Abstract
Context: Catharanthus roseus (L.) G. Don (Apocynaceae) is a medicinal plant that produces more than 130 alkaloids, with special attention given to the production of the anti-hypertensive monomeric indole alkaloids, serpentine and ajmalicine, and the antitumor dimeric alkaloids, vinblastine and vincristine.
Objective: This study evaluated the cytotoxic activity of the indole alkaloid-enriched bioactive extract obtained from suspension cultured-cells of C. roseus elicited with methyl jasmonate (MJ) and cyclodextrins (CDs) in three cell lines: JURKAT E.6 human lymphocytic leukemia, THP-1 human monocytic leukemia and BL 1395 non-tumor human B-cell line.
Materials and methods: An indole alkaloid-enriched bioactive extract was obtained from C. roseus cell cultures elicited with MJ and CDs. The indole alkaloids were identified using an HPLC-diode array system coupled to a time-of-flight mass spectrometer using electrospray ionization (ESI) source. The cytotoxic assays were made using the colorimetric assay 2, 3-bis (2-methoxy-4-nitro-5-sulfophenyl)-S-[(phenylamino)carbonyl]-2 tetrazolium hydroxide (XTT).
Results: Four indole alkaloids were identified (catharanthine, ajmalicine, tabersonine and lochnericine) but only catharanthine and ajmalicine were quantified. The concentration of the indole alkaloid-enriched bioactive extract that inhibited cell growth by 50% was 211 and 210 ng/mL for the JURKAT E.6 and THP-1 cell lines, respectively.
Discussion and conclusion: The results confirm that the powerful antitumor activity of this indole alkaloid-enriched bioactive extract is not due to the effect of a single compound but depends on the synergistic action of the four compounds identified.
Introduction
Catharanthus roseus (L.) G. Don (Apocynaceae) is a medicinal plant that has been long used to treat cerebral and memory disorders in European folk medicine and which produces more than 130 alkaloids, some of which have a high added value because of their wide spectrum of pharmaceutical applications. The most important alkaloids are the antitumor dimeric alkaloids, vinblastine (used in the treatment of Hodgkin’s disease) and vincristine (used for children’s leukemia). These dimeric indole alkaloids are synthesized from vindoline and catharanthine. Likewise, much attention has also focused on the production of the anti-hypertensive monomeric indole alkaloids, ajmalicine and serpentine, which are used to combat heart arrhythmias and to improve the blood circulation in the brain. Such properties make these alkaloids excellent targets for production by biotechnological means.
During the last 30 years, several techniques have been developed to isolate and identify C. roseus metabolites, revealing at the same time their therapeutic potential, especially vinblastine and vincristine (CitationHisiger & Jolicoeur, 2007). Production levels of these metabolites are very low since it takes about 500 kg of dried C. roseus leaves to isolate 1 g of vinblastine (CitationNoble, 1990) making it a very expensive process. Improvements in the production of indole alkaloids by elicitation were described by CitationAlmagro et al. (2008, Citation2011). Thus, the combined treatment with two elicitors, cyclodextrins (CDs) and methyl jasmonate (MJ), in C. roseus cell cultures had a synergistic effect on the production of indole alkaloids, increasing the production of both catharanthine and ajmalicine. In fact, catharanthine levels (112 mg catharanthine/g dry weight [DW]) were 5.5 times those obtained when CDs (21 mg catharanthine/g DW) or MJ (20 mg catharanthine/g DW) were used separately. Ajmalicine production also increased in C. roseus cell cultures, reaching 108 mg/g DW when CDs and MJ were used together (CitationAlmagro et al., 2011).
In the present work, the cytotoxic activity of an extract obtained from C. roseus suspension cultured-cells elicited with CDs and MJ was evaluated in vitro against two leukemic cell lines (JURKAT E.6 and THP-1), and one non-tumor lymphocyte cell line (BL 1395).
Methods
Plant material
Plants of C. roseus were grown in Viveros Bermejo S.L., Murcia, Spain (2009). Calli were induced from young leaves in LS medium (CitationLinsmaier & Skoog, 1965) supplemented with thiamine (0.4 mg/L), myo-inositol (100 mg/L,) sucrose (30 g/L), naphthalene acetic acid (0.19 mg/L), 2,4-dichlorophenoxyacetic acid (0.22 mg/L) and 8 g/L agar. Friable calluses were cultured every month. Suspension cultured-cells were established from friable calluses in 250 mL flasks containing 100 mL of the same medium without agar. Cultures were maintained at 25°C in darkness on a rotary shaker (110 rpm) and cultured every 14 days by diluting 1:1 (v/v) with fresh culture medium.
Elicitation, extraction and analyses of the C. roseus bioactive extract
In order to obtain the bioactive extract, 20 g of washed cells (fresh weight) were transferred into 100 mL of sterile fresh culture medium containing 50 mM CDs (CAVASOL®, Wacker Quimica Ibérica, Barcelona, Spain) and 100 μM MJ (Duchefa, Spain). After 4 days of cell elicitation, C. roseus cells were separated from the culture medium under a gentle vacuum and the spent medium was used for extracting indole alkaloids by phase partitioning with ethyl acetate (v/v) after setting pH to 10.
The organic phase was collected and evaporated at 40°C in vacuum. After that, the solid residue was dissolved in 1 mL of methanol and this bioactive extract was filtered through 0.22 µm nylon filter.
Chromatographic separation of bioactive extract was carried out on a Mediterranean sea C-18 column (150 × 4.60 mm, 3 µm particle size, Teknokroma, Spain) at room temperature. Elution was performed with a mobile phase of methanol-acetonitrile-ammonium acetate (0.0025 mol/L) and triethylamine (0.0072 mol/L) (10:40:50:0.1) at a flow rate of 1 mL/min as described by CitationZhao et al. (2000) with minor modifications. The HPLC-diode array system (Jasco LC-NETII/ADC, Spain) was coupled to a time-of-flight mass spectrometer (TOF/MS, Agilent Technologies, Santa Clara, CA, USA) using an ESI-TOF/MS analysis was performed in the positive mode using full scan mode and the mass range was set at 100–1000 m/z.
The conditions of the electrospray ionization (ESI) source were carried out as follows: N2 drying gas flow rate, temperature, and pressure, 8.0 L/min, 350°C, and 40 psig, respectively. The main instrumental conditions were capillary voltage, 4000 V; fragmentor voltage, 215 V; skimmer voltage, 60 V; octopole DC1 and DC2 voltage, 34.0 and 35.2 V; octopole RF, 250 V. The instrument performed automatic autotuning using a dual nebulizer electrospray source with an automated calibrant delivery system, which introduced a calibrating solution containing the internal standard masses (ESI-TOF tuning mix solution, G1969-85000, Agilent Technologies, Santa Clara, CA, USA). Reference masses consisted of fluorinated compounds provided by the manufacturer (ESI-TOF reference mass solution kit, G1969-85001, Agilent Technologies, Santa Clara, CA, USA). MS was controlled and operated with Agilent Mass Hunter Workstation Software Ver. A.02.02 (Agilent Technologies, Santa Clara, CA, USA) and data analysis were performed with Applied Biosystems/ MSD-SCIEX Analyst QS Software version 1.1 (Applied Biosystems, Darmstadt, Germany).
Cell lines
The tumor cell lines used were JURKAT E.6, human lymphocytic leukemia line and THP-1, human monocytic leukemia line. Also, a non-tumor human B-cell line, BL 1395 was used as control. The first two cell lines were obtained from the European Collection of Cell Culture (UK), and the BL 1395 cell line was obtained from the American Type Culture Collection (US). BL 1395 is a human B-cell line derived from normal B lymphocytes transformed with Epstein–Barr virus.
Cell lines were cultured in RPMI 1640 medium (Sigma–Aldrich, Madrid, Spain) with 10% heat-inactivated fetal bovine serum (Sigma–Aldrich, Madrid, Spain), 2 mM glutamine, 100 g/mL streptomycin and 100 U/mL penicillin and they were maintained at 37°C in a 5% CO2 atmosphere with 95% humidity. The optimal plating density of the cell lines was determined to be 20,000 cells per well to ensure exponential growth throughout the experimental period and to ensure a linear relationship between absorbance at 450 nm and cell number when cell lines were analyzed by toxicity test, as described below.
Toxicity test
Cell proliferation was detected using the colorimetric assay XTT (Sigma–Aldrich, Madrid, Spain) and phenazine methosulfate (PMS) (Sigma–Aldrich, Madrid, Spain), a compound that enhances the cellular reduction of XTT (CitationScudiero et al., 1988). For the assay, cells were centrifuged (150 g at 24°C for 10 min), resuspended and counted by Trypan blue exclusion in a hemocytometer and diluted in RPMI 1640 medium without phenol red. Next, 100 μL/well of these cell suspensions were seeded on 96-well microtiter plates (20,000 cells/well) and incubated to allow cell attachment. After 3 h, cells were treated with different concentrations of bioactive extract ranging from 7.2 to 7200 ng/mL. These concentrations were calculated from the total concentration of indole alkaloids quantified (catharanthine plus ajmalicine). In each microplate assay, 10% (v/v) dimethyl sulfoxide was included as positive internal control, while culture medium and 0.5% methanol (v/v) were included as negative and solvent controls, respectively. These serial dilutions of indole alkaloid-enriched bioactive extract meant that the final mixture used for treating the cells contained no more than 0.13% of methanol at the highest concentration (7200 ng/mL) of the extract. The final volume in each well was 200 μL. At the end of the exposure time, 50 μL of PMS-XTT solution (final concentration, 50 μg of XTT and 0.38 μg of PMS per well) were added to each well. The XTT absorbance obtained after reduction of this compound was measured 4 h later using a multi-plate reader (Labsystems Multiskan MCC/340). The effect of the indole alkaloid-enriched bioactive extract on the cell lines was quantified as the percentage of viability (measured as the reduced dye, at 450 nm) relative to non-treated cell viability (control) (% cell viability = (Absorbance test/Absorbance control) × 100). An antitumor analysis of commercial catharanthine and ajmalicine was also performed at the same concentrations as those used for the indole alkaloid-enriched bioactive extract.
Statistical analysis
The data, presented as mean ± SD, were obtained from two independent experiments with six replicates each. Statistical analysis was performed by ANOVA test. Differences were considered significant at p < 0.05. The 50% growth inhibitory concentration (IC50) was calculated from the temporal study obtained by plotting the percentage of absorbance (compared to the control) versus the indole alkaloid-enriched bioactive extract concentrations.
Results
Analysis of the bioactive extract
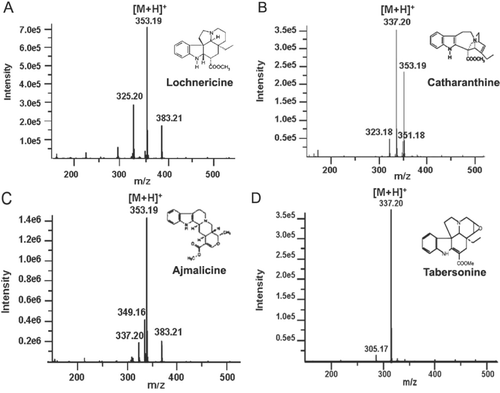
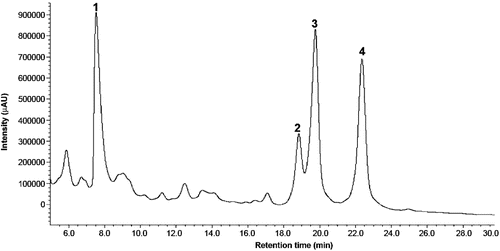
In the chromatogram (), four indole alkaloids were identified by HPLC-DAD-ESI-TOF/MS from the retention time and mass spectra in the extracellular media. The compounds with retention time 7.52 (Peak 1), 18.82 (Peak 2), 19.74 (Peak 3) and 22.34 min (Peak 4), were identified as putative lochnericine, catharanthine, ajmalicine and putative tabersonine, respectively.
Figure 1. Chromatogram of one indo alkaloid-enriched bioactive extract. Peaks identified with commercial patterns were: peak 2 (catharanthine) and peak 3 (ajmalicine). Identification based on both absorbance spectrum and retention time gave peak 1 (putative lochnericine) and peak 4 (putative tabersonine).
As shown in , lochnericine was identified by its retention time and by its absorption spectrum described by CitationHisiger and Jolicoeur (2007). Its presence was also confirmed by its mass spectrum described by CitationFerreres et al. (2010). Although lochnericine () has the same molecular weight as ajmalicine (), the molecular ion [M+H]+ 325.20 is characteristic of this molecule and it is not present in the ajmalicine mass spectrum.
In the same way, tabersonine () which has an identical molecular weight to catharanthine, was identified by its retention time () and its absorption spectrum described by CitationHisiger and Jolicoeur (2007). Its presence was also validated by identification of a molecular ion of [M+H]+ 305.17, which is characteristic of this molecule, as described by CitationFerreres et al. (2010) and which is not present in the catharanthine mass spectrum (). Likewise, catharanthine and ajmalicine were quantified in the extracts by performing a calibration curve with commercial catharanthine and ajmalicine. Since each concentration corresponds to an integrated peak area, extrapolating these data in the calibration curve gave the concentrations of both compounds in the extracellular media were 0.17 and 0.38 mg/mL for catharanthine and ajmalicine, respectively. However, the amounts of these compounds inside the cells were very low in comparison with those found in the culture medium after elicitation (data not shown).
Dose-response study of the indole alkaloid-enriched bioactive extract versus cell viability of the tumor cell lines
Before carrying out a dose-response study of the indole alkaloid-enriched bioactive extract versus cell viability, the growth of the tumor cell lines was characterized in order to ascertain the concentration of cells per well that should be cultured to carry out the cytotoxicity study, and the lineal relation between the cell concentration and their absorbance. The concentration of cells per well chosen was 20,000 cells/well. The indole alkaloid-enriched bioactive extract from C. roseus was evaluated in assays that assessed cytotoxicity against two human cancer cell lines (JURKAT E.6 and THP-1) and one human non-tumor cell line (BL 1395) ().
Figure 3. Cytotoxic effect of different concentrations of indole alkaloid-enriched bioactive extract on the cell lines, JURKAT E.6 (diamond), THP-1 (triangle) and BL 1395 (circle). Mean values ± SD based on six replicate samples obtained in a single experiment.
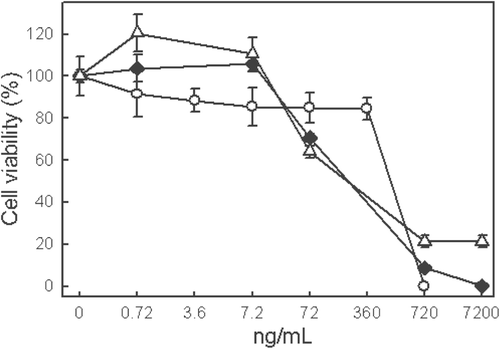
The concentration of the indole alkaloid used for the cytotoxic assay was between 0.72 and 7200 ng/mL. As can be observed in , the viability of the JURKAT E.6 and THP-1 cell lines fell at concentrations above 7.2 ng/mL. Lower concentrations did not decrease the viability with respect to the control, while concentrations of 720 ng/mL and above were practically lethal for both cell lines. The 72 ng/mL concentration, which affected the cell viability of the tumor cell lines, did not affect the cell viability of non-tumor cell BL 1395. Moreover, the concentration of 360 ng/mL decreased the viability by 60 ± 2.3% in JURKAT E.6 and THP-1 cell lines but not in the BL 1395 cell line. In contrast, the concentration of 720 ng/mL reduced viability significantly for all the cell lines.
Temporal study of indole alkaloid-enriched bioactive extract versus cell viability and determination of IC50
The time-related effect of different concentrations of the indole alkaloid-enriched bioactive extract on the THP-1 and JURKAT E.6 cell lines is shown in and , respectively. The concentration of 360 ng/mL was lethal for the THP-1 at all incubation times, while a dose five times lower (72 ng/mL) decreased slightly cell viability after 96 h incubation. The other concentrations did not significantly affect THP-1 cell viability at any of the incubation times used (). By lineal regression of the 72 h incubation data (which pointed to cell exponential growth), the IC50 for THP-1 cell line was found to be 210 ± 6.5 ng/mL.
Figure 4. Time course of cytotoxic effect of different concentrations of indole alkaloid-enriched bioactive extract on THP-1 cell line. Mean values ± SD based on six replicate samples obtained in a single experiment. *p < 0.05 in relation to the control.
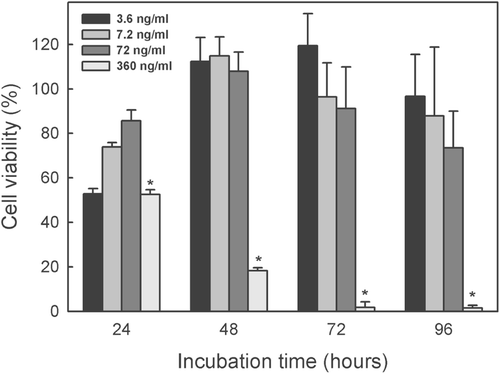
Figure 5. Time course of cytotoxic effect of different concentrations of indole alkaloid-enriched bioactive extract on JURKAT E.6 cell line. Mean values ± SD based on six replicate samples obtained in a single experiment. *p < 0.05 in relation to the control.
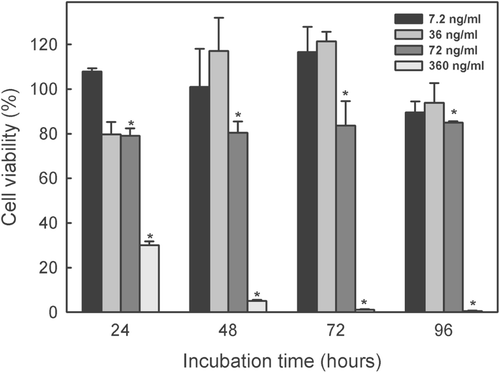
Extract concentration of 360 ng/mL was lethal against the JURKAT E.6 cell line (). The 72 ng/mL dose inhibited growth at all the incubation times compared with the control (100% of viability) (p < 0.05) while the 36 and 7.2 ng/mL doses did not significantly affect cell viability at any of the incubation times used. By lineal regression of the 72 h incubation data, the IC50 for JURKAT E.6 cell line was found to be 211 ± 5.1 ng/mL.
These results are cause for optimism, since the criterion of the American National Cancer Institute for considering a crude extract as a potential source of compounds for use in the treatment of cancer is of an IC50 below 30 μg/mL (CitationSuffness & Pezzuto, 1990).
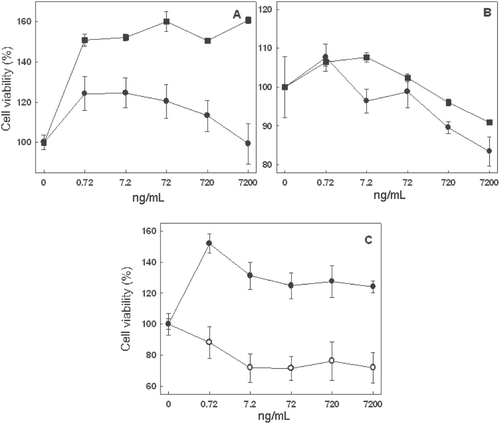
Using an indole alkaloid-enriched extract begs the question as to which specific compounds are responsible for any antitumor activity. Given that only catharanthine and ajmalicine are commercially available, we assayed whether they were responsible for the antitumor activity noted. However, although the commercial standards were used in the same concentrations as they occur in the extract, the results bore no resemblance to the results obtained with the indole alkaloid-bioactive enriched extract. As shown in , none of the concentrations of ajmalicine and catharanthine tested on JURKAT E.6 () and THP-1 () cell lines produced a decrease in cell viability compared with the control. Besides, the joint addition of both catharanthine and ajmalicine at the same concentrations used in the assays had no effect on the THP-1 cell line (). In contrast, the viability of the JURKAT E.6 cell line fell slightly above a concentration of 7.2 ng/mL (). In fact the concentration of 7200 ng/mL, which was lethal, decreased viability to below 71.9 ± 1.5%. It can therefore be affirmed that the addition of catharanthine and ajmalicine, separately or together, is not responsible for the strong antitumor activity that the indole alkaloid-enriched bioactive extract has.
Figure 6. Cytotoxic activity of commercial ajmalicine (square) and catharanthine (circle) at different concentrations on JURKAT E.6 (A) and THP-1 (B) cell lines. Cytotoxic effect of the joint action of commercial ajmalicine and catharanthine (C) at different concentrations on cell lines, JURKAT E.6 (white circle) and THP-1 (black circle). Mean values ± SD based on six replicate samples obtained in a single experiment.
Discussion
In search for antiproliferative compounds against human cancer cells, we evaluated the cytotoxic activity of indole alkaloid-enriched bioactive extracts obtained from suspension cultured-cells of C. roseus elicited with MJ and CDs. The extracts had a strong cytotoxic activity since the IC50 values were 211 and 210 ng/mL for JURKAT E.6 and THP-1 cell lines, respectively.
Previous studies have described the cytotoxic potential of 412 extracts from Brazilian plants used in traditional medicine (Citationde Mesquita et al., 2009). These extracts were tested against human colon carcinoma (HCT-8), melanoma (MDA-MB-435), brain (SF-295) tumor cell lines and (HL-60) human promyelocytic leukemia after 72 h incubation. The strongest cytotoxic activity was found for an extract of Casearia sylvestris, which had an IC50 of 1.3 μg/mL against the HL-60 cell line, and for an extract of Simarouba versicolor, which had an IC50 of 1.1 μg/mL against the HL-60 cell line (). C. sylvestris and S. versicolor are two plants whose extracts contain indol alkaloids as the major metabolites. In our study, the IC50 values obtained were 6.2- and 5.2-times lower than the results obtained using extracts from C. sylvestris and S. versicolor, respectively, against leukemia cell line HL-60 of similar origin to the line used in our trial ().
Table 1. IC50 values obtained from vegetable extract enriched in different types of alkaloid.
The indole alkaloid fraction of a methanolic extract obtained from Peganum harmala was tested in vitro on two tumor cell lines: Med-mek (fibrosarcoma) and UCP-Med (hepatocarcinoma). Cell proliferation was significantly reduced at all the tested concentrations (20–120 μg/mL) during the first 24 h of incubation (CitationLamchouri et al., 1999). The IC50 after 24 h incubation was 18.4 μg/mL for Med-mek and 33.7 μg/mL for UCP-Med carcinoma. CitationVijayan et al. (2002) tested the in vitro cytotoxicity of the total indole alkaloid fraction of a methanolic extract from unripe fruits of Solanum pseudocapsicum on HEp-2 (immortalized cell tracheal), Vero (kidney epithelial carcinoma) and RD (rhabdomyosarcoma) cell lines. The IC50 was found to be 6.3, 12.0, and 1.6 µg/mL for the HEp-2, Vero and RD cell lines, respectively. In our study, the IC50 values obtained were lower than the results obtained by CitationLamchouri et al. (1999) and CitationVijayan et al. (2002). Bearing in mind the good results obtained by other authors using indole alkaloid-enriched extracts against epithelial cell lines, it seems highly likely that our extract, whose cytotoxic effect was demonstrated against lymphocytic and monocytic cell lines, would also be effective against epithelial cell lines.
Not only indole alkaloids have antitumor activity, but also other alkaloids, such as the glyco-alkaloids present in extracts of Solanum crinitum, which were capable of inhibiting the growth of the cells belonging to murine Ehrlich carcinoma and human K562 leukemia () by 50% at concentrations of 19.5 and 13.6 µg/mL, respectively, after 48 h incubation (CitationSouza et al., 2002).
In addition, acridone alkaloid-enriched extracts of fruits of Actinidia polygamous had an IC50 of 0.5 mg/mL against a promyelocytic leukemia cell line (HL-60) after 72 h incubation. This cytotoxic activity was less potent against LS-174T colon carcinoma and non-tumor dermal cell lines (CitationYoshizawa et al., 2002).
The cytotoxic activity of our extract is 68.25-times higher than that of the glyco-alkaloid-enriched extracts used by CitationSouza et al. (2002) against the K562 leukemia cell line. Furthermore, acridone alkaloid-enriched extracts obtained by CitationYoshizawa et al. (2002) presented a cytotoxic activity 2.5 times lower than our extract again a leukemia cell line (HL-60). Our results strongly suggest that indole alkaloid-enriched bioactive extract has a higher cytotoxic activity against leukemia cell lines than other alkaloids such as glyco-alkaloids and acridone alkaloids ().
Conclusions
The results confirm the powerful antitumor activity of the indole alkaloid-enriched bioactive extract obtained from suspension cultured-cells of C. roseus elicited with MJ and CDs. The addition of catharanthine and ajmalicine, separately or together, is not responsible for this strong antitumor activity. No cytotoxic activity has been described in the literature for lochnericine. However, the antitumor activity of tabersonine has been described, showing an IC50 value of 5.4 µM (i.e., 1.82 µg/mL) against an HL-60 cell line (CitationFeng et al., 2010). Tabersonine is structurally similar to vindoline, which, in turn, binds to catharanthine to give the dimeric indole alkaloid, vinblastine, so that the joint action of tabersonine and catharanthine could be responsible for the powerful antitumor activity of the indole alkaloid-enriched bioactive extract obtained from C. roseus. Another possibility is that the cytotoxic effect of the bioactive extract is due to the synergistic action of the four compounds presented in the extract. The synergistic effect between bioactive compounds has been described by other authors and it is used as a strategy for cancer treatment (CitationJiang et al., 2010). Further pharmacological studies are needed in order to confirm this synergism.
Acknowledgments
We are grateful to Dr. José Muñoz from the Servicio de Apoyo a las Ciencias Experimentales (SACE) of the University of Murcia and to Dr Antonio José López Pérez for carrying out the cell elicitation experiments and Viveros Bermejo S.L for providing C. roseus plants.
Declaration of interest
The authors report no declarations of interest. This work has been partially supported by the Consejería de Educación, Ciencia e Investigación de la Región de Murcia (BIO BVA 07 01 0003) and Fundación Séneca (08799/PI/08). Francisco Fernández Pérez and Lorena Almagro held a grant from the Ministerio de Ciencia e Innovación.
References
- Almagro L, Ló pez Perez AJ, Pedreñ o MA. (2011). New method to enhance ajmalicine production in Catharanthus roseus cell cultures based on the use of cyclodextrins. Biotechnol Lett, 33, 381–385.
- Almagro L, Sabater-Jara AB, Pedreñ o MA. (2008). Combined use of cyclodextrins and methyl jasmonate to increase the production of indole alkaloids. ES patent: P200802437.
- de Mesquita ML, de Paula JE, Pessoa C, de Moraes MO, Costa-Lotufo LV, Grougnet R, Michel S, Tillequin F, Espindola LS. (2009). Cytotoxic activity of Brazilian Cerrado plants used in traditional medicine against cancer cell lines. J Ethnopharmacol, 123, 439–445.
- Feng T, Li Y, Liu YP, Cai XH, Wang YY, Luo XD. (2010). Melotenine A, a cytotoxic monoterpenoid indole alkaloid from Melodinus tenuicaudatus. Org Lett, 12, 968–971.
- Ferreres F, Pereira DM, Valentã o P, Oliveira JM, Faria J, Gaspar L, Sottomayor M, Andrade PB. (2010). Simple and reproducible HPLC-DAD-ESI-MS/MS analysis of alkaloids in Catharanthus roseus roots. J Pharm Biomed Anal, 51, 65–69.
- Hisiger S, Jolicoeur M. (2007). Analysis of Catharanthus roseus alkaloids by HPLC. Phytochem Rev, 6, 207–234.
- Jiang H, Shang X, Wu H, Huang G, Wang Y, Al-Holou S, Gautam SC, Chopp M. (2010). Combination treatment with resveratrol and sulforaphane induces apoptosis in human U251 glioma cells. Neurochem Res, 35, 152–161.
- Lamchouri F, Settaf A, Cherrah Y, Zemzami M, Lyoussi B, Zaid A, Atif N, Hassar M. (1999). Antitumour principles from Peganum harmala seeds. Therapie, 54, 753–758.
- Linsmaier EM, Skoog F. (1965). Organic growth factor requirements of tobacco tissue cultures. Physiol Plant, 18, 100–127.
- Noble RL. (1990). The discovery of the vinca alkaloids–chemotherapeutic agents against cancer. Biochem Cell Biol, 68, 1344–1351.
- Scudiero DA, Shoemaker RH, Paull KD, Monks A, Tierney S, Nofziger TH, Currens MJ, Seniff D, Boyd MR. (1988). Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res, 48, 4827–4833.
- Souza AE, da Silva TMS, Alves CCF, de Carvalho MG, Filhob RB, Echevarria A. (2002). Cytotoxic activities against Ehrlich Carcinoma and human K562 leukaemia of alkaloids and flavonoid from two Solanum species. J Braz Chem Soc, 13, 838–842.
- Suffness M, Pezzuto JM. (1990). Assays related to cancer drug discovery. Methods Plant Biochem, 6, 71–133.
- Vijayan P, Kumar SV, Dhanaraj SA, Badami S, Suresh B. (2002). In vitro cytotoxicity and anti-tumor properties of the total alkaloid fraction of unripe fruits of Solanum pseudocapsicum. Pharm Biol, 40, 456–460.
- Yoshizawa Y, Fukiya Y, Izumi Y, Hata K, Iwashita J, Murofushi N, Abe T. (2002). Induction of apoptosis with an extract of Actinidia polygamous fruit in the promyelocytic leukemia cell line HL-60. J Health Sci, 48, 303–309.
- Zhao J, Zhu WH, Hu Q, He XW. (2000). Improved alkaloid production in Catharanthus roseus suspension cell cultures by various chemicals. Biotechnol Lett, 22, 1221–1226.