Abstract
Context: The β-amyloid (Aβ) peptide aggregation with accompanying oxidative stress plays the major role in the pathogenesis of Alzheimer’s disease (AD). Some natural compounds, including borneol, shed promising light on AD treatment.
Objective: The present study was designed to investigate the antioxidative, antiapoptotic effects, and neuroprotection of borneol in human neuroblastoma cells (SH-SY5Y).
Materials and methods: Oxidative stress was induced by administering 50 µM Aβ into SH-SY5Y cells. Neuroprotective effect of commercially available borneol was examined by determining cell viability with the MTT assay. Intracellular reactive oxygen species (ROS) generation was measured using a fluorometer with further examination of heme oxygenase-1 (HO-1) and nuclear factor-erythroid 2 p45-related factor 2 (Nrf2) expression. Apoptosis was examined by measuring the ratio of B-cell lymphoma 2 (Bcl-2)/Bcl-2-associated X protein (Bax).
Results: Our data indicated that Aβ-induced cell cytotoxicity was inhibited by 100 µM of (−) and (+) borneol treatment. Treatment of borneol significantly decreased ROS generation (P < 0.01). The expression of HO-1 and nuclear translocation of Nrf2 were increased by Aβ treatment. This nuclear translocation of Nrf2 was further increased by administration of borneol. Compared with the Aβ treated group, the (+) borneol treated group significantly increased Bcl-2 expression with decreased expression of Bax.
Discussion and conclusion: Borneol protected SH-SY5Y cells against Aβ-induced toxicity, exerted an antioxidative effect and suppressed apoptosis. It increases our knowledge about neuroprotective mechanism of borneol, and it is hopeful to be a candidate compound for developing therapeutic drug for the prevention and treatment of AD and other Aβ-related neurodegenerative diseases.
Introduction
Plants contain volatile organic essential oils that include a wide array of low molecular weight terpenes in a highly concentrated form. Borneol (C10H18O) is a bicyclic monoterpene present in several medicinal plants. It is known to promote mental alertness by relieving symptoms of anxiety, fatigue and insomnia (CitationSilva-Filho et al., 2011). CitationHan et al. (2011) showed that salvianic borneol ester, synthesized by salvianic acid A and borneol, destabilized β-amyloid (Aβ) peptide aggregates and demonstrated the neuroprotective effects against hydrogen peroxide-induced toxicity in human neuroblastoma cells and motor neuron hybridoma cells. CitationSaravanakumar et al. (2012) investigated the protective effect of borneol on liver metabolism in a nitric oxide deficient rat model of hypertension and suggested that borneol had the potential to be used as a novel defense on altered hepatic metabolism. Additionally, the vasorelaxant effect of borneol was reported to be attributed to the calcium influx blockade through ion channels, calcium immobilization from intracellular stores and potassium channel activation (CitationSilva-Filho et al., 2011). Similar to other essential oils, borneol was shown to have free radical scavenging activity (CitationMkaddem et al., 2009), but this antioxidative property was not manifested when aqueous and ethanol solutions of borneol were assayed (CitationSlamenová et al., 2009), probably due to different source and different extraction procedure of borneol.
Recently, we reported that SuHeXiang Wan essential oil alleviates Aβ-induced memory impairment through inhibition of tau protein phosphorylation in mice (CitationJeon et al., 2011). Previously, we found that borneol is the most abundant component of SuHeXiang Wan essential oil (CitationKoo et al., 2004). The progressive deposition starting with monomeric, then oligomeric Aβ to final form of insoluble plaques in the brain is the central figure of neuropathology of Alzheimer’s disease (AD). AD as the major public health problem is characterized by loss of memory and cognition as well as behavioral instability in the elderly with no clinically accepted treatment to cure it or to stop its progression. Considerable evidence indicates that fibrillar aggregation of Aβ is associated with AD neurotoxicity (CitationIrie et al., 2005; CitationCerpa et al., 2008). In this regard, borneol was investigated as one of alternative agents aiming at interfering with Aβ aggregation as a potential for prevention and treatment of AD. Thus, in this study, a human neuroblastoma cell line was used to investigate the neuroprotective, antioxidative and antiapoptotic effect of borneol against the damaging effects of Aβ.
Materials and methods
Reagents
Dulbecco’s modified Eagle minimum essential medium (DMEM), fetal bovine serum (FBS) and penicillin/streptomycin were purchased from Hyclone Laboratories Inc. (Logan, UT, USA). Glutamine, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), 2,7-dichlorodihydrofluorescein diacetate (H2DCF-DA), potassium phosphate, (+) borneol (420247), (−) borneol (139114) were from Sigma-Aldrich (St. Louis, MO, USA). Aβ1–42 was purchased from Biosource International (Camarillo, CA, USA).
Cell culture
SH-SY5Y cells, human neuroblastoma cell line, were obtained from the American Type Culture Collection (Rockville, MD, USA). Cells were maintained in DMEM supplemented with 10% FBS and 100 unit/mL penicillin, 100 µg/mL streptomycin in condition of 95% air and 5% CO2 at 37°C.
Cell viability
Cell viability was determined by the MTT assay according to manufacturer’s method. Briefly, cells were plated into 96-well plates. After cells were treated as described in the figure legend, 100 µL of 5 mg/mL MTT solution was added to each well of the 96-well plates and incubated for 4 h at 37°C. The medium containing MTT was then discarded and 200 µL of dimethyl sulfoxide (DMSO) was added to each well. The absorbance was read with a Versa max ELISA reader (Molecular Devices, Sunnyvale, CA, USA) at a wavelength of 570 nm.
Reactive oxygen species measurement
Intracellular reactive oxygen species (ROS) generation was measured using a fluorometer. The ROS-sensitive dye, H2DCF-DA, was passively entered, converted to dichlorofluorescin diacetate (DCFH), reacted with ROS and formed the fluorescent product, dichlorofluorescin (DCF). The treated cells were incubated with 30 µM H2DCF-DA for 30 min. The fluorescence intensity was measured at 480 nm excitation and 530 nm emission using a fluorescence microplate reader (SpectraMax Gemini EM, Molecular Device; Sunnyvale, CA, USA).
Western blotting analysis
The nucleic components were isolated using a nucleic/cytosolic fractionation kit (BioVision, Mountain View, CA, USA) according to the manufacturer’s instructions. The protein content of each cellular extract was quantified by the Bradford assay. An equal amount of protein from each sample was separated by SDS-poly-acrylamide gel electrophoresis (10% gel) and transferred to a Hybond ECL nitrocellulose membrane. The membranes were blocked with 5% skim milk and incubated with anti-Bax or anti-Bcl-2 antibody (Santa-Cruz Scientific, USA) and HRP-conjugated secondary antibody (Assay Design, USA) followed by enhanced chemiluminescence detection (Thermo scientific, USA). The nuclear fraction was incubated with rabbit anti-Nrf2 (Abcam, Cambridge, UK), rabbit anti-heme oxygenase-1 (anti-HO-1) antibody (Abcam, Cambridge, UK), rabbit anti-Lamin B antibody (Santa-Cruz Scientific, USA) or mouse anti-β actin antibody (Santa-Cruz Scientific, USA) for normalization of loading control.
Data analysis
All statistical analyses were conducted with SPSS (ver. 19, Somers, NY, USA). Values were expressed as mean ± SEM. All data were analysed using Student’s t-test and one way ANOVA. Statistical significance was accepted at a P value less than 0.05.
Results
Neuroprotective activity
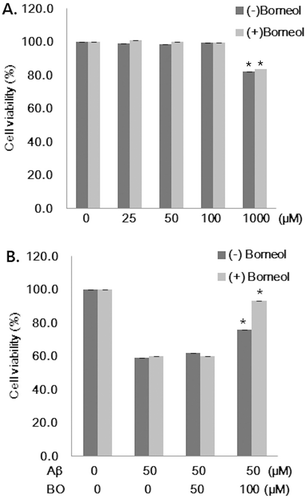
shows the results of cytotoxicity testing of borneol in SH-SY5Y cells by means of the MTT assay. Borneol had no cytotoxic effect on cells up to 100 µM, but 1000 µM of (+) and (−) borneol-induced cell death by 18% (). Thus, to examine the neuroprotective effect of borneol, 50 and 100 µM of borneol were pretreated in SH-SY5Y cells before adding 50 µM of Aβ. Aβ had cytotoxicity showing 60% cell viability compared with control (). Pretreatment of 50 µM borneol to the medium containing Aβ did not inhibit the Aβ-induced cytotoxic cell death. However, 100 µM of (−) and (+) borneol significantly increased cell viability with 76.0 ± 3.09% and 93.3 ± 2.72%, respectively, compared to that of Aβ treated cells (). These data indicated that Aβ-induced cell cytotoxicity was inhibited by 100 µM of (−) and (+) borneol treatment in human neuroblastoma cells.
Figure 1. Effect of borneol on Aβ1–42 induced cytotoxicity in SH-SY5Y cells. (A) Cells were treated with various concentrations of borneol (25, 50, 100 and 1000 µM) without Aβ for 24 h. Cell viability was then determined by MTT assay. (B) Cells were pretreated with 50 and 100 µM of borneol for 30 min and then exposed to the medium containing 50 µM Aβ1–42 for 24 h. Cell viability was then determined by MTT assay. Values are presented as mean ± SEM of six determinations. *P < 0.05 vs. Aβ only, BO; borneol.
Antioxidative activity
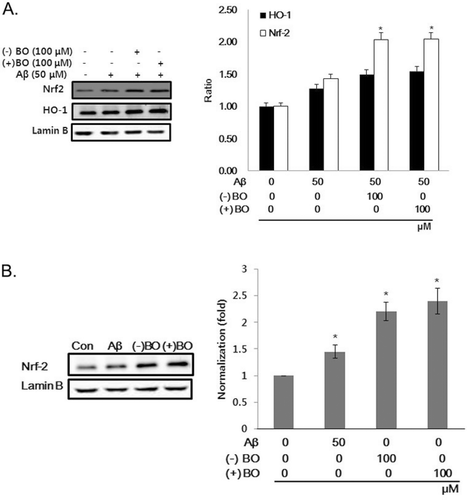
Since Aβ-induced oxidative stress is related to neuron damage, the antioxidant effect of borneol was examined by measuring ROS production in the cells. As shown in , Aβ treatment significantly increased the level of ROS reactive fluorescent dye by ten times compared to that of control group in SH-SY5Y cells. However, treatment of 100 µM of (−) and (+) borneol significantly decreased Aβ-induced ROS generation (P < 0.01). The involvement of ROS in Aβ-induced oxidative damage was further assessed by examining the expression of HO-1 and nuclear factor-erythroid 2 p45-related factor 2 (Nrf2) in the nuclear fraction. Moreover, the expression of lamin B, as a loading control nuclear protein, was examined. Because HO-1, which is regulated by Nrf2, is induced as a defense against oxidative stress, we assessed whether borneol could regulate expression of HO-1 as well as nuclear translocation of Nrf2. The expression levels of HO-1 and Nrf2 in the nuclear fraction were increased by Aβ treatment compared to that of control. This upregulation of Nrf2 was further increased by administration of Aβ plus borneol in the neuroblastoma cells (). Moreover, only borneol treatment also induced Nrf2 translocation into nucleus (). Thus, it is possible that borneol actually acts as a cell irritant to protect cell. These data imply that borneol may exert an antioxidative effect through the nuclear translocation of Nrf2.
Figure 2. Inhibitory effect of borneol on Aβ1–42 induced ROS overproduction in SH-SY5Y cells. The cells were treated with borneol for 30 min before 50 µM Aβ1–42 stimulation for 1 h. ROS generation was measured by the fluorescence intensity of DCF. RFU (relative fluorescence units). Values are indicated as mean ± SEM. **P < 0.01 vs. Aβ only.
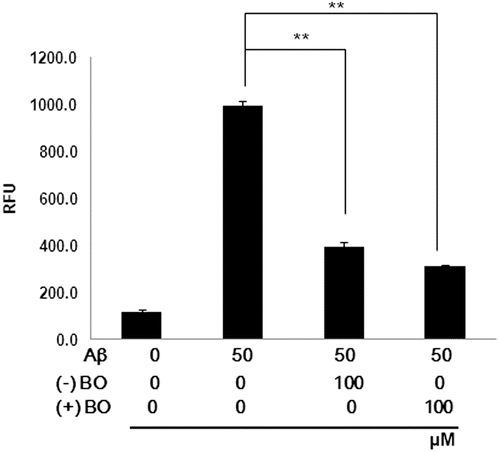
Figure 3. Effect of borneol on HO-1 and Nrf2 expression in Aβ1–42 treated SH-SY5Y cells. (A) The cells were treated with 100 µM borneol for 30 min before 50 µM of Aβ1–42 treatment for 16 h and then measured for HO-1 and Nrf2 translocation by immunoblotting. (B) The cells were treated with 100 µM of (−), (+) borneol or 50 µM of Aβ1–42 for 16 h and then measured for Nrf2 translocation by immunoblotting. The intensity of the protein bands was quantitated by densitometry and normalized versus lamin B. The data are expressed as mean ± SEM of three experiments. *P < 0.05 vs. Aβ only.
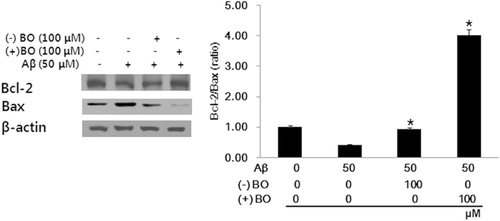
Antiapoptotic activity
Bcl-2 inhibits apoptosis by preventing mitochondrial membrane depolarization, whereas Bax promotes apoptosis by inducing mitochondrial membrane depolarization and cytochrome c release. Therefore, to investigate the mechanisms of borneol on the antiapoptotic effect, we examined the expression level of Bcl-2 and Bax in SH-SY5Y cells. Borneol treatment significantly increased Bcl-2 expression with decreased expression of Bax compared with that of Aβ treated group (). This suggests that borneol suppressed the Aβ-induced apoptosis of SH-SY5Y cells by upregulating Bcl-2 expression.
Discussion
Oxidative stress is a hallmark in the pathogenesis of AD. In humans, the antioxidant response elements (AREs) regulate the expression of a number of cytoprotective antioxidant enzymes and free radical scavengers which contribute to the endogenous defense against oxidative insults (CitationLiu et al., 2007). Genes regulated by AREs include HO-1 which belongs to a family of cytoprotective and detoxification genes. The AREs are cis-acting elements located in regulatory regions of antioxidant and phase II detoxification genes, and are activated by ROS with unknown molecular mechanism (CitationNguyen et al., 2003). The main transcription factor involved in the induction of phase II genes is Nrf2. Nrf2 deficient mice exhibit lower expression of cytoprotective genes (CitationAleksunes et al., 2008; CitationReisman et al., 2009) and enhanced susceptibility to carcinogenesis (CitationRamos-Gomez et al., 2001). The present study shows that monoterpene borneol demonstrated the antioxidative effect against Aβ-induced neurotoxicity in human neuroblastoma cells. Furthermore, this antioxidative effect of borneol was found to be through nuclear translocation of Nrf2 in the current study. It is in accordance with the previous in vitro study (CitationCao et al., 2005) which confirms that increasing Nrf2 activity may provide a useful way to combat oxidative damage. In fact, some of phytochemicals and edible plant extracts that activate Nrf2 were proven as potent inducers of cytoprotective genes. For example, the kavalactones methysticin, yangonin, and kavain were able to protect neurons against Aβ toxicity in vitro by activating Nrf2 and mediating an upregulation of a battery of genes encoding detoxifying and antioxidative enzymes (CitationWruck et al., 2008). The carnosol derived from rosemary (CitationMartin et al., 2004), a phlorotannin eckol found in Ecklonia cava (CitationKim et al., 2010), a red pigment brazilin obtained from the wood of the brazilwood family (CitationChoi & Kim, 2008) and the phytoestrogen puerarin as the main isoflavone glycoside found in the root of Pueraria lobata (CitationHwang & Jeong, 2008) activated Nrf2-mediated HO-1 induction in various kinds of cells via extracellular regulated kinase (Erk) and phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB, Akt) signaling. Erk and PI3K/Akt contribute to ARE-driven HO-1 expression, and Nrf2 is a critical regulator of HO-1. The activation of Nrf2 starts with dissociation of Nrf2:INrf2 (inhibitor of Nrf2) complex at the level of cytoplasm. Then stabilized Nrf2 translocates to the nucleus and induces its downstream genes encodes cytoprotective proteins that neutralize reactive molecules.
In addition, borneol reduced Aβ-induced apoptotic cell death in SH-SY5Y cells. Apoptosis or programmed cell death is an extremely fine-tuned regulatory network consisting of pro- and antiapoptotic factors (CitationChen et al., 2011). Bcl-2 family proteins are critical regulators of apoptosis which consists of three subfamilies of Bcl-2, Bax and Bik. While Bcl-2 is one of antiapoptotic members of the family, Bax is a proapoptotic member. The ratio between antiapoptotic and proapoptotic members of the Bcl-2 family may determine the susceptibility of a cell to apoptosis (CitationNiture & Jaiswal, 2011). Our results clearly demonstrated that Aβ had a significant apoptotic effect in SH-SY5Y cells. However, the inhibitory effect of borneol against apoptosis has been well proven by the increase of Bcl-2/Bax expression. These findings are in accordance with previous publication showing that altered Bcl-2/Bax ratio induced the release of cytochrome c for the activation of terminal cascade of apoptosis by inducing mitochondrial membrane depolarization (CitationSalvucci et al., 2001). Interestingly enough, INrf2 mediates degradation of endogenous antiapoptotic Bcl-2 protein leading to decreased ratio of Bcl-2/Bax with subsequent enhanced apoptosis in lung cancer cells (CitationNiture & Jaiswal, 2011). Furthermore, apoptosis in colon cancer cells was demonstrated by enhanced hypodiploid DNA content, increased level of Bax, decreased level of Bcl-2 and increased release of cytochrome c (CitationBat-Chen et al., 2010).
Of the many structural types of Aβ aggregates, it is generally believed that soluble oligomeric or protofibrillar Aβ is the most neurotoxic. However, it is aggregation from each individual Aβ species that induces toxicity in AD (CitationHärd, 2011). Therefore, it is important to control this nucleated polymerization since it is the rate of aggregation that determines toxicity and neuronal apoptosis in AD. A synthetic compound based on medicinal plant which contains borneol destabilized Aβ oligomers (CitationHan et al., 2011). Thus, natural herbs using the root of Salvia miltiorhiza Bunge (Lamiaceae) (CitationRen et al., 2004), the whole plant of Ginkgo biloba L. (Ginkgoaceae) (CitationDas et al., 2002) or extracted herbal compounds (CitationMukherjee et al., 2007; CitationLin et al., 2008; CitationGuo et al., 2010) can provide the rationale for the pharmacotherapy of AD.
Collectively, our findings demonstrated that borneol has a significant neuroprotective effect on SH-SY5Y cells insulted by Aβ via nuclear translocation of Nrf2 and upregulation of Bcl-2. This indicates that borneol has potential as a therapeutic agent for Aβ accumulation.
Conclusion
In this study, we showed borneol protected SH-SY5Y cells against Aβ-induced toxicity, exerted an antioxidative effect and suppressed apoptosis. Taken together, the data presented here increases our knowledge about the neuroprotective mechanism of borneol, which may be a candidate compound for developing therapeutic drug for the prevention and treatment of AD and other Aβ-related neurodegenerative diseases.
Declaration of interest
This study was supported by a grant of the Oriental Medicine R&D Project, Ministry for Health & Welfare & Family Affairs, Republic of Korea (B090068).
References
- Aleksunes LM, Slitt AL, Maher JM, Augustine LM, Goedken MJ, Chan JY, Cherrington NJ, Klaassen CD, Manautou JE. (2008). Induction of Mrp3 and Mrp4 transporters during acetaminophen hepatotoxicity is dependent on Nrf2. Toxicol Appl Pharmacol, 226, 74–83.
- Bat-Chen W, Golan T, Peri I, Ludmer Z, Schwartz B. (2010). Allicin purified from fresh garlic cloves induces apoptosis in colon cancer cells via Nrf2. Nutr Cancer, 62, 947–957.
- Cao TT, Ma L, Kandpal G, Warren L, Hess JF, Seabrook GR. (2005). Increased nuclear factor-erythroid 2 p45-related factor 2 activity protects SH-SY5Y cells against oxidative damage. J Neurochem, 95, 406–417.
- Cerpa W, Dinamarca MC, Inestrosa NC. (2008). Structure-function implications in Alzheimer’s disease: Effect of Aβ oligomers at central synapses. Curr Alzheimer Res, 5, 233–243.
- Chen Z, Niu X, Li Z, Yu Y, Ye X, Lu S, Chen Z. (2011). Effect of ubiquitin carboxy-terminal hydrolase 37 on apoptotic in A549 cells. Cell Biochem Funct, 29, 142–148.
- Choi BM, Kim BR. (2008). Upregulation of heme oxygenase-1 by brazilin via the phosphatidylinositol 3-kinase/Akt and ERK pathways and its protective effect against oxidative injury. Eur J Pharmacol, 580, 12–18.
- Das A, Shanker G, Nath C, Pal R, Singh S, Singh H. (2002). A comparative study in rodents of standardized extracts of Bacopa monniera and Ginkgo biloba: Anticholinesterase and cognitive enhancing activities. Pharmacol Biochem Behav, 73, 893–900.
- Guo AJ, Xie HQ, Choi RC, Zheng KY, Bi CW, Xu SL, Dong TT, Tsim KW. (2010). Galangin, a flavonol derived from Rhizoma Alpiniae Officinarum, inhibits acetylcholinesterase activity in vitro. Chem Biol Interact, 187, 246–248.
- Han M, Liu Y, Zhang B, Qiao J, Lu W, Zhu Y, Wang Y, Zhao C. (2011). Salvianic borneol ester reduces β-amyloid oligomers and prevents cytotoxicity. Pharm Biol, 49, 1008–1013.
- Härd T. (2011). Protein engineering to stabilize soluble amyloid β-protein aggregates for structural and functional studies. FEBS J, 278, 3884–3892.
- Hwang YP, Jeong HG. (2008). Mechanism of phytoestrogen puerarin-mediated cytoprotection following oxidative injury: Estrogen receptor-dependent up-regulation of PI3K/Akt and HO-1. Toxicol Appl Pharmacol, 233, 371–381.
- Irie K, Murakami K, Masuda Y, Morimoto A, Ohigashi H, Ohashi R, Takegoshi K, Nagao M, Shimizu T, Shirasawa T. (2005). Structure of β-amyloid fibrils and its relevance to their neurotoxicity: Implications for the pathogenesis of Alzheimer’s disease. J Biosci Bioeng, 99, 437–447.
- Jeon S, Hur J, Jeong HJ, Koo BS, Pak SC. (2011). SuHeXiang Wan essential oil alleviates amyloid β induced memory impairment through inhibition of tau protein phosphorylation in mice. Am J Chin Med, 39, 917–932.
- Kim KC, Kang KA, Zhang R, Piao MJ, Kim GY, Kang MY, Lee SJ, Lee NH, Surh YJ, Hyun JW. (2010). Up-regulation of Nrf2-mediated heme oxygenase-1 expression by eckol, a phlorotannin compound, through activation of Erk and PI3K/Akt. Int J Biochem Cell Biol, 42, 297–305.
- Koo BS, Lee SI, Ha JH, Lee DU. (2004). Inhibitory effects of the essential oil from SuHeXiang Wan on the central nervous system after inhalation. Biol Pharm Bull, 27, 515–519.
- Lin HQ, Ho MT, Lau LS, Wong KK, Shaw PC, Wan DC. (2008). Anti-acetylcholinesterase activities of traditional Chinese medicine for treating Alzheimer’s disease. Chem Biol Interact, 175, 352–354.
- Liu Y, Kern JT, Walker JR, Johnson JA, Schultz PG, Luesch H. (2007). A genomic screen for activators of the antioxidant response element. Proc Natl Acad Sci USA, 104, 5205–5210.
- Martin D, Rojo AI, Salinas M, Diaz R, Gallardo G, Alam J, De Galarreta CM, Cuadrado A. (2004). Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J Biol Chem, 279, 8919–8929.
- Mkaddem M, Bouajila J, Ennajar M, Lebrihi A, Mathieu F, Romdhane M. (2009). Chemical composition and antimicrobial and antioxidant activities of Mentha (longifolia L. and viridis) essential oils. J Food Sci, 74, M358–M363.
- Mukherjee PK, Kumar V, Houghton PJ. (2007). Screening of Indian medicinal plants for acetylcholinesterase inhibitory activity. Phytother Res, 21, 1142–1145.
- Niture SK, Jaiswal AK. (2011). INrf2 (Keap1) targets Bcl-2 degradation and controls cellular apoptosis. Cell Death Differ, 18, 439–451.
- Nguyen T, Sherratt PJ, Pickett CB. (2003). Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol, 43, 233–260.
- Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW. (2001). Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in Nrf2 transcription factor-deficient mice. Proc Natl Acad Sci USA, 98, 3410–3415.
- Reisman SA, Buckley DB, Tanaka Y, Klaassen CD. (2009). CDDO-Im protects from acetaminophen hepatotoxicity through induction of Nrf2-dependent genes. Toxicol Appl Pharmacol, 236, 109–114.
- Ren Y, Houghton PJ, Hider RC, Howes MJ. (2004). Novel diterpenoid acetylcholinesterase inhibitors from Salvia miltiorhiza. Planta Med, 70, 201–204.
- Salvucci O, Carsana M, Bersani I, Tragni G, Anichini A. (2001). Antiapoptotic role of endogenous nitric oxide in human melanoma cells. Cancer Res, 61, 318–326.
- Saravanakumar M, Manivannan J, Sivasubramanian J, Silambarasan T, Balamurugan E, Raja B. (2012). Molecular metabolic fingerprinting approach to investigate the effects of borneol on metabolic alterations in the liver of nitric oxide deficient hypertensive rats. Mol Cell Biochem, 362, 203–209.
- Silva-Filho JC, Oliveira NN, Arcanjo DD, Quintans-Júnior LJ, Cavalcanti SC, Santos MR, Oliveira RD, Oliveira AP. (2011). Investigation of mechanisms involved in (−)-borneol-induced vasorelaxant response on rat thoracic aorta. Basic Clin Pharmacol Toxicol, 110, 171–177.
- Slamenová D, Horváthová E, Wsólová L, Sramková M, Navarová J. (2009). Investigation of anti-oxidative, cytotoxic, DNA-damaging and DNA-protective effects of plant volatiles eugenol and borneol in human-derived HepG2, Caco-2 and VH10 cell lines. Mutat Res, 677, 46–52.
- Wruck CJ, Götz ME, Herdegen T, Varoga D, Brandenburg LO, Pufe T. (2008). Kavalactones protect neural cells against amyloid β peptide-induced neurotoxicity via extracellular signal-regulated kinase ½-dependent nuclear factor erythroid 2-related factor 2 activation. Mol Pharmacol, 73, 1785–1795.