Abstract
Context: Diabetic nephropathy is one of the important microvascular complications of diabetes; however, the main problem remains is the control of progression of nephropathy in diabetes. Chebulic acid was selected, as tannins from Terminalia chebula are used as antidiabetic, renoprotective, antioxidant, hypotensive and an α-glucosidase inhibitor.
Objective: In this study, we evaluated the effect of chebulic acid on ischemia reperfusion induced biochemical alteration in diabetic rats.
Materials and methods: Chebulic acid (CA) was isolated from T. chebula; LD50 and acute toxicity studies of CA were done. Renal ischemia and reperfusion technique was used to induce nephropathy in diabetic rats. Glibenclamide (10 mg/kg) was used as diabetic standard; CA at doses of 25 and 50 mg/kg were administered for 28 days and various biochemical parameters were monitored.
Results: The LD50 was found to be 251 mg/kg; 25 and 50 mg/kg doses were selected as no toxic symptoms were observed at both doses, except slight diarrhea. CA significantly (p < 0.001) reduced the glucose, creatinine, urea nitrogen, glycosylated hemoglobulin, proteinuria, urine albumin excretion, glomerular filtration rate (GFR), and increased serum insulin and glycogen level. CA also restored glucose 6-phosphate dehydrogenase, glutathione, superoxide dismutase, catalase and malondialdehyde levels. Improvement in kidney was also noted in histopathological studies.
Conclusions: The statistical data indicated that chebulic acid at both doses (25 and 50 mg/kg) improves biochemical alterations caused by renal ischemia in diabetic rats.
Keywords::
Introduction
Diabetic nephropathy is one of the important microvascular complications of diabetes mellitus and the most common cause of chronic kidney disease (CKD) and end-stage renal disease (ESRD) in many countries (CitationHa et al., 2008). Nephropathy usually becomes clinically evident after 15–25 years of diabetes (CitationRabkin, 2003). Nephropathy develops in about 20–40% of all diabetic patients, with approximately 40% of patients with type 1 diabetes and 5–15% of patients with type 2 diabetes (DeFronzo, Citation1995). The exact cause of diabetic nephropathy is unknown, but a variety of factors contribute to the renal damage; hyperglycemia is a common etiologic factor in diabetic patients with nephropathy, but a genetic predisposition and smoking contribute as well, hypertension and microalbuminuria are also familial marker of risk (CitationEvans & Capell, 2000; CitationDe Boer et al., 2011).
Terminalia chebula Retz. (Combretaceae), native to India and Southeast Asia, has been used traditionally to treat various diseases (CitationLee et al., 2007). In the modern era it has been used as a cytoprotective (CitationSaleem et al., 2002), antidiabetic (CitationMurali et al., 2004; CitationKumar et al., 2006), renoprotective (CitationRao & Nammi, 2006), antioxidant (CitationSabu & Kuttan, 2002; CitationNaik et al., 2004), and cardioprotective (CitationSuchalatha & Devi, 2005).
Previous studies showed that tannins isolated from T. chebula are cytoprotective (CitationLee et al., 1995), antioxidant (CitationKlika et al., 2004), hypotensive (CitationLin et al., 1993), and α-glucosidase inhibitors (CitationGao et al., 2007). Chebulic acid (CA) has been used as hepatoprotective (CitationLee et al., 2007) and it causes reduction of advanced glycation end products in epithelial cells (CitationLee et al., 2010). Although antidiabetic and renoprotective effect of extract have been verified, the effect of CA on ischemia reperfusion induced biochemical alteration in diabetic rats has not been published. Therefore, the present study investigates the protective effect of CA (25 and 50 mg/kg) on animal model of diabetic nephropathy.
Materials and methods
Chemicals
All the chemicals, reagents, and solvents used in the activity were of analytical grade. Diagnostic kits used were procured from Lab Care Diagnostic (India) Pvt. Ltd. Serum insulin was analyzed by commercial pathology laboratory (Choksi Laboratories Limited, Indore, India).
Extraction and isolation
Dried fruits of T. chebula were purchased from a local market, Mandsaur, India, identified by Prof. Gyanendra Tiwari, Scientist, Government College of Horticulture, Mandsaur (Madhya Pradesh, India) and a voucher specimen (BRNCP/TC/009/2009) was deposited in the herbarium of Department of Pharmacognosy, B. R. Nahata College of Pharmacy, Mandsaur (Madhya Pradesh, India). T. chebula fruits (10 kg) were coarsely powdered; defatted with petroleum ether (60–80°C); dried and filled in a Soxhlet apparatus for extraction with absolute ethanol as solvent. The extraction was carried out for a period of 72 h. The extract obtained was dried in vacuum to remove excess solvent. The ethanol extract obtained was resuspended in H2O, and then extracted successively with n-hexane, chloroform, ethyl acetate, and n-butanol. Extract obtained after extraction with ethyl acetate was dried. Dried compound was subjected to column chromatography (silica gel) and eluted by methanol in ethyl acetate (30:70, v/v). The obtained fraction was further purified by Sephadex LH-20 column chromatography using methanol as the eluent. RP-HPLC (Waters, USA) with C-18 column (5 µm, 250 × 4 mm, Lichro Cart, Merck, Germany) was used for further separation and estimation; formic acid and acetonitrite were used as eluent and quantification of compound was done using calibration curve of standard compound. Data of FTIR, 1H NMR and MS spectroscopy of CitationLee et al. (2007), were used to identify the compound.
Animals
All the animals were procured from animal house of B.R.N.C.P. Mandsaur. Healthy adult Wistar albino rats of either sex weighing between 200–250 g were used for ischemia-reperfusion (ESRD) model. The animals were stabilized for 1 week; they were maintained in standard condition at room temp; 60 ± 5% relative humidity and 12 h light–dark cycle. They had been given standard pellet diet and water ad libitum throughout the course of the study. All the animal studies were carried out in Department of Pharmacology and Toxicology, B.R.N.C.P. Mandsaur; with permission from Institutional animal ethical committee (IAEC reg. no. 918/AC/05/CPCSEA). The animals were handled gently to avoid stress, which could result in an increased adrenal output.
Acute oral toxicity study and LD50
The Miller and Tainter (graphical) method was used to calculate LD50. The Probit values were plotted against log dose and then dose corresponding to Probit 5 was calculated. 1/5th and 1/10th of LD50 was selected as dose for further study (CitationGhosh, 2008; CitationRandhawa, 2009).
The rats were randomly divided into three equal groups (n = 6/group); Group I served as control and received distilled water; Group II received single oral dose of CA (25 mg/kg) and Group III received single oral dose of 50 mg/kg of CA. During the treatment, animal were observed daily for overt signs of toxicity (salivation, lachrymation, squinted eyes, writhing, convulsions, tremors, yellowing of fur, loss of hair), stress (erection of fur and exophthalmia), behavioral abnormalities (impairment of spontaneous movement, climbing, cleaning of face and ataxia and other postural changes) and aversive behavior (biting and scratching behavior, licking of tail, paw and penis, intense grooming behavior and vocalization) and diarrhea for 24 h and then daily for 14 days. Food consumption was monitored daily and body weights were recorded weekly. On the 14th day, animals were sacrificed and all the organs were removed for gross pathological examination.
Induction of diabetes
Animals were fasted overnight; a single i.p. injection of freshly prepared streptozotocin (50 mg/kg in 0.1 M citrate buffer pH 4.5) was injected. Following the STZ injection, rats were given drinking water supplemented with sucrose (15 g/L) for 48 h, to limit early mortality as stores of insulin are released from damaged pancreatic islets. The diabetes was confirmed by estimation of blood glucose level (BGL) at the third day. Rats having BGL more than 250 mg/dL were used for future studies. Diabetic rats should be given daily subcutaneous injections of long-acting insulin (2–4 U/rat, Human Mixtard, Abbott India Ltd., Mumbai, India) to maintain blood glucose levels in a desirable range. A relapse period of 2 weeks was give to rats before surgery (CitationNangle et al., 2006; CitationKumar & Subramanian, 2008; CitationTesch & Allen, 2007).
Induction of ischemia
The animals were anesthetized by an i.p. injection of ketamine (80 mg/kg; Neon Lab Ltd., Mumbai). During the operation the animals were placed on a thermo pad, which kept the body temperature at 37.5°C. An incision slightly deviated from midline towards left side was made and the left renal artery was located and dissected free from its surrounding structures. After a recovery period of 10 min, renal ischemia was evoked by clamping the left renal artery for 30 min. Subsequently the abdomen was sutured and the animals were returned to their cages. Animals were watched for 1 h after surgery for the recovery. Animals were given 1–2 mL normal saline and Pethidine HCl (10 mg/kg, Neon Lab. Ltd., Mumbai, India) after recovery. Aseptic conditions were maintained for 1 week with special care. Treatment was started next day of surgery. Animals were treated for 28 days (CitationMelin et al., 1997; CitationWongmekiat et al., 2007).
The animals were randomly divided into the seven groups (n = 6); the treatment to each group was as described below.
Group I: Normal control rats (NC).
Group II: Diabetic control (DC).
Group III: Nephropathy control (NeC); unilateral ischemia in normal rats.
Group IV: Diabetic nephropathy control (DNC); unilateral ischemia in diabetic rats.
Group V: Diabetes standard (DStd); Diabetes + ischemia rats given glibenclamide (10 mg/kg body weight/day/rat) in aqueous solution orally for 28 days.
Group VI: Treatment group I (CA 25); Diabetes + ischemia rats given chebulic acid (25 mg/kg body weight/day/rat) in aqueous solution orally for 28 days.
Group VII: Treatment group II (CA 50); Diabetes + ischemia rats given chebulic acid (50 mg/kg body weight/day/rat) in aqueous solution orally for 28 days.
Biochemical analysis
Blood urea nitrogen (BUN), urinary urea nitrogen (UUN), plasma (PC) and urine creatinine (UC), urine protein (UP), urinary albumin excretion (UAE), urinary glucose (UG), glycosylated hemoglobulin (GHb) and glucose 6-phosphate dehydrogenase (G6PDH) were assessed using commercially available diagnostic kits. Blood glucose level (BG) was assessed using glucose strips (Dr. Morphen’s glucostrip). Serum insulin was analyzed by commercial pathology laboratory (Choksi Laboratories Limited; Indore, India).
The method of CitationMaiti et al. (2004) was used to estimate hepatic glycogen level. Kidneys were isolated and washed with chilled normal saline; CitationAslan et al. (2007) and CitationWang et al. (2008) methods were used to estimate reduced glutathione (GSH) and lipid peroxidation (MDA), respectively. The methods of CitationDhir and Kulkarni (2008) and CitationSharma et al. (2007) were adopted to estimate superoxide dismutase and catalase.
Histopathological studies
Kidney were collected and immediately fixed in 10% formalin, dehydrated in a graded ethanol (50–100%) series, cleared in xylene, and embedded in paraffin. Sections (4–5 µm) were prepared and stained with hematoxylin and eosin (HE) dyes for photomicroscopic observations. Sections were examined in a blinded fashion in all treatment groups.
Statistical analysis
All values are presented as mean ± SEM. CA (treated groups) were compared with all set of diseased control groups (DC, NeC, DNC). Statistical analysis of data was performed by one way analysis of variance (ANOVA) followed by Tukey’s t-test; p value <0.05 was considered as statistically significant.
Results
Isolation and characterization
The compound was isolated from T. chebula as yellow powder with a yield of 0.07% w/w (735 mg), purity of 97.04% w/w, and melting point of 195–208°C. The IR spectrum showed the main peaks at 3560-3500, 3000–3100, 1600, 725-680 (data not shown). 1H NMR data showed (400 MHz, deuterium oxide) δ 6.75 (s, 1H), 5.50-5.41 (m, 2H), 3.98 (t, J = 5.1 Hz, 1H), 3.38 (q, J = 5.0 Hz, 1H), 3.06 (dd, J = 12.5, 5.1 Hz, 1H), 2.73 (dd, J = 12.5, 5.1 Hz, 1H). From these data, the compound was identified as chebulic acid.
Acute toxicity and LD50
LD50 of chebulic acid was found to be 251 mg/kg (Log LD50 = 2.40); 25 and 50 mg/kg doses were used for entire study. In an acute oral toxicity study, CA treated animals did not show any change in their behavioral, neurological and autonomic pattern at both doses. Mild diarrhea was observed at both doses. There was no significant difference in the body weights and food consumption when compared to the vehicle treated group. Also, no gross pathological changes were seen. Thus, it was concluded that CA was safe at both doses (25 and 50 mg/kg).
Effect of CA on biochemical parameters
shows that the blood glucose level of DC and DNC group was increased by nearly 5-fold above the normal range; no change was observed in the NeC group. Treatment with CA at both dose levels showed a significant reduction in blood glucose level. CA (50 mg/kg) exhibited a maximum glucose lowering effect (78.88%) at the end of study when compared to DNC.
Table 1. Effect of Chebulic acid on the biochemical profile of blood.
There was a significant increment in serum blood urea nitrogen of the diseased group (DC, NeC and DNC) as compared to the normal animals; after 28 days treatment, CA 50 mg/kg (18.33 ± 0.55) brought BUN almost to the of normal level (17.67 ± 0.67).
CA showed a dose-related significant (p < 0.001) reduction in serum creatinine, GHb and G6PDH compared to diseased groups (). Insulin level was decreased in streptozotocin treated groups; it was decreased up to 1/3rd in DC (5.83 ± 0.79) and DNC groups (5.50 ± 0.43) whereas no effect was seen in the NeC group (14.33 ± 0.67). Administration of CA (25 and 50 mg/kg) caused a significant increase in serum insulin level at the end of study. Almost a 50% depletion of glycogen was observed in diseased animals (DC and DNC); Administration of CA at doses of 25 and 50 mg/kg for 28 days resulted in a significant (p < 0.001) increase in the glycogen level. However, the values were not restored to normal at any dose level ().
depicts the effect on the levels of creatinine, protein, albumin, urea nitrogen and glucose in urine of treated animals. After the 28 days treatment, a significant (p < 0.001) reduction was observed in excretion of biochemicals in the urine of treated groups. A fall of 69% in UC, 63% in UP, 40% in UAE, 36% in UUN, 73% in UG and 88% in GFR was observed after 28 days of treatment with CA (50 mg/kg) as compared to the DNC group.
Table 2. Effect of chebulic acid on the biochemical profile of urine.
Effect of CA on in vivo antioxidant
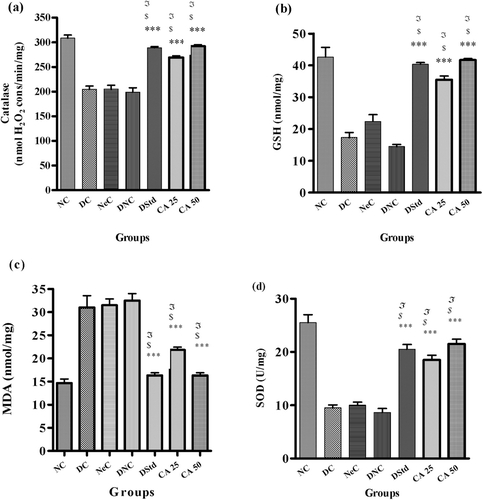
After 28 days, it was observed that the diseased animals (DC, NeC, DNC) showed a significant reduction in catalase, GSH and SOD levels; whereas lipid peroxidation was increased as compared to NC groups (–). When compared to diseased rats, CA at both doses showed significant (p < 0.001) but dose-dependent effect. CA 50 mg/kg caused an increase of 47% in catalyze level, 65% in GSH level, and 59% in SOD level whereas MDA level was decreased by 53% as compared to DNC rats.
Effect of CA on histology of kidney
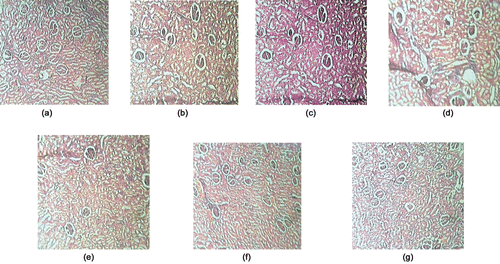
Histological examination of kidney sections showed necrosis of glomerulous with damaged proximal and convoluted tubules; diffused mesangium and thicker basal membrane of DC, NeC and DNC (–) as compared to normal rats. Treatment with CA (25 and 50 mg/kg) showed reduction in glomerular damage improvement in tubules and a lesser degree of mesangial matrix increment and basal membrane thickening was observed ( and ).
Figure 2. Effect of chebulic acid on histopathology of kidney (H and E × 100); (a) NC, normal glomeruli; (b) DC, diabetic control; (c) NeC, nephropathy control; (d) DNC, diabetic nephropathy control; (e) DStd, glibenclamide treated group (10 mg/kg); (f) CA 25, chebulic acid treated group (25 mg/kg); and (g) CA 50, chebulic acid treated group (50 mg/kg).
Discussion
The present manuscript describes the protective effect of chebulic acid on ischemia reperfusion induced biochemical alteration in diabetic rats. The LD50 of CA was found to be 251 mg/kg. No serious toxic and adverse effects were noted at the 25 and 50 mg/kg doses of CA.
In the animal model STZ caused features resembling human diabetes, whereas ischemia caused symptoms resembling nephropathy and also increased oxidative burden on the body (CitationSteffes et al., 1978).
Results of this study have shown that CA attenuated the cachectic condition along with polydipsia, polyuria and polyphagia which were commonly observed in diabetic nephropathy (CitationKawanishi et al., 1994; CitationCasey et al., 2005).
Hyperglycemia is directly linked to the development of diabetic nephropathy as it causes series of biochemical disturbances in kidney. Hyperglycemia causes activation of the polyol pathway, nonenzymatic glycation, glucose autoxidation and de novo synthesis of diaglycerol leading to protein kinase C and phospholipase A2 activation, resulting in hyperfusion and hyperfilteration and affecting glomerular permeability. Strict control of blood glucose level was found to be beneficial in controlling and preventing functional as well as structural abnormalities (CitationReddi & Camerini-Davalos, 1990; CitationLarkins & Dunlop, 1992). The results of this study showed a significant (p < 0.001) reduction in blood glucose levels attribute to the antihyperglycemic effect of CA.
Hyperglycemia also leads to nonenzymatic glycation, resulting in structural and functional changes in soluble and insoluble protein molecules such as hemoglobin in blood. Uncontrolled GHb augments development and progression of microvasular and macrovascular complications. Glycation itself generates oxygen derived free radicals, further increasing the risk of proteinuria and renal failure. Strict control of glucose causes a subsequent reduction in GHb level leading to reduction in inflammatory mediator accumulation in kidney matrix as well as less accumulation of mesangial matrix and less thickening of glomerular basement membrane (CitationJovanovic & Peterson, 1981; CitationKrishnamurti & Steffes, 2001). CA showed a prominent effect on reduction of glycosylate hemoglobulin, which further confirms an antidiabetogenic effect.
A reduction in the concentration of catalyses, GSH, SOD, and G6PDH, and an elevation in lipid peroxidation (MDA) was observed in animals with diabetes and its complications. Ameliorating oxidative stress by normalized activity of G6PDH, GSH, MDA, SOD, catalyze and resorting NADPH can be beneficial in diabetic nephropathy and prevents its progression (CitationSharma et al., 2006; CitationLiu et al., 2008). In our study, administration of CA causes a significant increase in catalyze, GSH and SOD whereas G6PDH and MDA were significantly decreased. Thus, it is reasonable to conclude that CA restored antioxidant potential of treated animals.
A low level of insulin and hyperglycemia causes derangement of glycoprotein metabolism and thickening of basement membrane (CitationSpiro & Spiro, 1971). A previous study showed that the antihyperglycemic effect of T. chebula was due to an increase in insulin level suggesting its insulinogenic activity due to stimulation of insulin secretion from remnant β cells or from regeneration of β cells (CitationChattopadhyay, 1999; CitationPari & Latha, 2002; CitationKumar et al., 2006). From these results, it can not be ignored that CA increases secretory β cells in islets and reduces blood glucose level in diabetic control and DNC rats.
Conversion of glucose to glycogen in liver is dependent on extracellular glucose concentration and on availability of insulin. CA significantly restored the level of hepatic glycogen, which further confirms the insulinogenic effect of CA and regulation of glycogenesis by CA (CitationKumar & Subramanian, 2008).
Alteration of urinary albumin, blood and urinary nitrogen urea, creatinine, urinary protein and glucose excretion was usually observed in diabetic nephropathy indicating progressive damage to glomerular and tubular cells resulting in decline in GFR (CitationRossing et al., 1994; CitationLiu et al., 2008). In the present study, the treatment group showed a significant decrease in urea, creatinine and urinary excretion. The results contribute to the renoprotective effect of CA.
Significant reduction was observed in glucose and albumin excretion of CA treated animals. CA at both doses was unable to abolish the damage fully suggesting that the treatment regimen is only preventive and altered glomerular activity cannot be revert totally.
Glomerular filtration rate (GFR) and creatinine clearance are directly dependent on hemodynamic factors. Renal damage is due to hyperglycemia and oxidative stress is caused an elevation in GFR (CitationHostetter et al., 1981; CitationKelly et al., 2000). Animals treated with CA indicated a significant decrease in GFR which could be due to improvement in renal function and decreased kidney burden.
In present study, histopathology showed damaged proximal convoluted tubules, mesangial cells, dilated tubules and glomerular necrosis in kidney of diseased (diabetic, nephropatic and diabetic nephropathy) rats. CA was able to diminish the necrosis of glomerular, proximal convoluted tubule, mesangial cell, endothelial cells etc., but neither of the treatment regimens was able to normalize the renal damage. The observed effect may be due to promising control of hyperglycemia and urinary burden by chebulic acid.
Conclusion
The present study shows that chebulic acid was effective in controlling elevated metabolic parameters, oxidative stress and renal damage, supporting its beneficial effect in diabetic nephropathy. Further studies are in progress to determine exact mechanism of action(s) responsible for the activity.
Acknowledgments
The authors would like to express their gratitude to Ret. Prof. Dr. D. N. Srivasatva, Ex-Head, Department of Pharmacology and Veterinary Sciences, J. N. K. V., Jabalpur, Madhya Pradesh, India for their expert opinion on the histopathology.
Declaration of interest
The authors declare no conflicts of interest.
References
- Aslan M, Deliorman Orhan D, Orhan N, Sezik E, Yesilada E. (2007). In vivo antidiabetic and antioxidant potential of Helichrysum plicatum ssp. plicatum capitulums in streptozotocin-induced-diabetic rats. J Ethnopharmacol, 109, 54–59.
- Casey RG, Joyce M, Roche-Nagle G, Chen G, Bouchier-Hayes D. (2005). Pravastatin modulates early diabetic nephropathy in an experimental model of diabetic renal disease. J Surg Res, 123, 176–181.
- Chattopadhyay RR. (1999). Possible mechanism of antihyperglycemic effect of Azadirachta indica leaf extract: Part V. J Ethnopharmacol, 67, 373–376.
- De Boer IH, Rue TC, Cleary PA, Lachin JM, Molitch ME, Steffes MW, Sun W, Zinman B, Brunzell JD. (2011). Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: An analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Arch Intern Med, 171, 412–420.
- DeFronzo R. (1995). Diabetic nephropathy: Etiologic and therapeutic considerations. Diabetes Rev, 3, 510–564.
- Dhir A, Kulkarni SK. (2008). Venlafaxine reverses chronic fatigue-induced behavioral, biochemical and neurochemical alterations in mice. Pharmacol Biochem Behav, 89, 563–571.
- Evans TC, Capell P. (2000). Diabetic nephropathy. Clin Diabetes, 18, 1–11.
- Gao H, Huang YN, Xu PY, Kawabata J. (2007). Inhibitory effect on α-glucosidase by the fruits of Terminalia chebula Retz. Food Chem, 105, 628–634.
- Ghosh MN. (2008). Fundamentals of Experimental Pharmacology. Kolkata, India: Hilton and Company.
- Ha H, Hwang IA, Park JH, Lee HB. (2008). Role of reactive oxygen species in the pathogenesis of diabetic nephropathy. Diabetes Res Clin Pract, 82 Suppl 1, S42–S45.
- Hostetter TH, Troy JL, Brenner BM. (1981). Glomerular hemodynamics in experimental diabetes mellitus. Kidney Int, 19, 410–415.
- Jovanovic L, Peterson CM. (1981). The clinical utility of glycosylated hemoglobin. Am J Med, 70, 331–338.
- Kawanishi K, Ishida T, Kajikawa T, Tada S. (1994). [Clinical laboratory tests in diabetes mellitus]. Rinsho Byori, 42, 779–785.
- Kelly DJ, Allen TJ, Cooper ME. (2000). Experimental diabetic nephropathy: Is it relevant to human disease. Nephrology, 5, 177–185.
- Klika KD, Saleem A, Sinkkonen J, Kahkonen M, Loponen J, Tahtinen P, Pihlaja K. (2004). The structural and conformational analyses and antioxidant activities of chebulinic acid and its thrice-hydrolyzed derivative, 2,4-chebuloyl-β-d-glucopyranoside, isolated from the fruit of Terminalia chebula. Arkivoc, 7, 83–105.
- Krishnamurti U, Steffes MW. (2001). Glycohemoglobin: A primary predictor of the development or reversal of complications of diabetes mellitus. Clin Chem, 47, 1157–1165.
- Kumar GPS, Arulselvan P, Kumar DS, Subramanian SP. (2006). Antidiabetic activity of fruits of Terminalia chebula on streptozotocin induced diabetic rats. J Health Sci, 52, 283–291.
- Kumar GPS, Subramanian SP. (2008). Biochemical studies on the effect of Terminalia chebula on the levels of glycoproteins in streptozotocin induced experimental diabetes in rats. J Appl Biomed, 6, 105–115.
- Larkins RG, Dunlop ME. (1992). The link between hyperglycaemia and diabetic nephropathy. Diabetologia, 35, 499–504.
- Lee HS, Jung SH, Yun BS, Lee KW. (2007). Isolation of chebulic acid from Terminalia chebula Retz. and its antioxidant effect in isolated rat hepatocytes. Arch Toxicol, 81, 211–218.
- Lee HS, Koo YC, Suh HJ, Kim KY, Lee KW. (2010). Preventive effects of chebulic acid isolated from Terminalia chebula on advanced glycation endproduct-induced endothelial cell dysfunction. J Ethnopharmacol, 131, 567–574.
- Lee SH, Ryu SY, Choi SU, Lee CO, No Z, Kim SK, Ahn JW. (1995). Hydrolysable tannins and related compound having cytotoxic activity from the fruits of Terminalia chebula. Arch Pharmacal Res, 18, 118–120.
- Lin TC, Hsu FL, Cheng JT. (1993). Antihypertensive activity of corilagin and chebulinic acid, tannins from Lumnitzera racemosa. J Nat Prod, 56, 629–632.
- Liu HR, Tang XY, Dai DZ, Dai Y. (2008). Ethanol extracts of Rehmannia complex (Di Huang) containing no Corni fructus improve early diabetic nephropathy by combining suppression on the ET-ROS axis with modulate hypoglycemic effect in rats. J Ethnopharmacol, 118, 466–472.
- Maiti R, Jana D, Das UK, Ghosh D. (2004). Antidiabetic effect of aqueous extract of seed of Tamarindus indica in streptozotocin-induced diabetic rats. J Ethnopharmacol, 92, 85–91.
- Melin J, Hellberg O, Akyürek LM, Källskog O, Larsson E, Fellström BC. (1997). Ischemia causes rapidly progressive nephropathy in the diabetic rat. Kidney Int, 52, 985–991.
- Murali YK, Chandra R, Murthy PS. (2004). Antihyperglycemic effect of water extract of dry fruits of Terminalia chebula in experimental diabetes mellitus. Indian J Clin Biochem, 19, 202–204.
- Naik GH, Priyadarsini KI, Naik DB, Gangabhagirathi R, Mohan H. (2004). Studies on the aqueous extract of Terminalia chebula as a potent antioxidant and a probable radioprotector. Phytomedicine, 11, 530–538.
- Nangle MR, Gibson TM, Cotter MA, Cameron NE. (2006). Effects of eugenol on nerve and vascular dysfunction in streptozotocin-diabetic rats. Planta Med, 72, 494–500.
- Pari L, Latha M. (2002). Effect of Cassia auriculata flowers on blood sugar levels, serum and tissue lipids in streptozotocin diabetic rats. Singapore Med J, 43, 617–621.
- Rabkin R. (2003). Diabetic nephropathy. Clin Cornerstone, 5, 1–11.
- Randhawa MA. (2009). Calculation of LD50 values from the method of Miller and Tainter, 1944. J Ayub Med Coll Abbottabad, 21, 184–185.
- Rao NK, Nammi S. (2006). Antidiabetic and renoprotective effects of the chloroform extract of Terminalia chebula Retz. seeds in streptozotocin-induced diabetic rats. BMC Complement Altern Med, 6, 17.
- Reddi AS, Camerini-Davalos RA. (1990). Diabetic nephropathy. An update. Arch Intern Med, 150, 31–43.
- Rossing P, Hommel E, Smidt UM, Parving HH. (1994). Reduction in albuminuria predicts a beneficial effect on diminishing the progression of human diabetic nephropathy during antihypertensive treatment. Diabetologia, 37, 511–516.
- Sabu MC, Kuttan R. (2002). Anti-diabetic activity of medicinal plants and its relationship with their antioxidant property. J Ethnopharmacol, 81, 155–160.
- Saleem A, Husheem M, Härkönen P, Pihlaja K. (2002). Inhibition of cancer cell growth by crude extract and the phenolics of Terminalia chebula retz. fruit. J Ethnopharmacol, 81, 327–336.
- Sharma A, Bhardwaj S, Mann AS, Jain A, Kharya MD. (2007). Screening method of antioxidant activity: An overview. Phcog Rev, 1, 232–238.
- Sharma S, Kulkarni SK, Chopra K. (2006). Curcumin, the active principle of turmeric (Curcuma longa), ameliorates diabetic nephropathy in rats. Clin Exp Pharmacol Physiol, 33, 940–945.
- Spiro RG, Spiro MJ. (1971). Effect of diabetes on the biosynthesis of the renal glomerular basement membrane. Studies on the glucosyltransferase. Diabetes, 20, 641–648.
- Steffes MW, Brown DM, Mauer SM. (1978). Diabetic glomerulopathy following unilateral nephrectomy in the rat. Diabetes, 27, 35–41.
- Suchalatha S, Devi CS. (2005). Protective effect of Terminalia chebula against lysosomal enzyme alterations in isoproterenol-induced cardiac damage in rats. Exp Clin Cardiol, 10, 91–95.
- Tesch GH, Allen TJ. (2007). Rodent models of streptozotocin-induced diabetic nephropathy. Nephrology (Carlton), 12, 261–266.
- Wang H, Gao XD, Zhou GC, Cai L, Yao WB. (2008). In vitro and in vivo antioxidant activity of aqueous extract from Choerospondias axillaris fruit. Food Chem, 106, 888–895.
- Wongmekiat O, Thamprasert K, Lumlertgul D. (2007). Renoprotective effect of trolox against ischaemia-reperfusion injury in rats. Clin Exp Pharmacol Physiol, 34, 753–759.