Abstract
Context: In the present investigation, acute and subacute toxicity of the biogenic Se nanoparticles (Se NPs) has been reported.
Objective: To characterize the Se NPs produced by a bacterium species and to evaluate their toxicity and impact on clinical chemistry and hematological parameters of NMRI mice.
Materials and methods: The Se NPs were prepared by Bacillus sp. MSh-1 in a culture medium containing SeO2 (1.26 mM) and their physiochemical properties investigated using TEM, XRD and FT-IR. The LD50 of Se NPs and SeO2 were determined and the subacute toxicity evaluated by orally administration of 0, 2.5, 5, 10 and 20 mg kg−1 of Se NPs to male mice for 14 consecutive days. Parameters of blood cells, AST, ALT, ALP, creatinine, BUN, cholesterol, bilirubin, triglyceride and CPK were experimentally measured.
Results: The XRD and TEM analyses showed that the spherical NPs were amorphous, in the size range of 80–220 nm. The toxicological evaluation showed that the LD50 values of SeO2 and Se NPs were 7.3 and 198.1 mg kg−1, respectively. No biochemical changes were observed from the administration of 2.5, 5 and 10 mg kg−1 of Se NPs, but a dose of 20 mg kg−1 was accompanied with signs of toxicity including lower body weight and changes in clinical chemistry and hematological parameters.
Conclusion: The biogenic Se NPs were less toxic than synthetic Se NPs and much less (26-fold) toxic than the SeO2, which demonstrates the important role of Bacillus sp. MSh-1 in conversion of a highly toxic Se compound to the less toxic Se NPs.
Introduction
An important feature of nanotechnology concerns the development of experimental procedure for the arrangement of a nanoscale materials with special properties (CitationMurray et al., 2000). Although different procedures for assembly of nanomaterials have been extended, in recent years production of nanoparticles (NPs) by bio-mimetic methods, as novel techniques, has been an attractive subject with increasing attention (CitationMandal et al., 2006). Furthermore, growing demands to expand green chemistry for nanomaterial synthesis has resulted in researchers focusing on biosynthetic methods (CitationMohanpuria et al., 2008). Among these methods, green synthesis of NPs by biological organisms such as bacteria can participate with other methods attributable to its clean, nontoxic and eco-friendly aspects (CitationBhattacharya & Gupta, 2005). Some micro-organisms have good potential to reduce different metal and metalloid ions to nanoparticles and the ability of various bacterias to reduce selenium ions have been previously reported (CitationKessi et al., 1999; CitationYee et al., 2007; CitationJafari-Fesharaki et al., 2010). However, limited research has been reported on the isolation, characterization and employment of the biogenic Se NPs for various applications. On the other hand, nanotoxicology is rising as an important part of nanotechnology and the majority of studies in this field have focused on health effects of exposure to nanoparticles (CitationFischer & Chan, 2007; CitationAillon et al., 2009).
Selenium is a micronutrient metalloid element with broad functions in biologic systems, including antioxidant effects, immune modulation, cancer prevention and antiviral activities (CitationBeck et al., 2003; CitationHoffmann & Berry, 2008; Zeng & Combs, 2008). There is a very thin border between the lowest acceptable levels of intake and toxicity (CitationSchrauzer & Surai, 2009). Selenium toxicity is related to chemical forms in which exposure to selenious acid or selenium oxide has caused serious toxicity (CitationKöppel et al., 1986). Oxidation states of selenium including selenate (Se+6), selenite (Se+4), selenide (Se−2) and elemental selenium (Se0) are commonly found in the environment. Generally, Se0 is prepared on a nanoscale by reduction of higher oxidation states to other allotropic forms (CitationYang et al., 2008; CitationRaevskaya et al., 2008).
Elemental selenium at the redox state of zero is insoluble in aqueous media and generally considered to be biologically inert (CitationZhang et al., 2005). In addition, based on several in vivo and in vitro studies, the lower toxicity of synthetic elemental selenium in comparison to other oxidative states has previously been established (CitationZhang et al., 2001, 2007). Some animal studies have indicated that liver is the main target organ of selenium toxicity (CitationDiskin et al., 1979). Damage to the structural integrity of the liver is assessed by elevated serum levels of enzymes such as ALT, AST and ALP. Nevertheless, there is no report on the acute lethal dose of the biogenic Se NPs as novel nanoparticles derived from bacteria.
In this investigation, we prepared biogenic Se NPs by employing the Bacillus sp. MSh-1 as a potent bacterium in selenium reduction. In the next step, cells containing intracellular Se NPs were disrupted by the liquid nitrogen method. Subsequently, to separate and purify Se NPs from the whole-cell lysate, we used a liquid-liquid two phase partitioning method. After characterization of the purified Se NPs, the median lethal dose of the Se NPs were determined in mice and compared with selenium dioxide. Finally, the subacute toxicity of Se NPs was tested by measuring serum biochemical parameters.
Materials and methods
Materials
Selenium dioxide (SeO2), nutrient broth, nutrient agar, n-octyl alcohol, sodium dodecyl sulfate (SDS) and Tris base were purchased from Merck Chemicals (Germany). All other chemicals and solvents were of analytical grade. The micro-organism used in this study was identified as Bacillus sp. MSh-1 by the methods described earlier (CitationShakibaie et al., 2010). The organism was continuously conserved on nutrient agar plates complemented with the 1.26 mM SeO2 using continuous sub-culturing every 14 days.
Biosynthesis of Se NPs
Synthesis of Se NPs was carried out by Bacillus sp. MSh-1 using a recently described method (CitationShakibaie et al., 2010). Nutrient broth was supplemented with Se4+ ions (100 mg L−1; which is equal to 1.26 mM SeO2) and sterilized by a filtration process using a Millipore filter apparatus (0.22 µm). The medium was inoculated (1% v/v) with the fresh inoculums (OD600, 0.1) and for aerobic cultivation; the flasks were plugged with cotton and incubated at 30°C in a shaker incubator (150 rpm). In a similar trial, inoculated media without Se4+ ions were used as controls. After 14 h, the bacterial cells and Se NPs were removed from the culture medium by centrifugation at 4000g for 10 min. Red is the color of precipitated Se0 NPs, thus serving as a provisional marker in which micro-organism reduces the Se4+ ions to Se NPs (CitationOremland et al., 2004).
Isolation and recovery of the Se NPs
The resulting pellets were washed with 0.9% NaCl solution, centrifuged and transferred to a mortar. By adding liquid nitrogen, the pellets were frozen and disrupted with a pestle. The resulting slurry was ultrasonicated at 100 W for 5 min and washed three times by sequential centrifugation (10000g, 5 min) using Tris-HCl buffer (1.5 M, pH 8.3) containing 1% sodium dodecyl sulfate (SDS) and deionized water, respectively. The pellets were suspended in deionized water, and the resulting suspension containing Se NPs and cell debris was collected. The suspension (4 mL) was then transferred to test tubes, and 2 mL of n-octyl alcohol was added to each tube. The assortments were shaken vigorously, the mixed phases were separated by centrifugation at 2000g for 5 min and were stored at 4°C for 24 h. Following the time period, the generated Se NPs can be observed at the bottom of the tubes and the cell debris remained between two phases. The lower and upper phases were discarded, and settled NPs were washed with chloroform, ethyl alcohol and distilled water, respectively (CitationShakibaie et al., 2010). For further characterizations and biological experiments, the cleaned NPs were then resuspended in deionized water and stored at 4°C.
Characterization of the Se NPs
The properties of Se NPs were further confirmed using a number of instrumental techniques. For transmission electron microscopy, an aqueous suspension containing the Se NPs was dispersed ultrasonically, and a drop of the suspension was placed on carbon-coated copper TEM (transmission electron microscope) grids and dried under an IR lamp. Micrographs were obtained using a TEM (Zeiss 902A) operated at an accelerating voltage of 80 kV. Related size distribution pattern of Se NPs was plotted by manual counting of 200 individual particles from different TEM images. The crystalline structure of the Se NPs was checked by the XRD (X-ray diffraction) technique using an X-ray diffractometer (Philips PW1710) with CuKα radiation (λ = 1.5405 Å) over a scanning range of Bragg angles from 20° to 80°. The surface chemistry of oven-dried Se NPs (37°C, 48 h) extracted with the two solvent phase system has been also investigated by Fourier transform infrared (FT-IR) with a infrared spectrophotometer (Shimadzu IR-470, Japan) at a resolution of 4 cm−1 in KBr pellet.
Animals and treatments
Male NMRI mice (body weight 22 ± 2 g) were used in this study. The mice were purchased from the Pasteur Institute of Iran (Tehran, Iran) and were housed in plastic cages (four to six each) in a colony room with controlled temperature (22 ± 1°C) and humidity (50 ± 10%) with a 12/12 h light/dark cycle. The mice had free access to tap water and food. The experimental procedures carried out in this study were in compliance with the guidelines of the Tehran University of Medical Science (Tehran, Iran) for the care and use of laboratory animals.
Acute toxicity
Ninety six mice were randomly divided into 12 groups with 8 mice per group. A bolus of selenium dioxide and Se NPs dispersed in 0.9% apyrogenic sterile NaCl was administered orally by gavage at the doses indicated in . In a similar trial, the control group received 0.9% apyrogenic sterile NaCl. The animals were observed for viability and clinical signs of toxicity on the day of dosing and then daily for 14 days. Cumulative mortality within 24 h after the treatment was used for the calculation of median lethal dose (LD50).
Table 1. Acute lethal effect of selenium dioxide and biogenic Se NPs in mice.
Evaluation of subacute toxicity
Fifty mice were randomly divided into five groups with 10 mice per group. The first group (control) was administrated 0.9% saline orally (PO), and the second to fifth groups were orally administrated Se NPs at the doses of 2.5, 5, 10 and 20 mg kg−1, respectively, for 14 consecutive days.
Determination of clinical chemistry and hematological parameters
Following the experimental period, animals were fasted overnight and anaesthetized by Ketamine-Xylizine, then the abdomen was opened, and blood samples were collected from the heart. For hematological studies, total blood was collected into tubes containing ethylenediamine tetraacetic acid (EDTA) anticoagulant, and biochemical parameters, including hemoglobin, hematocrit, white blood cell counts, red blood cell counts, and platelet counts were measured. To measure clinical chemistry parameters in serum, blood was collected into tubes containing no anticoagulant, allowed to clot, and serum was separated by centrifugation at 2000g for 20 min. The assays of aspartate amino transferase (AST), alanine amino transferase (ALT), alkaline phosphatase (ALP), creatinine, BUN, cholesterol, bilirubin (direct and total), triglyceride and creatine phosphokinase (CPK) were performed using Ellitech diagnostic kits (Sees, France).
Statistical analysis
The results were presented as mean ± SE. The difference between groups was evaluated by ANOVA which followed by Tukey test and p values less than 0.05 were considered significant.
Results
Green synthesis
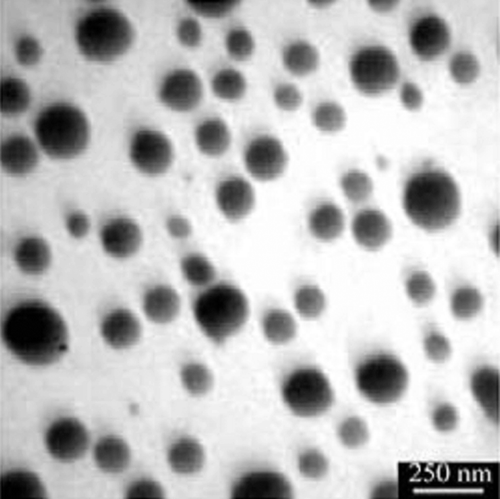
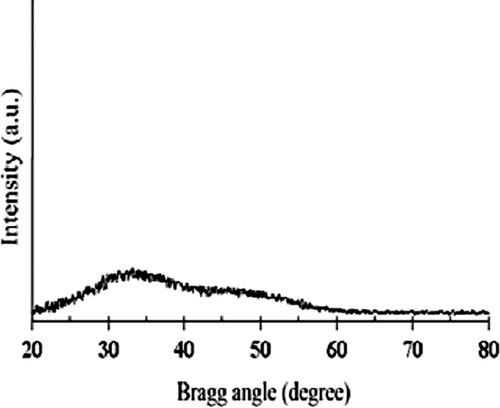
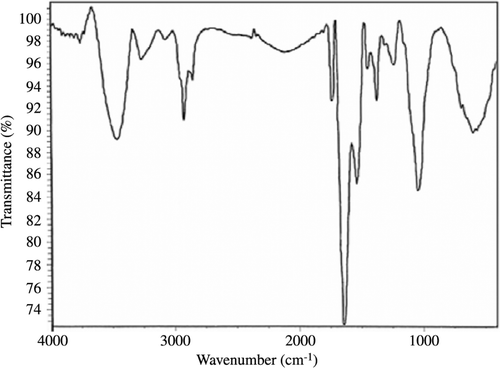
Bacillus sp. MSh-1 culture exhibited a gradual change in color toward red when it was incubated with SeO2. As a result of the Se+4 ions reduction to Se0 nanoparticles, the color of inoculated nutrient broth media was changed to intense red after 14 h of incubation at 30°C. Control (inoculated media without Se+4 ions) did not exhibit any color change of the culture media. According to , which shows the TEM image, Se NPs have a spherical shape. The TEM micrograph clearly illustrates individual Se NPs with a small amount of aggregation. Size distribution measured from manual counting of 200 individual particles from different TEM images showed that the NPs with the size of 105 to 130 nm had the most frequency. shows the X-ray diffraction profile of Se NPs synthesized using Bacillus sp. MSh-1. The XRD pattern of Se NPs showed the presence of broad peaks without any clear lattice parameters. FTIR spectrum of Se NPs showed main peaks at 3462 and 1663 cm−1. A strong peak at 3462 cm−1 could correspond to O-H or N-H and this indicated an alcohol or N-H containing amide, while the strong peak at 1663 cm−1 could be related to a carbonyl group (). These results might be attributable to the connection of proteins on the surface of nanoparticles during the formation of the Se NPs.
Acute lethal dose (LD50)
shows the result of acute Se NPs and SeO2 toxicity in mice. SeO2 caused complete mortality at the dose of 24 mg kg−1. However, biogenic Se NPs showed no acute toxicity at this dose. According to the data presented in , the LD50 of SeO2 and Se NPs were 7.35 mg kg−1 (with 95% confidence limits of 4.39–10.38 mg kg−1) and 194.6 mg kg−1 (with 95% confidence limits of 152.4–242.29 mg kg−1), respectively.
Clinical chemistry and hematology parameters determination
Based on the results for acute lethal dose (LD50), doses of 2.5, 5, 10 and 20 mg kg−1 of Se NPs were chosen. No death was observed in doses of 2.5, 5 and 10 mg kg−1, while 20% death was observed in the group receiving 20 mg kg−1 of Se NPs. The results of the clinical chemistry and hematological parameters following Se NPs administration (14 days) are presented in and . No change was found attributed to the administration of Se NPs at the employed doses of 2.5, 5 and 10 mg kg−1 over the control.
Table 2. Clinical chemistry parameters in serum.
Table 3. Hematology parameters in total blood.
Body weight
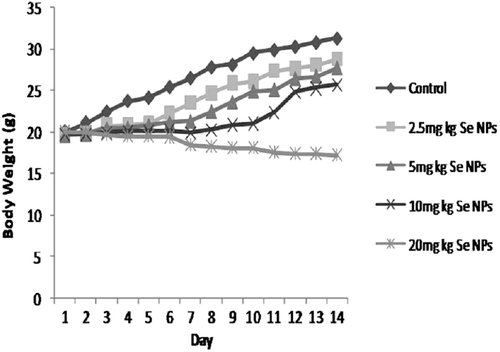
Mean body weights were shown in . The body weight of male mice receiving Se NPs at the dose of 20 mg kg−1 were significantly decreased (p < 0.05), but in other groups, similar to control, body weights increased daily.
Discussion
In this study, Se NPs were prepared by a previously isolated bacterium (Bacillus sp. MSh-1) (CitationShakibaie et al., 2010), purified and their properties were confirmed using a number of instrumental techniques. The purified Se NPs exhibited a relatively wide size range between 80 and 220 nm. Also, biogenic Se NPs prepared by Bacillus sp. MSh-1 did not show any clear lattice parameters in XRD pattern, thus classified as amorphous materials (). FTIR measurements of Se NPs were applied to identify the functional group existing on the surface of nanoparticles. In biological systems, such as Bacillus sp. MSh-1, while the Se NPs are grown under the reductive reactions, some compounds might be entrapped within the layers and their functional groups were exposed in the surface of the nanoparticles (). As a novel idea in further studies, each of the mentioned functional groups existing on the surface of the nanoparticles could be used for the formation of covalent binding between the various active compounds and biogenic nanoparticles. While the lower toxicity of the synthetic Se NPs has been previously reported, in comparison to other selenium compounds such as selenomethionine (CitationZhang et al., 2008), to the best of our knowledge and according to a survey of the literature, no report is available on the acute lethal effect of biologic Se NPs. Comparing the measured LD50 for SeO2 and biogenic Se NPs revealed that the Bacillus sp. MSh-1 converts the soluble SeO2 to insoluble Se particles at nano size which are approximately 26-fold less toxic in mice (LD50 198.1 and 7.35 mg kg−1, respectively). Zhang et al. reported that their synthetic Se NPs have a 7-fold lower acute toxicity than sodium selenite in Kunming mice (LD50 113 and 15 mg kg−1, respectively) (CitationZhang et al., 2001). Furthermore, Wang et al. reported that their Se NPs have a 3.5-fold lower acute toxicity than selenomethionine in Kunming mice (LD50 92.1 and 25.6 mg kg−1, respectively) (CitationWang et al., 2007). It seems that the chemical form, oxidative state and solubility of the selenium compounds play an important role in the acute toxicity of selenium.
Growth retardation is a main characteristic in selenium-intoxicated animals in which the American National Research Council concluded that inhibition of growth might be the best indicator for the toxic effects of selenium (CitationThorlacius-Ussing, 1990; CitationOrskov & Flyvbjerg, 2000). In the present study, Se NPs at the dose of 20 mg kg−1 significantly reduced body weight of mice, while body weight of the animals receiving Se NPs at the doses of 2.5, 5 and 10 mg kg−1 were increased similar to the controls (). Se causes growth retardation by reducing growth hormone and somatomedin C production (CitationKöppel et al., 1986), insulin-like growth factor-binding proteins and insulin-like growth factor-I (CitationGrønbaek et al., 1995).
Liver enzyme activities such as ALT, AST and ALP are the major characteristics of liver function. Continuous 14 days administration of Se NPs at the doses of 2.5, 5 and 10 mg kg−1 caused no significant change on liver functions (p > 0.05). In addition, no change in levels of creatine phosphokinase (CPK), blood urea nitrogen (BUN) and creatinine was observed. Similarly, other clinical chemistry parameters, including hemoglobin, hematocrit, bilirubin, cholesterol, triglyceride, white blood cell counts, red blood cell counts and platelet counts were at normal ranges in Se NPs treated groups at the doses of 2.5, 5 and 10 mg kg−1 with no significant difference.
At the dose of 2.5 mg kg−1, Selenium dioxide (SeO2) caused a high death rate in the subacute study, while at the similar dose with biogenic Se NPs, no death was observed. The surviving animals in the SeO2 group showed clinical symptoms of poisoning, including skin color change and lower mobility, appetite and body weight.
Conclusion
Despite variations existing between species, our study demonstrated that Se NPs synthesized by Bacillus sp. MSh-1 are less toxic than synthetic Se NPs and much less toxic than SeO2. These results might be due to this fact that the physiochemical stability of metalloid NPs formed biologically by micro-organisms, are higher than that of NPs obtained by ordinary synthetic methods (CitationOremland et al., 2004). At present, the reason for this difference is not clearly known and merits further investigation.
Acknowledgments
The authors wish to thank the Biotechnology Research Center, TUMS, Pharmaceutics research center, Kerman University of medical sciences and the Iranian Nanotechnology Initiative Council for their admirable participation in this study.
Declaration of interest
This study was funded and supported by Tehran University of Medical Sciences (TUMS); Grant no. 10825.
References
- Aillon KL, Xie Y, El-Gendy N, Berkland CJ, Forrest ML. (2009). Effects of nanomaterial physicochemical properties on in vivo toxicity. Adv Drug Deliv Rev, 61, 457–466.
- Beck MA, Levander OA, Handy J. (2003). Selenium deficiency and viral infection. J Nutr, 133, 1463S–1467S.
- Bhattacharya D, Gupta RK. (2005). Nanotechnology and potential of microorganisms. Crit Rev Biotechnol, 25, 199–204.
- Diskin CJ, Tomasso CL, Alper JC, Glaser ML, Fliegel SE. (1979). Long-term selenium exposure. Arch Intern Med, 139, 824–826.
- Fischer HC, Chan WC. (2007). Nanotoxicity: The growing need for in vivo study. Curr Opin Biotechnol, 18, 565–571.
- Grønbaek H, Frystyk J, Orskov H, Flyvbjerg A. (1995). Effect of sodium selenite on growth, insulin-like growth factor-binding proteins and insulin-like growth factor-I in rats. J Endocrinol, 145, 105–112.
- Hoffmann PR, Berry MJ. (2008). The influence of selenium on immune responses. Mol Nutr Food Res, 52, 1273–1280.
- Jafari-Fesharaki P, Nazari P, Shakibaie M, Rezaee S, Banoee M, Abdollahi M, Shahverdi AR. (2010). Biosynthesis of selenium nanoparticles using Klebsiella pneumoniae and their recovery by a simple sterilization process. Braz Microbiol, 41, 461–466.
- Kessi J, Ramuz M, Wehrli E, Spycher M, Bachofen R. (1999). Reduction of selenite and detoxification of elemental selenium by the phototrophic bacterium Rhodospirillum rubrum. Appl Environ Microbiol, 65, 4734–4740.
- Köppel C, Baudisch H, Beyer KH, Klöppel I, Schneider V. (1986). Fatal poisoning with selenium dioxide. J Toxicol Clin Toxicol, 24, 21–35.
- Mandal D, Bolander ME, Mukhopadhyay D, Sarkar G, Mukherjee P. (2006). The use of microorganisms for the formation of metal nanoparticles and their application. Appl Microbiol Biotechnol, 69, 485–492.
- Mohanpuria P, Rana NK, Yadav SK. (2008). Biosynthesis of nanoparticles: Technological concepts and future applications. J Nanoparticles Res, 10, 510–517.
- Murray CB, Kangan CR, Bawendi MG. (2000). Synthesis and characterization of monodisperse nanocrystals and closed-packed nanocrystal assemblies. Ann Rev Mater Sci, 30, 545–610.
- Oremland RS, Herbel MJ, Blum JS, Langley S, Beveridge TJ, Ajayan PM, Sutto T, Ellis AV, Curran S. (2004). Structural and spectral features of selenium nanospheres produced by Se-respiring bacteria. Appl Environ Microbiol, 70, 52–60.
- Orskov H, Flyvbjerg A. (2000). Selenium and human health. Lancet, 356, 942–943.
- Raevskaya AE, Stroyuk AL, Kuchmiy SY, Dzhagan VM, Zahn DRT, Schulze S. (2008). Annealing-induced structural transformation of gelatin-capped Se nanoparticles. Solid State Commun, 145, 288–292.
- Schrauzer GN, Surai PF. (2009). Selenium in human and animal nutrition: Resolved and unresolved issues. A partly historical treatise in commemoration of the fiftieth anniversary of the discovery of the biological essentiality of selenium, dedicated to the memory of Klaus Schwarz (1914-1978) on the occasion of the thirtieth anniversary of his death. Crit Rev Biotechnol, 29, 2–9.
- Shakibaie M, Khorramizadeh MR, Faramarzi MA, Sabzevari O, Shahverdi AR. (2010). Biosynthesis and recovery of selenium nanoparticles and the effects on matrix metalloproteinase-2 expression. Biotechnol Appl Biochem, 56, 7–15.
- Thorlacius-Ussing O. (1990). Selenium-induced growth retardation. Histochemical and endocrinological studies on the anterior pituitaries of selenium treated rats. Dan Med Bull, 37, 347–358.
- Wang H, Zhang J, Yu H. (2007). Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: Comparison with selenomethionine in mice. Free Radic Biol Med, 42, 1524–1533.
- Yang LB, Shen YH, Xie AJ, Liang JJ, Zhang BC. (2008). Synthesis of Se nanoparticles by using TSA ion and its photocatalytic application for decolorization of cango red under UV irradiation. Mater Res Bull, 43, 572–582.
- Yee N, Ma J, Dalia A, Boonfueng T, Kobayashi DY. (2007). Se(VI) reduction and the precipitation of Se(0) by the facultative bacterium Enterobacter cloacae SLD1a-1 are regulated by FNR. Appl Environ Microbiol, 73, 1914–1920.
- Zeng H, Combs GF Jr. (2008). Selenium as an anticancer nutrient: Roles in cell proliferation and tumor cell invasion. J Nutr Biochem, 19, 1–7.
- Zhang JS, Gao XY, Zhang LD, Bao YP. (2001). Biological effects of a nano red elemental selenium. Biofactors, 15, 27–38.
- Zhang J, Wang H, Yan X, Zhang L. (2005). Comparison of short-term toxicity between Nano-Se and selenite in mice. Life Sci, 76, 1099–1109.
- Zhang J, Wang X, Xu T. (2008). Elemental selenium at nano size (Nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: Comparison with Se-methylselenocysteine in mice. Toxicol Sci, 101, 22–31.