJoe et al., 1999; CitationJoe & Wilder, 1999). In the rat RA model, the rat right hind foot swelled, and the inflammatory reaction spread to another hind foot and even the front foot after a period of time. In addition, the expression levels of rat IL-6 and IL-12 rose continuously. This study explored the influence of two SCPs on expression levels of rat IL-6 and IL-12, which further play an important role in the RA inflammation process including autoantibody generation and inflammatory cells in joint cavities and synovia. Taking the dosage (Shark Cartilage Capsules US time, 720 mg mucopolysaccharides/d) of shark cartilage capsules in human as the reference, the conversion factor between human and rat was 6.3, the dosage of the rat was calculated as follow: 720 mg/d ÷ 60 kg × 6.3 × 0.12 kg = 9 mg/d. The results showed that SCP-1 and SCP-2 could significantly reduce paw edema in rats and attenuate weight loss caused by inflammatory response in rats. In addition, SCP-1 and SCP-2 could significantly inhibit the expression of IL-6 and IL-12 in the RA model.
IL-6 is a glycoprotein with a molecular weight of 19–28 kD and activated monocytes are the mainly source of IL-6 in the blood. Interestingly, the ability of monocytes to synthetise IL-6 decreases when they differentiate into macrophages (CitationHuang et al., 2003). In RA pathogenesis, IL-6 can stimulate B cells to proliferate, differentiate into plasma cells and secrete Ig and rheumatoid factor and other autoantibodies. In addition, IL-6 can activate endothelial cells to secrete IL-1, activate fibroblasts to express TH and MHC molecules, and induce inflammatory cell aggregation, infiltration, involved in inflammatory and immune responses, which enhance the role of IL-6 to induce synovitis. Furthermore, IL-6 can induce liver cells to synthesize acute phase proteins, activate synovial macrophages and STAT proteins, induce IL-1, IFN and TNF increasing, thus causing acute synovitis response and pain. IL-6 can activate various cells, such as APC, T cells, B cells, synovial and cartilage cells, and can present the antigen to T cells to start the inflammatory and immune responses. IL-6 can recruit inflammatory cells into the joint cavity and synovial,and promote the production and secretion of inflammatory mediators, resulting in immune-mediated synovial inflammation (CitationIshihara & Hirano, 2002). Therefore, investigation of the effects of IL-6 on RA from the perspective of molecular immunology may provide a new target for the drug treatment. In this study, the effects of two kinds of SCP on the expression levels of IL-6 of peritoneal macrophage culture medium in the rat model of RA were studied. The results showed that, the expression of IL-6 of peritoneal macrophage supernatant in the model group was significantly improved. Two kinds of SCPs both exhibited an inhibitory effect on the expression of IL-6 in rat peritoneal macrophages culture supernatant with different degrees. Different concentrations of SCP-1 and SCP-2, SCP-2 exhibited a stronger inhibitory effect, suggesting SCP-2 play a better role in the treatment of RA. Meanwhile, the inhibitory effect was dose-dependent, when the drug concentration was 125 μg/mL, the two shark cartilage polysaccharides showed the strongest inhibition.
IL-12 is a heterodimeric glycoprotein with the molecular weight of 75 kD, which is mainly activated by macrophages, dendritic cells and neutrophils and has proinflammatory and immunoregulatory effects (CitationOzenci et al., 2000). In RA pathogenesis, IL-12 can induce T cells (CD4+) proliferation, differentiation and activate T cells, which can secrete more cytokines to induce an inflammatory response. IL-12 has the ability of attracting and recruiting inflammatory cells into the joint cavity and synovium, promoting the production and secretion of various inflammatory mediators and leading to the degradation and synthesis decreased of cartilage proteoglycan. IL-12 can reduce apoptosis and promote the proliferation of synovial cells. Furthermore, it can promote the degradation and destruction of cartilage cells. In addition, IL-12 can induce Th0 cells to differentiate into Th1 cells, but also promote the proliferation of the CTL and activate its cytotoxicity (CitationSakkas et al., 1998; CitationWatford et al., 2003). Hence, inhibiting the activity of IL-12 is conducive to treatment of RA. In this study, the effects of two kinds of SCP on the expression levels of IL-12 of peritoneal macrophage culture supernatant in the rat model of RA were investigated. It can be seen from the results that the expression of IL-12 of peritoneal macrophage supernatant in the RA model rats was significantly increased. SCP-1 and SCP-2 could inhibit the expression of IL-12 with different degrees. Different concentrations of SCP-1 and SCP-2, SCP-2 exhibited a stronger inhibitory effect, suggesting that SCP-2 play a better role in the treatment of RA. Meanwhile, the inhibitory effect was dose-dependent, when the drug concentration was 125 μg/mL, SCPs showed the strongest inhibition on the expression of IL-12. When the drug concentration is higher or lower than the drug concentration, the inhibition decreased, which was consistent with the inhibitory effect of shark cartilage polysaccharides on the expression of IL-6.
Conclusions
The current study demonstrated that oral administration of SCP effectively blocked the process of RA by inhibiting joint inflammation and joint damage progression, improving radiologic change of bone, increasing weight loss and inhibiting swelling index of paw. The inflammatory process in RA rats without treatment was shown to lead to substantial increases in the levels of the pro-inflammatory cytokines IL-6 and IL-12, meanwhile SCP showed significant inhibition of the over-production of IL-6 and IL-12. In sum, we examined the effect of the two kinds of shark cartilage polysaccharides on paw swelling and the secretion of IL-6 and IL-12 in the rat RA model. Studies have shown that SCP-1 and SCP-2 can relieve paw swelling and inhibit the expression of IL-6 and IL-12 of RA rats. SCP-2 showed stronger effect than SCP-1 in inhibiting the expression of IL-6 and IL-12, and the effect was dose-dependent. When the drug concentration was 125 μg/mL, the inhibition was strongest.
Declaration of interest
The authors declared no conflict of interest.
References
- Chen JS, Chang CM, Wu JC, Wang SM. (2000). Shark cartilage extract interferes with cell adhesion and induces reorganization of focal adhesions in cultured endothelial cells. J Cell Biochem, 78, 417–428.
- Huang M, Pang X, Karalis K, Theoharides TC. (2003). Stress-induced interleukin-6 release in mice is mast cell-dependent and more pronounced in apolipoprotein E knockout mice. Cardiovasc Res, 59, 241–249.
- Ishihara K, Hirano T. (2002). IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev, 13, 357–368.
- Jikko A, Wakisaka T, Iwamoto M, Hiranuma H, Kato Y, Maeda T, Fujishita M, Fuchihata H. (1998). Effects of interleukin-6 on proliferation and proteoglycan metabolism in articular chondrocyte cultures. Cell Biol Int, 22, 615–621.
- Joe B, Griffiths MM, Remmers EF, Wilder RL. (1999). Animal models of rheumatoid arthritis and related inflammation. Curr Rheumatol Rep, 1, 139–148.
- Joe B, Wilder RL. (1999). Animal models of rheumatoid arthritis. Mol Med Today, 5, 367–369.
- Liu M, Mao W, Guan H, Li L, Wei B, Li P. (2011). Effects of taurochenodeoxycholic acid on adjuvant arthritis in rats. Int Immunopharmacol, 11, 2150–2158.
- Maruotti N, Cantatore FP, Crivellato E, Vacca A, Ribatti D. (2006). Angiogenesis in rheumatoid arthritis. Histol Histopathol, 21, 557–566.
- McNiff PA, Stewart C, Sullivan J, Showell HJ, Gabel CA. (1995). Synovial fluid from rheumatoid arthritis patients contains sufficient levels of IL-1 beta and IL-6 to promote production of serum amyloid A by Hep3B cells. Cytokine, 7, 209–219.
- Merly L, Simjee S, Smith SL. (2007). Induction of inflammatory cytokines by cartilage extracts. Int Immunopharmacol, 7, 383–391.
- Nishimoto N, Kishimoto T. (2004). Inhibition of IL-6 for the treatment of inflammatory diseases. Curr Opin Pharmacol, 4, 386–391.
- Ozenci V, Kouwenhoven M, Press R, Link H, Huang YM. (2000). IL-12 elispot assays to detect and enumerate IL-12 secreting cells. Cytokine, 12, 1218–1224.
- Pearson W, Orth MW, Karrow NA, Maclusky NJ, Lindinger MI. (2007). Anti-inflammatory and chondroprotective effects of nutraceuticals from Sasha’s Blend in a cartilage explant model of inflammation. Mol Nutr Food Res, 51, 1020–1030.
- Ping Wang, Junmin Tang. (2009). Solvent-free mechanochemical extraction of chondroitin sulfate from shark cartilage. Chemical Engineering and Processing: Process Intensification, 48, 1187–1191.
- Ratel D, Glazier G, Provençal M, Boivin D, Beaulieu E, Gingras D, Béliveau R. (2005). Direct-acting fibrinolytic enzymes in shark cartilage extract: Potential therapeutic role in vascular disorders. Thromb Res, 115, 143–152.
- Romagnani S. (2004). Immunologic influences on allergy and the TH1/TH2 balance. J Allergy Clin Immunol, 113, 395–400.
- Sakkas LI, Johanson NA, Scanzello CR, Platsoucas CD. (1998). Interleukin-12 is expressed by infiltrating macrophages and synovial lining cells in rheumatoid arthritis and osteoarthritis. Cell Immunol, 188, 105–110.
- Taylor PC. (2003). Antibody therapy for rheumatoid arthritis. Curr Opin Pharmacol, 3, 323–328.
- Walsmith J, Roubenoff R. (2002). Cachexia in rheumatoid arthritis. Int J Cardiol, 85, 89–99.
- Watford WT, Moriguchi M, Morinobu A, O’Shea JJ. (2003). The biology of IL-12: Coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev, 14, 361–368.
- Weyand CM, Fulbright JW, Goronzy JJ. (2003). Immunosenescence, autoimmunity, and rheumatoid arthritis. Exp Gerontol, 38, 833–841.
- Wilson C, Tiwana H, Ebringer A. (2000). Molecular mimicry between HLA-DR alleles associated with rheumatoid arthritis and Proteus mirabilis as the aetiological basis for autoimmunity. Microbes Infect, 2, 1489–1496.
- Wong PK, Quinn JM, Sims NA, van Nieuwenhuijze A, Campbell IK, Wicks IP. (2006). Interleukin-6 modulates production of T lymphocyte-derived cytokines in antigen-induced arthritis and drives inflammation-induced osteoclastogenesis. Arthritis Rheum, 54, 158–168.