Abstract
Context: Dracocephalum moldavica Linn (Labiatae) is one of the ethnomedicinal drugs of Uygur in Xinjiang, China. This herb is mainly used in treating cardiovascular diseases, such as atherosclerosis. However, the mechanism of pharmacological activity of D. moldavica has been poorly studied.
Objective: To explore the pharmacological mechanism of D. moldavica in treating atherosclerosis by investigating the effects of total flavonoids from the aerial portion of D. moldavica on rat vascular smooth muscle cells (VSMCs).
Materials and methods: The proliferation and migration of VSMCs were evaluated via a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay and transwell chamber experiment, respectively. The expression of proliferating cell nuclear antigen (PCNA), nuclear factor κB p65 (NF-κB p65), intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) of VSMCs was determined using immunohistochemistry staining and quantitative real-time PCR (qRT-PCR).
Results: Total flavonoids (IC50 = 145.63 μg/mL) significantly inhibited tumor necrosis factor-α (TNF-α) induced VSMC proliferation at concentrations of 25, 50, and 100 μg/mL. Treatment with 50 and 100 μg/mL of total flavonoids also significantly inhibited TNF-α-induced VSMC migration, whereas 25 μg/mL of total flavonoids did not elicit any significant inhibitory effect. In addition, the effects of total flavonoids on inflammatory molecule expression of cells were tested by immunohistochemistry staining, showing that TNF-α-induced expression of PCNA, NF-κB p65, ICAM-1, and VCAM-1 in VSMCs was dose-dependently suppressed by total flavonoids. Furthermore, qRT-PCR data confirmed the inhibition of mRNA expressions of these inflammatory molecules.
Discussion and conclusion: These data suggest that total flavonoids from D. moldavica exhibit anti-inflammatory activities, which is probably one of the underlying mechanisms of D. moldavica for clinical treatment of atherosclerosis.
Introduction
In the past decades, atherosclerosis has been regarded as an excessive inflammatory response associated with vascular injury (CitationLusis, 2000; CitationLibby et al., 2010). After vascular injury, monocytes, platelets, and lymphocytes adhere to the walls of blood vessels and release a series of inflammatory factors, including cytokines and peptide growth factors (CitationTedgui & Mallat, 2006; CitationOsterud & Bjorklid, 2003). Tumor necrosis factor-α (TNF-α), one of the key regulatory factors in inflammatory response, plays an important role in the initiation and development of atherosclerosis (CitationMcKellar et al.,2009). TNF-α can accelerate excessive proliferation and migration of vascular smooth muscle cells (VSMCs) from arterial media to intima, which are the major causes of neointimal hyperplasia, contributing to atherosclerosis development and progression (CitationGlass & Witztum, 2001; CitationHansson, 2005; CitationGerthoffer, 2007). TNF-α also induces the expression of cellular adhesion molecules on VSMCs, including intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) (CitationHuo & Ley, 2001). Such expression of cell adhesion molecules promotes the interaction between VSMCs and inflammatory leukocytes, thereby facilitating the transmigration and accumulation of leukocytes at inflammatory sites, which play a very important role in further exacerbating the disease (CitationHuo & Ley, 2001; CitationBraun et al., 1999).
Hence, factors affecting the proliferation, migration, and adhesion molecule expression of VSMCs are important in regulating the vascular inflammatory processes of atherosclerosis. Currently, several attempts have been conducted to develop drugs that may regulate the proliferation, migration, and adhesion molecule expression of VSMCs for potential therapeutic purposes (CitationRajesh et al., 2008; CitationChoi et al., 2010).
Dracocephalum moldavica Linn (Labiatae) is an annual herb which has approximately 60 species (32 species in China) distributed mainly in temperate region of Asia (CitationLi et al., 2010). Very few Dracocephalum species are distributed in Central and North Europe, but are found chiefly on high mountains and semiarid areas (CitationGu et al., 2005). D. moldavica is used primarily as a traditional Uygur folk medicine in Xinjiang, China for the treatment of cardiovascular diseases, such as atherosclerosis, coronary heart disease, angina pectoris, hypertension, and myocardial ischemia (CitationGuo et al., 2005).
Modern pharmacological studies have shown that the active components in D. moldavica exhibit a protective effect on ischemic myocardium and reduce oxygen free radicals injuries on platelets in patients with coronary heart disease (CitationGu et al., 2005). In recent years, drug composition analysis has revealed that D. moldavica mainly contains volatile oil, trace elements, terpenoids, flavonoids, and polysaccharides. Flavonoids are one of the major effective components (CitationSong et al., 2010). Epidemiological studies have reported a reduced risk of coronary heart disease in subjects with a high flavonoid intake (CitationGrassi et al., 2009; CitationGeleijnse et al., 1999). Flavonoids possess protective effects, including antioxidative, anti-inflammatory, and antithrombotic activities. These compounds are also involved in reducing blood fat and inhibiting platelet aggregation (CitationGonzález et al., 2011; CitationEl Haouari & Rosado, 2011; CitationTerao, 2009; CitationBenavente-García & Castillo, 2008). However, little is known about the pharmacological action mechanism by which total flavonoids from D. moldavica leads to its effects in atherosclerosis.
Hence, this study has investigated the effects of total flavonoids from the aerial portion of D. moldavica on the proliferation, migration, and adhesion molecule expression of rat VSMCs induced by TNF-α, exploring the pharmacological mechanism of D. moldavica in treating atherosclerosis.
Materials and methods
Drugs and reagents
Total flavonoids extracted from the aerial part of D. moldavica in ethanol (Patent No. CN 200710203385.1) were prepared by Xinjiang Xibu Jiasite Pharmaceutical Company (Urumqi, China) and identified by Dr. Jun Zhao in Xinjiang Institute of Materia Medica. The voucher specimen (20100708) was deposited in the Laboratory of Pharmaceutical Preparations of Xinjiang Institute of Materia Medica. The content of total flavonoid in the extract was 57% and dissolved in a small volume of dimethyl sulfoxide (DMSO). Dulbecco’s modified Eagle’s medium (DMEM, high glucose) and fetal bovine serum (FBS) were purchased from GIBCO/BRL, Maryland, USA; Recombinant rat TNF-α from PeproTech, New Jersey, USA; 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) from Sigma-Aldrich, St. Louis, MO, USA; antirat ICAM-1, VCAM-1, nuclear factor κB p65 (NF-κB p65), and proliferating cell nuclear antigen (PCNA) antibodies from Santa Cruz, Santa Cruz, CA, USA; antirat α-smooth muscle actin (α-SMA), SABC-Cy3, SABC immunohistochemistry kit from Wuhan Boster, Wuhan, China; RNAprep pure Cell/Bacteria Kit from Tiangen Biotech, Beijing, China; ReverTra Ace qPCR RT Kit from Toyobo, Osaka, Japan; Real Master Mix (EVAGreen™) from NewBio Industry, Beijing, China. All other reagents used were of analytical grade obtained commercially.
Primary culture and purity identification of rat VSMCs
Explants from the thoracic aorta of Wistar rats aged 6–8 weeks were prepared after removing the adventitia using 0.1% type II collagenase digestion. Small fragments were prepared and placed in 25 cm2 culture dishes in DMEM supplemented with 10% FBS, l-glutamine (1.6 mM), penicillin G (100 U/mL), and streptomycin sulfate (100 μg/mL), and maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2. The VSMCs were passaged at a ratio of 1:3, until confluence was reached. The morphology of VSMCs was observed under an inverted microscope, and cell purity was identified using fluorescence immunohistochemistry methods. In brief, the cells grown on microscope slides were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) and blocked with 10% normal goat serum in PBS for 1 h. The cells were first incubated with primary antibodies against α-SMA (100 ×dilution) at 4°C overnight, followed by incubation with Cy3-conjugated goat antimouse IgG for 1 h at room temperature. Finally, the specimens were observed under a fluorescence microscope (BX41 microscope; Olympus, Tokyo, Japan), and the red fluorescence represented the positive antigen of α-SMA.
Cell treatment and cell vitality assay
VSMCs in passages six to eight were seeded in cell culture plates at a density of 2 × 105 cells/mL. When grown to 80% confluence, the cells were growth-arrested for 24 h in a serum-free medium, and then transferred back into a complete medium containing 10% FBS for the subsequent experiments. Subsequently, the MTT assay showed that the IC50 value of total flavonoid-mediated inhibition on VSMC proliferation was 145.63 μg/mL. The cells were pretreated without or with various concentrations (i.e., 25, 50, and 100 μg/mL) of total flavonoids from D. moldavica in DMEM culture medium for 2 h and then stimulated with TNF-α (10 ng/mL). The control group was treated only with DMEM culture medium containing 0.1% DMSO, which did not cause any toxic effects.
The Trypan blue exclusion test was conducted to evaluate the cytotoxic effect of drug on VSMCs and detect cell viability. The results showed that the different concentrations of total flavonoids did not exhibit any toxicity on VSMCs. The cell survival rate was more than 95%.
Cell proliferation assay
VSMC were seeded into 96-well plates in 100 μL complete medium, with three replications per group. After 12 h of incubation, MTT was added to a final concentration of 0.5 mg/mL and then incubated for 4 h. The insoluble formazan product was dissolved in DMSO. Absorbance of the samples were read using a microplate reader at 490 nm (BIO-TEK, USA).
Cell migration assay
VSMC migration was assayed using a modified Boyden’s chamber method with the Transwell system (Corning, NY, USA). The lower side of a polyvinylpyrrolidone-free polycarbonate membrane with a pore size of 8.0 μm was used. VSMC were resuspended in a serum-free medium to a concentration of 1 × 105 cells/mL. Cell suspension (200 μL) was added to the upper wells of the Boyden’s chamber and 5% FBS medium was added to the lower chamber of the plate. Migration was allowed to proceed for 4 h at 37°C in a 5% CO2 humidified atmosphere. The remaining cells on the upper surface of the membrane were then scraped off using cotton sticks. Cells that migrated to the lower surface were fixed with paraformaldehyde and stained with hematoxylin. Migration capacity was determined by the average number of stained cells in six random fields of view (400× magnification) per membrane.
Cell immunohistochemistry
For PCNA, NF-κB p65, ICAM-1, and VCAM-1 immunohistochemical assay, VSMC were grown on microscope slides for 24 h, fixed in 4% paraformaldehyde solution, and pretreated with 0.5% Triton X-100 in PBS (for PCNA and NF-κB assay). The specimens were then placed in 3% H2O2 for 5 min to inhibit endogenous peroxidase activity, and placed in PBS with 10% normal goat serum for 20 min to block nonspecific binding sites. The cells were initially incubated with primary antirat antibodies against PCNA, NF-κB p65, ICAM-1, or VCAM-1 at 4°C overnight and then incubated with biotinylated goat antimouse IgG or goat antirabbit IgG for 20 min at 4°C. Negative controls were made by replacing the primary antibodies with homologous nonimmune IgG. After incubation in streptavidin-biotin-peroxidase complex for 20 min at 4°C and in diaminobenzidine chromogen for 10 min, the slides were restained with hematoxylin.
Quantification of NF-κB p65, ICAM-1, and VCAM-1 expression was conducted on an automated image analysis system (Image-Pro Plus 5.0). The positively stained area was measured in at least 10 high-power fields (400× magnification). Quantification of PCNA expression was determined by calculating the percentage of positive stained cells per high-power field.
Quantitative real-time PCR (qRT-PCR)
We performed qRT-PCR to detect the mRNA expression level of PCNA, NF-κB p65, ICAM-1, and VCAM-1 of VSMCs and confirm the immunohistochemical assay results. After the cells in each group were cultured for 12 h, total RNA was extracted using RNAprep pure cell/bacteria kit according to the manufacturer’s instructions. The quality of RNA was confirmed via 1% agarose gel electrophoresis and quantified spectrophotometrically. The total RNA was reverse transcribed into cDNA using a ReverTra Ace qPCR RT kit according to the manufacturer’s instructions. The primers used for qRT-PCR were designed according to the mRNA sequences of the target genes in GenBank databases. The primer sequences purchased from Invitrogen are listed in . Each cDNA sample (100 ng) was amplified in triplicate using Real Master Mix (EVAGreen™). The thermocycler parameters were as follows: 95°C for 30 s, 40 cycles at 95°C for 5 s and 60°C for 30 s. The relative mRNA amounts of target genes were normalized to the values of β-actin. The results were expressed as fold changes of threshold cycle (Ct) value relative to the controls using the 2-ΔΔCt method.
Table 1. Primer sequences for real-time PCR
Statistical analysis
Values were presented as mean ± standard error of mean (SEM). Multiple group means of measurement data were compared using one-way ANOVA, and q test was used for pairwise comparisons. The data on rate and enumeration were analyzed through χ2 tests. All values were considered significant when p < 0.05.
Results
Purity identification of cultured VSMCs
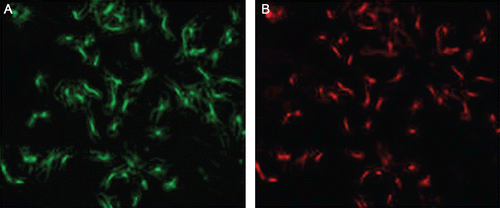
As observed under an inverted microscope, the cells exhibited characteristic spindle shapes and a typical appearance of “valley and hill” after confluence. As determined through fluorescence immunohistochemical staining with primary antibodies against α-SMA, more than 95% of cells emitted red Cy3 fluorescence under a fluorescence microscope ().
Figure 1. The purity identification of rat primary cultured VSMCs. Isolated VSMCs were fixed and subjected to immunofluorescence with antibodies to α-SMA. (A) The cells were observed under an optical microscope; (B) cells were observed under a fluorescence microscope, and the red fluorescence represented the positive antigen of α-SMA.
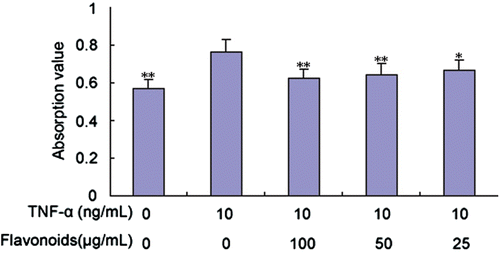
Effect of total flavonoids from D. moldavica on TNF-α-induced VSMC proliferation
We investigated the effect of total flavonoids on VSMCs by measuring their proliferation level using a MTT assay. As expected, TNF-α (10 ng/mL) significantly stimulated VSMC proliferation compared with the control group. As shown in , the TNF-α-induced VSMC proliferation was significantly suppressed by total flavonoids at noncytotoxic concentrations of 25, 50, and 100 μg/mL.
Figure 2. Effect of total flavonoids from Dracocephalum moldavica on TNF-α-induced rat VSMCs proliferation. Quantitative analysis of VSMC proliferation was measured by MTT assay. Values are represented as mean ± SEM (n = 6). Asterisk indicates a significant difference as compared to TNF-α alone group. *p < 0.05; **p < 0.01.
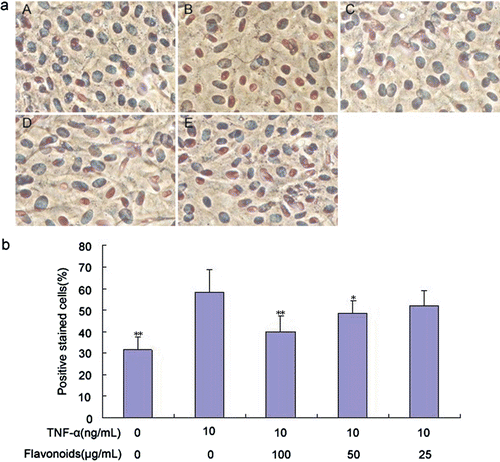
Effect of total flavonoids from D. moldavica on TNF-α-induced PCNA expression of VSMCs
We examined the effect of total flavonoids on TNF-α-induced PCNA expression of VSMCs to investigate the possible mechanism associated with the inhibitory effect of total flavonoids on TNF-α-induced VSMC proliferation. As detected via cell immunohistochemistry, treatment with TNF-α significantly induced the intracellular expression of PCNA compared with the control group, whereas total flavonoids decreased TNF-α-induced PCNA expression in a concentration-dependent manner ().
Figure 3. Effect of total flavonoids from Dracocephalum moldavica on TNF-α-induced PCNA expression in rat VSMCs. (A) Immune staining for PCNA expression in VSMCs in culture. (i) Cells with no treatment; (ii) cells treated with TNF-α (10 ng/mL); (iii) cells treated with TNF-α (10 ng/mL) and total flavonoids (100 μg/mL); (iv) cells treated with TNF-α (10 ng/mL) and total flavonoids (50 μg/mL); (v) cells treated with TNF-α (10 ng/mL) and total flavonoids (25 μg/mL). (B) Quantification of PCNA expression was determined by calculating the percentage of positive stained cells per high-power field. Values are represented as mean ± SEM (n = 6). Asterisk indicates a significant difference as compared to TNF-α alone group. *p < 0.05; **p < 0.01.
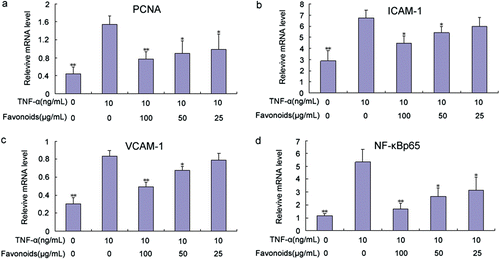
Total cellular RNA was isolated from VSMCs and analyzed via qRT-PCR using PCNA-specific primers to confirm the possibility that total flavonoids likely inhibited PCNA expression by regulating at the mRNA level. VSMCs were pretreated with various concentrations of total flavonoids for 2 h, and then stimulated with TNF-α. The treatment of VSMCs with total flavonoids significantly inhibited the TNF-α-induced mRNA expression of PCNA (). The level of mRNA inhibition was comparable with that of intracellular protein expression.
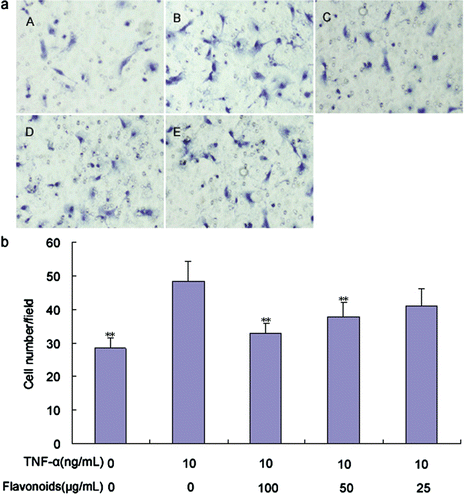
Effect of total flavonoids from D. moldavica on TNF-α-induced VSMC migration
The effect of total flavonoids on TNF-α-induced VSMC migration was detected using the transwell chamber experiment. TNF-α (10 ng/mL) significantly promoted VSMC migration compared with the control group (). Treatment with 50 and 100 μg/mL of total flavonoids significantly inhibited TNF-α-induced VSMC migration, whereas 25 μg/mL of total flavonoids did not elicit any significant inhibitory effect on cell migration. This result indicates that the inhibitory effect is dose-dependent.
Figure 4. Effect of total flavonoids from Dracocephalum moldavica on TNF-α-induced rat VSMCs migration. VSMCs migration was assayed by using a modified Boyden’s chamber method with the Transwell system. (A) The representative images of VSMCs migration in different groups. (i) Cells with no treatment; (ii) cells treated with TNF-α (10 ng/mL); (iii) cells treated with TNF-α (10 ng/mL) and total flavonoids (100 μg/mL); (iv) cells treated with TNF-α (10 ng/mL) and total flavonoids (50 μg/mL); (v) cells treated with TNF-α (10 ng/mL) and total flavonoids (25 μg/mL). (B) VSMCs migration capacity was identified by the average number of stained cells. Values are represented as mean ± SEM (n = 6). Asterisk indicates a significant difference as compared to TNF-α alone group. *p < 0.05; **p < 0.01.
Effect of total flavonoids from D. moldavica on TNF-α-induced ICAM-1 and VCAM-1 adhesion molecule expression of VSMCs
VSMCs were pretreated without or with various concentrations of total flavonoids for 2 h and then stimulated with TNF-α (10 ng/mL) to examine the effect of total flavonoids on TNF-α-induced adhesion molecule expression levels. As detected via cell immunohistochemistry, treatment with TNF-α significantly induced the cell surface expression of ICAM-1 and VCAM-1 compared with the control group, whereas total flavonoids decreased TNF-α-induced ICAM-1 and VCAM-1 expression in a concentration-dependent manner ( and ).
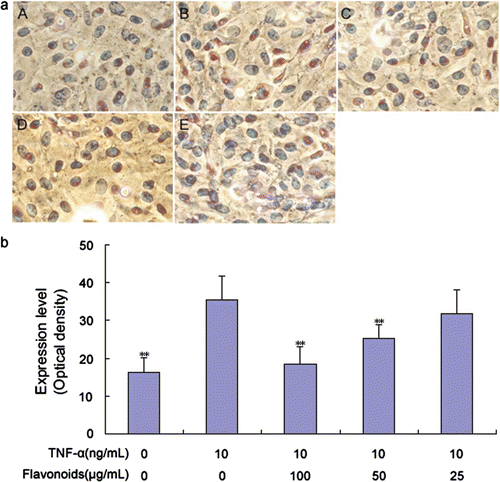
Figure 5. Effect of total flavonoids from Dracocephalum moldavica on TNF-α-induced ICAM-1 expression in rat VSMCs. (A) Immune staining for ICAM-1 expression in VSMCs in culture. (i) Cells with no treatment; (ii) cells treated with TNF-α (10 ng/mL); (iii) cells treated with TNF-α (10 ng/mL) and total flavonoids (100 μg/mL); (iv) cells treated with TNF-α (10 ng/mL) and total flavonoids (50 μg/mL); (v) cells treated with TNF-α (10 ng/mL) and total flavonoids (25 μg/mL). (B) Staining was quantified as optical density using an automated image analysis system. Values are represented as mean ± SEM (n = 6). Asterisks indicate a significant difference as compared to TNF-α alone group. *p < 0.05; **p < 0.01.
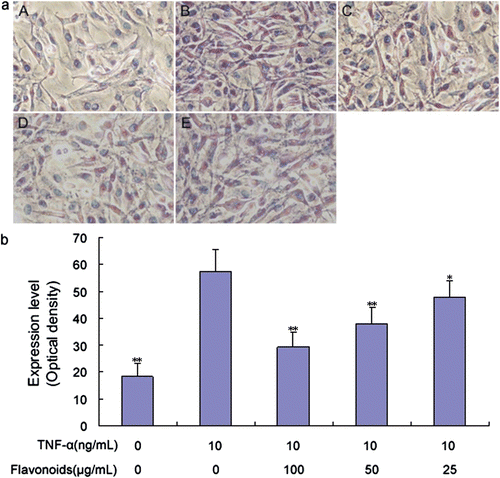
Figure 6. Effect of total flavonoids from Dracocephalum moldavica on TNF-α-induced VCAM-1 expression in rat VSMCs. (A) Immune staining for VCAM-1 expression in VSMCs in culture. (i) Cells with no treatment; (ii) cells treated with TNF-α (10 ng/mL); (iii) cells treated with TNF-α (10 ng/mL) and total flavonoids (100 μg/mL); (iv) cells treated with TNF-α (10 ng/mL) and total flavonoids (50 μg/mL); (v) cells treated with TNF-α (10 ng/mL) and total flavonoids (25 μg/mL). (B) Staining was quantified as optical density using an automated image analysis system. Values are represented as mean ± SEM (n = 6). Asterisk indicates a significant difference as compared to TNF-α alone group. *p < 0.05; **p < 0.01.
Total cellular RNA was isolated from VSMCs and analyzed via qRT-PCR using ICAM-1 and VCAM-1-specific primers, respectively, to confirm the possibility that total flavonoids inhibited ICAM-1 and VCAM-1 expressions by modulating the mRNA levels of these adhesion molecules. VSMCs were pretreated with various concentrations of total flavonoids for 2 h and then stimulated with TNF-α. The treatment of VSMCs with total flavonoids significantly inhibited the TNF-α-induced mRNA expression of ICAM-1 and VCAM-1 ( and ). The level of mRNA inhibition was comparable with that of cell surface protein expression.
Effect of total flavonoids from D. moldavica on TNF-α-induced on NF-κB expression of VSMCs
We examined the effect of total flavonoids on TNF-α-induced NF-κB expression of VSMCs to investigate the possible mechanism involved in the inhibitory effect of total flavonoids on TNF-α-induced adhesion molecule expression of VSMCs. As detected via cell immunohistochemistry, treatment with TNF-α significantly induced the intracellular expression of NF-κB p65 compared with the control group (), whereas total flavonoids reduced TNF-α-induced NF-κB p65 expression in a concentration-dependent manner.
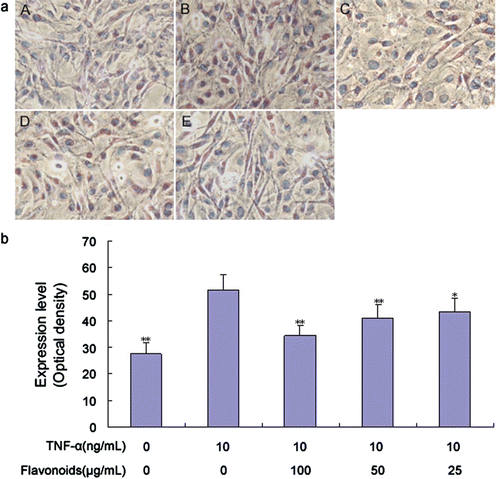
Figure 7. Effect of total flavonoids from Dracocephalum moldavica on TNF-α-induced NF-κB expression in rat VSMCs. (A) Immune staining for NF-κB p65 expression in VSMCs in culture. (i) Cells with no treatment; (ii) cells treated with TNF-α (10 ng/mL); (iii) cells treated with TNF-α (10 ng/mL) and total flavonoids (100 μg/mL); (iv) cells treated with TNF-α (10 ng/mL) and total flavonoids (50 μg/mL); (v) cells treated with TNF-α (10 ng/mL) and total flavonoids (25 μg/mL). (B) Staining was quantified as optical density using an automated image analysis system. Values are represented as mean ± SEM (n = 6). Asterisk indicates a significant difference as compared to TNF-α alone group. *p < 0.05; **p < 0.01.
Total cellular RNA was isolated from VSMCs and analyzed via qRT-PCR using NF-κB p65-specific primers to determine the possibility that total flavonoids inhibited NF-κB p65 expression by adjusting the mRNA level. VSMCs were pretreated with various concentrations of total flavonoids for 2 h and then stimulated with TNF-α. The treatment of VSMCs with total flavonoids significantly inhibited the TNF-α-induced mRNA expression of NF-κB p65 (). The level of mRNA inhibition was comparable with that of intracellular protein expression.
Figure 8. Effect of total flavonoids from Dracocephalum moldavica on TNF-α-induced PCNA (A), ICAM-1(B), VCAM-1(C), and NF-κB p65 (D) mRNA expression in rat VSMCs. Expression of mRNA was analyzed by quantitative real-time PCR. Values are represented as mean ± SEM (n = 3). Asterisk indicates a significant difference as compared to TNF-α alone group. *p < 0.05; **p < 0.01.
Discussion
Research indicates that consuming flavonoids in foods and beverages may decrease the risk of atherosclerosis (CitationReed, 2002). In vitro and in vivo experiments with flavonoids demonstrated that these compounds are dietary antioxidants that can inhibit low-density lipoprotein (LDL) oxidation, inflammatory response in atherosclerosis, platelet aggregation and adhesion, and enzymes involved in lipid and lipoprotein metabolism. Moreover, they can induce endothelium-dependent vasodilation, increase reverse cholesterol transport, and decrease total and LDL cholesterol (CitationReed, 2002).
D. moldavica is used primarily as a traditional Uygur folk medicine in Xinjiang, China for the treatment of cardiovascular diseases, such as atherosclerosis (CitationGuo et al., 2005). In recent years, drug composition analysis has shown that flavonoids are one of the principal effective components in D. moldavica (CitationSong et al., 2010). However, the mechanism involved in the pharmacological activity of total flavonoids from D. moldavica on atherosclerosis remains poorly understood.
In this study, we first detected the effect of total flavonoids from D. moldavica on the proliferation and migration of rat VSMCs. Excessive proliferation and migration of VSMCs from arterial media to intima are the major causes of neointimal hyperplasia, which contributes to the development and progression of vascular pathologies, such as atherosclerosis (CitationKim et al., 2009), and is directly implicated in the failure of clinical interventions used in treating patients with coronary heart disease (CitationRajesh et al., 2008). TNF-α is one of the most potent factors accelerating excessive proliferation and migration of VSMCs (CitationLee et al., 2009). Therefore, VSMC proliferation and migration in this study were induced by adding TNF-α in cultured cells. We found that total flavonoids significantly reduced TNF-α-induced VSMC proliferation and migration. Testing drug concentrations in this study exhibited no effect on cell attachment, and the cell survival rate was more than 95%. Therefore, we can rule out the possibility that the inhibitory effect of total flavonoids on VSMC proliferation and migration resulted from drug toxicity.
The mechanism of action of antiproliferative agents may involve effects on cell cycle regulatory proteins. PCNA is a nonhistone nuclear protein, widely used as a “proliferation marker” both in normal and diseased states and expressed mainly in the S-phase of the cell cycle, and is consistent with the proliferation results (CitationRanganna et al., 2000). We investigated the effect of total flavonoids on the PCNA expression of VSMCs to understand the possible mechanism associated with the inhibitory effect on cell proliferation. Results indicated that total flavonoids inhibited PCNA expression in a concentration-dependent manner after stimulation with TNF-α at the protein and mRNA levels. Our data suggest that the inhibiting effect of total flavonoids on VSMC proliferation can be mediated by inhibiting PCNA expression.
VSMCs express the cellular adhesion molecules ICAM-1 and VCAM-1 in many inflammatory diseases, including atherosclerosis (CitationBraun et al., 1999). These adhesion molecules are transmembrane glycoproteins that mediate cell-to-cell and cell-to-extracellular matrix interactions, and play crucial roles in regulating leukocyte recruitment into inflammatory sites (CitationHope & Meredith, 2003). The expression of these adhesion molecules on VSMCs is prominent in the fibrous caps of advanced atherosclerotic plaques and is also associated with disease severity (CitationKasper et al., 1996). In addition, VCAM-1 and ICAM-1 expressions are induced in responses to inflammatory cytokines, including TNF-α in vascular cells (CitationHuo & Ley, 2001). Thus, factors affecting the expression of adhesion molecules on VSMCs are important in regulating vascular inflammatory processes.
In this study, we evaluated the effects of total flavonoids from D. moldavica on ICAM-1 and VCAM-1 expression of VSMCs. Total flavonoids inhibited the expression of adhesion molecules in a concentration-dependent manner after TNF-α stimulation at the protein and mRNA levels. The expression of adhesion molecules on VSMCs may facilitate the accumulation of transmigrated leukocytes within the vascular wall. Based on these findings, our data suggest that total flavonoids from D. moldavica may play certain roles on delaying atherosclerosis development, stabilizing atherosclerotic plaques, and decreasing local inflammatory reactions.
TNF-α performs its inflammatory function through many downstream factors, particularly the inflammation-related transcription factor NF-κB (CitationWajant et al., 2003). NF-κB is a pleiotropic transcription factor that plays a critical role in the regulation of multiple gene expression involved in inflammatory responses, including ICAM-1 and VCAM-1 (CitationBrasier, 2010). NF-κB binds to the promoter regions of target genes as a dimer of two Rel family proteins, most frequently p50 and p65 (CitationYamamoto & Gaynor, 2001). It has been shown that NF-κB binds to the promoter regions of both ICAM-1 and VCAM-1 to induce their expression (CitationMacKenzie et al., 2007). We investigated the effect of total flavonoids on the NF-κB expression of VSMCs to understand the possible mechanism associated with the inhibitory effect on ICAM-1 and VCAM-1 expression. Our studies indicated that total flavonoids inhibited NF-κB expression in a concentration-dependent manner after TNF-α stimulation at the protein and mRNA levels. Our data suggest that the inhibiting effect of total flavonoids on ICAM-1 and VCAM-1 expression of VSMCs can be at least partially mediated by inhibiting NF-κB expression.
In summary, the results of the present study demonstrate that total flavonoids from D. moldavica can inhibit the proliferation, migration, and expression of ICAM-1 and VCAM-1 of VSMCs. Inhibiting proliferation and adhesion molecules expression could result from the suppression of PCNA expression and NF-κB activation, respectively. These data suggest that total flavonoids from D. moldavica exhibit anti-inflammatory activities, which is probably one of the underlying mechanisms of D. moldavica for clinical treatment of atherosclerosis.
Declaration of interest
This work was supported by the National Natural Science Foundation of China (No. 201091156), the National Key Scientific and Technological Project of New Drug Innovation (No. 2012ZX09102201-009), People’s Republic of China. The authors alone are responsible for the content and writing of the paper.
References
- Benavente-García O, Castillo J. (2008). Update on uses and properties of citrus flavonoids: New findings in anticancer, cardiovascular, and anti-inflammatory activity. J Agric Food Chem, 56, 6185–6205.
- Brasier AR. (2010). The nuclear factor-κB-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc Res, 86, 211–218.
- Braun M, Pietsch P, Schrör K, Baumann G, Felix SB. (1999). Cellular adhesion molecules on vascular smooth muscle cells. Cardiovasc Res, 41, 395–401.
- Choi KW, Park HJ, Jung DH, Kim TW, Park YM, Kim BO, Sohn EH, Moon EY, Um SH, Rhee DK, Pyo S. (2010). Inhibition of TNF-α-induced adhesion molecule expression by diosgenin in mouse vascular smooth muscle cells via downregulation of the MAPK, Akt and NF-κB signaling pathways. Vascul Pharmacol, 53, 273–280.
- El Haouari M, Rosado JA. (2011). Modulation of platelet function and signaling by flavonoids. Mini Rev Med Chem, 11, 131–142.
- Geleijnse JM, Launer LJ, Hofman A, Pols HA, Witteman JC. (1999). Tea flavonoids may protect against atherosclerosis: The Rotterdam Study. Arch Intern Med, 159, 2170–2174.
- Gerthoffer WT. (2007). Mechanisms of vascular smooth muscle cell migration. Circ Res, 100, 607–621.
- Glass CK, Witztum JL. (2001). Atherosclerosis. the road ahead. Cell, 104, 503–516.
- González R, Ballester I, López-Posadas R, Suárez MD, Zarzuelo A, Martínez-Augustin O, Sánchez de Medina F. (2011). Effects of flavonoids and other polyphenols on inflammation. Crit Rev Food Sci Nutr, 51, 331–362.
- Grassi D, Desideri G, Croce G, Tiberti S, Aggio A, Ferri C. (2009). Flavonoids, vascular function and cardiovascular protection. Curr Pharm Des, 15, 1072–1084.
- Gu HF, Chen RY, Sun YH, Xing JG. (2005). Studies on chemical constituents in herbs of Dracocephalum moldavica II. Zhongguo Zhong Yao Za Zhi, 30, 677–679.
- Guo CM, Wu RL, Feng S, Wang JD. (2005). Extraction and determination of Dracocephalum moldavica polysaccharides and its effects on reducing the free radical oxygen (in Chinese). Shi Pin Yu Fa Jiao Gong Ye 31, 129–132.
- Hansson GK. (2005). Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med, 352, 1685–1695.
- Hope SA, Meredith IT. (2003). Cellular adhesion molecules and cardiovascular disease. Part I. Their expression and role in atherogenesis. Intern Med J, 33, 380–386.
- Huo Y, Ley K. (2001). Adhesion molecules and atherogenesis. Acta Physiol Scand, 173, 35–43.
- Kasper HU, Schmidt A, Roessner A. (1996). Expression of the adhesion molecules ICAM, VCAM, and ELAM in the arteriosclerotic plaque. Gen Diagn Pathol, 141, 289–294.
- Kim HJ, Yoo EK, Kim JY, Choi YK, Lee HJ, Kim JK, Jeoung NH, Lee KU, Park IS, Min BH, Park KG, Lee CH, Aronow BJ, Sata M, Lee IK. (2009). Protective role of clusterin/apolipoprotein J against neointimal hyperplasia via antiproliferative effect on vascular smooth muscle cells and cytoprotective effect on endothelial cells. Arterioscler Thromb Vasc Biol, 29, 1558–1564.
- Lee B, Lee EJ, Kim DI, Park SK, Kim WJ, Moon SK. (2009). Inhibition of proliferation and migration by piceatannol in vascular smooth muscle cells. Toxicol In Vitro, 23, 1284–1291.
- Li JP, Li F, Guo WB, Zhou QZ, Liu CD, Mao XM, Tan WL, Zheng SB. (2010). Efficacy of low-dose tadalafil on ED assessed by Self-Esteem and Relationship Questionnaire. Zhonghua Nan Ke Xue, 16, 1147–1149.
- Libby P, Okamoto Y, Rocha VZ, Folco E. (2010). Inflammation in atherosclerosis: Transition from theory to practice. Circ J, 74, 213–220.
- Lusis AJ. (2000). Atherosclerosis. Nature, 407, 233–241.
- MacKenzie CJ, Ritchie E, Paul A, Plevin R. (2007). IKKα and IKKβ function in TNFα-stimulated adhesion molecule expression in human aortic smooth muscle cells. Cell Signal, 19, 75–80.
- McKellar GE, McCarey DW, Sattar N, McInnes IB. (2009). Role for TNF in atherosclerosis? Lessons from autoimmune disease. Nat Rev Cardiol, 6, 410–417.
- Osterud B, Bjorklid E. (2003). Role of monocytes in atherogenesis. Physiol Rev, 83, 1069–1112.
- Rajesh M, Mukhopadhyay P, Haskó G, Huffman JW, Mackie K, Pacher P. (2008). CB2 cannabinoid receptor agonists attenuate TNF-α-induced human vascular smooth muscle cell proliferation and migration. Br J Pharmacol, 153, 347–357.
- Ranganna K, Yatsu FM, Hayes BE, Milton SG, Jayakumar A. (2000). Butyrate inhibits proliferation-induced proliferating cell nuclear antigen expression (PCNA) in rat vascular smooth muscle cells. Mol Cell Biochem, 205, 149–161.
- Reed J. (2002). Cranberry flavonoids, atherosclerosis and cardiovascular health. Crit Rev Food Sci Nutr, 42, 301–316.
- Song R, Roberts BL, Lee EO, Lam P, Bae SC. (2010). A randomized study of the effects of t’ai chi on muscle strength, bone mineral density, and fear of falling in women with osteoarthritis. J Altern Complement Med, 16, 227–233.
- Tedgui A, Mallat Z. (2006). Cytokines in atherosclerosis: Pathogenic and regulatory pathways. Physiol Rev, 86, 515–581.
- Terao J. (2009). Dietary flavonoids as antioxidants. Forum Nutr, 61, 87–94.
- Wajant H, Pfizenmaier K, Scheurich P. (2003). Tumor necrosis factor signaling. Cell Death Differ, 10, 45–65.
- Yamamoto Y, Gaynor RB. (2001). Role of the NF-κB pathway in the pathogenesis of human disease states. Curr Mol Med, 1, 287–296.