Abstract
Context: Insects are a large, unexplored and unexploited source of potentially useful compounds for modern medicine. The larvae of the housefly (Musca domestica) have been used to study immune-induced molecules because they can survive in pathogenic environments.
Objective: The antiviral activity of a protein-enriched fraction (PEF) from the larvae of the housefly was evaluated in vitro and the possible antiviral mechanism was studied.
Materials and methods: PEF was isolated from the larvae of the housefly. The cytotoxicity of PEF was detected by the MTT assay. The in vitro antiviral activity of PEF against influenza virus was investigated. PEF was incubated with the virus and its target cells under various conditions, and its antiviral effects were examined by reduction in virus yield in cell cultures. Experiments with ribavirin were performed in parallel under the same conditions.
Results: The results indicated that PEF had minimal cytotoxicity against MDCK cells and the CC50 value was calculated to be 284.45 μg/ml. The antiviral results showed the loss of infectious capacity was more than two log (2) units in cell cultures compared with virus control. The effect of PEF was direct virucidal activity and the interference on the adsorption of cell and virus. The antiviral mechanism of PEF is different from ribavirin.
Conclusion: The results indicate that PEF showed strong antiviral activity against influenza virus at a very early stage of the interaction with virus particles or their entry into the cells. PEF has a great potential as a resource of healthy products.
Introduction
In the last 20 years, there have been many reports on the bioactivity of plants and their components in folk medicine, but there are few reports on the ethnopharmacological effects of insects (CitationHou et al., 2007). Insects are a large, unexplored and unexploited resource of potentially useful compounds for modern medicine (CitationChu et al., 2011). Insects have developed a complicated and effective innate immune system during evolution, which apparently differs from the adaptive immune system of vertebrates. Their defense mechanisms are rapid, only last up to a few days, but offer particularly powerful resistances to microbial infections (CitationHao et al., 2008). As invertebrates possess no acquired immunity and no interferons have been reported from invertebrates, it is speculated that invertebrates have other immune factors to fight against pathogens (CitationNakazawa et al., 2004). Many active proteins and peptides with antibacterial, antifungal and antiviral properties are found in insects (CitationHao et al., 2008; CitationFu et al., 2009; CitationJin et al., 2010). Identification and isolation of these active substances are very necessary for studying non-specific immune response mechanisms of insect against pathogen invasion. Exploitation and application of these substances will also ultimately benefit mankind in bio-pharmaceutical industry.
Housefly is an important medical insect which has a highly effective immune defense mechanism and is rarely infected even reared in large-scale, high-density conditions (CitationFu et al., 2009). It is well known that the domestic housefly can transmit many kinds of viruses such as Newcastle disease virus (NDV), turkey coronavirus, porcine reproductive and respiratory syndrome virus, rotavirus (CitationWanaratana et al., 2011). Previous studies showed that the housefly act as a mechanical vector for avian influenza (AI) H5N1 virus and the virus infection titer continuously decreased according to the increasing exposure time (CitationWanaratana et al., 2011). This indicated that the AI H5N1 virus could not replicate within the housefly, but its specific resistant mechanism is not known. Maybe there is some antiviral constitutes in the body of housefly.
In our previous study, we found that the homogenate of the larvae of the housefly inhibited the propagation of influenza virus in chicken embryo and destroyed the surface antigen of hepatitis B in vitro (CitationWang et al., 2006). However, the active constitutes of anti-influenza is still not clear. The present study describes a protein-enriched fraction (PEF) from the larvae of the housefly. The antiviral activity of PEF against human influenza H1N1 was investigated and the possible antiviral mechanism was studied. We hope that our findings can lay a foundation for studies of antiviral peptides or proteins from the housefly larvae, and provides insight into the antiviral immunity of housefly.
Materials and methods
Maintenance of housefly larvae
The larvae of houseflies were maintained according to the method we described before (CitationAi et al., 2008).
Preparation of PEF
Third-instar larvae (maggots) were collected, washed with distilled water, frozen and lyophilized. The lyophilized maggots were extracted with petroleum ether (bp 30–60°C) in a Soxhlet apparatus for 50 h. The defatted maggots (1 g) were pulverized at low temperature and were treated in cold (4°C) buffer (0.1 M citrate-Na2HPO4, 0.18 M NaCl, pH 7.0) for 0.5 h. After centrifugation at 1,800 g for 15 min, the supernatant was transferred to a new container and acidified to pH 5.8 with HCl. The extracts were subjected to graded precipitation with (NH4)2SO4 (below 65% saturation) and the precipitate dialyzed against distilled water. All the dialyzed extracts were concentrated and lyophilized. The yield of lyophilized supernatant (PEF) was about 52 mg and it was stored at −20°C until needed.
Cell and virus
Madin-Darby canine kidney (MDCK) cells used in this study were obtained from Hubei Provincial Center for Disease Control and Prevention. The cells were grown in Earle’s Minimum Essential Medium (MEM) supplemented with 10% fetal bovine serum, 100 mg ml−1 of streptomycin and 100 IU ml−1 of penicillin in a humidified atmosphere of 5% carbon dioxide (CO2) at 37°C. The cells were harvested using trypsin solution.
The influenza virus strain A/Shiyan/275/05 (H1N1) was obtained from Hubei Provincial Center for Disease Control and Prevention. Influenza virus was propagated in 10-day chick embryos. Allantoic fluids were harvested and stored at the freezer (−80°C). Virus titer was determined by cytopathic effect in MDCK cells and the infectious virus titer of this stock proved to be 1 × 107 TCID50/ml. Virus was stored at −70°C until used.
Cellular toxicity
The effect of PEF on the viability of MDCK cells was determined using a quantitative colorimetric MTT (3-[4,5-dimethylthiazol]-2,5-diphenyltetrazolium bromide) assay with minor modifications (CitationKimura et al., 2000). Briefly, PEF was diluted serially by 2-fold using MEM plus 2% FCS (fetal calf serum) (from 2500 to 4.88 μg/ml). The MDCK cells were seeded onto 96-well plate with a concentration of 1.0 × 105 cells per ml and a volume of 100 µl per well. Different concentrations of PEF were applied to culture wells in quadruplicate. The viability of the cells was detected using MTT after 3 days. The optical density (OD) in each well was determined using a 96-well plate reader at a wavelength of 570 nm. The median cytotoxic concentration (CC50) was calculated as the concentration of PEF which decreased the number of viable cells to 50% of the cell control.
Antiviral bioassays
Monolayers of MDCK cells were established in 96-well tissue culture plates by seeding 1 × 104 cells in each well. When the confluent monolayers were obtained, four different treatments were then applied according to modified protocols (CitationBelaid et al., 2002) as follows.
PEF treatment before virus adsorption/fusion
To determine the ability of PEF to directly inactivate influenza virus H1N1, 50 μl of virus suspension containing 200 TCID50 was mixed with 50 μl of dilution containing different concentrations of PEF (20, 50, 100, 150 μg/ml). Sham-treated virus was prepared similarly using maintenance medium without PEF. The mixtures were then added to monolayers of the MDCK cells in quadruplicate (100 μl of each mixture per well) after incubated at 37°C for 2 h. The virus was allowed to adsorb for 2 h at 37°C, then the virus inoculum was replaced with 0.1 ml of fresh medium. The cultures were incubated at 37°C for 3 days.
PEF treatments during virus adsorption/fusion
The inhibitory activity of PEF against the adsorption of influenza virus H1N1 to cells was examined during the contact period of the virus. 50 μl of virus suspension containing 200 TCID50 was mixed with 50 μl of serial dilution containing different concentrations of PEF. The mixtures were then added to monolayers of the MDCK cells in quadruplicate (100 μl per well). Sham-treated virus was prepared similarly using maintenance medium without PEF. After 2 h of virus adsorption at 37°C, the cells were washed with maintenance medium, and then pre-warmed maintenance medium without PEF was added to each well.
PEF treatments after virus adsorption/fusion
Confluent monolayers of MDCK cells were infected with influenza virus H1N1 at a multiplicity of infection of 100 TCID50/well in 50 μl of maintenance medium. Cultures were incubated at 37°C for 2 h to permit virus adsorption/fusion. Then virus inoculum was replaced with 100 μl of medium containing various concentrations of PEF. The fresh medium without PEF was conducted as control. The cultures were incubated at 37°C for 3 days.
PEF pre-treatment of cells alone
Cell monolayers were incubated in the presence of either fresh medium (controls) or culture medium containing different concentrations of PEF for 2 h. The cell monolayers were then washed three times with maintenance medium and inoculated with influenza virus H1H1 at a multiplicity of infection of 100 TCID50/well. The virus was allowed to adsorb for 2 h, then the virus inoculum was replaced with fresh medium. The cultures were incubated at 37°C for 3 days.
Experiments with ribavirin (1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide) were performed in parallel under the same conditions as described above. Monolayers of MDCK cells in 96-well tissue culture plates were treated with one of procedures as above, in the presence of virus and PEF or ribavirin, or in the presence of virus alone and cultured for 3 days at 37°C. The virus produced was harvested by freezing and thawing the cells three times. The virus virions released to the supernatant were titered by the hemagglutinin (HA) test as described elsewhere (CitationRimmelzwaan et al., 1998). HA titer was used as an indicator for infection of the cells in individual wells.
Results
Cytotoxicity of PEF
To assay for cytotoxic effect of PEF on MDCK cells, the cells were incubated with increasing concentrations of PEF. The viability of the treated cells was investigated using the MTT method. The CPE results showed that PEF up to 200 μg/ml concentration did not impair cell viability with respect to the corresponding untreated cells. The MTT method indicated that PEF had minimal cytotoxicity against MDCK cells and the CC50 value was calculated to be 284.45 μg/ml.
Virucidal effect of PEF on influenza virus
The preliminary studies on the inactivation of influenza virions indicated the loss of infectious capacity was more than two log (2) units () when the virus suspensions were pre-incubated for 2 h with different concentrations of PEF prior to the cell infection. In contrast, ribavirin showed little effect and there was almost no decrease in the HA titer of the supernatant compared with the virus control.
Figure 1. HA titers of influenza virus in the culture supernatant treated with PEF or ribavirin before virus adsorption/fusion. The virus virions released to the supernatant were titered by the hemagglutinin (HA) test. Values are means ± S.D. from three independent experiments. The bars indicate standard deviations. Where there is no bar, there is no standard deviation.
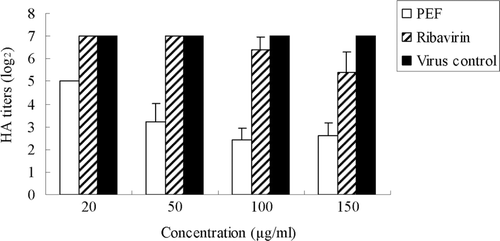
Effect of PEF on virus-cell interface
HA titers of the supernatant which contain PEF or ribavirin decreased compared to that of fresh medium. The HA results of the supernatant showed that both PEF and ribavirin inhibit the infectivity of the virus in the supernatant of the culture (). The logarithm value of HA titer declined significantly with increasing concentrations of PEF or ribavirin. This indicated that both of them effectively prevent the attachment of virus and cells.
Figure 2. HA titers of influenza virus in the culture supernatant treated with PEF or Ribavirin during virus adsorption/fusion. The virus virions released to the supernatant were titered by the hemagglutinin (HA) test. Values are means ± S.D. from three independent experiments. The bars indicate standard deviations. Where there is no bar, there is no standard deviation.
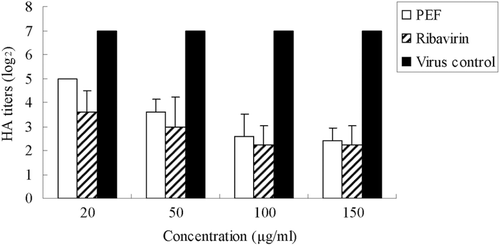
Effect of PEF addition after virus adsorption
Experiments in which PEF or ribavirin were added into the culture medium post-infection were conducted to determine whether they could affect virus growth at the later phases of the viral infectious cycle. As shown in , the logarithm value of HA titer declined significantly from 7 to 1 (50, 100, 150 μg/ml ribavirin), indicating ribavirin showed effective antiviral activity after virus adsorption. However, PEF did not show significant antiviral activity under the same condition.
Figure 3. HA titers of influenza virus in the culture supernatant treated with PEF or ribavirin after virus adsorption/fusion. The virus virions released to the supernatant were titered by the hemagglutinin (HA) test. Values are means ± S.D. from three independent experiments. The bars indicate standard deviations. Where there is no bar, there is no standard deviation.
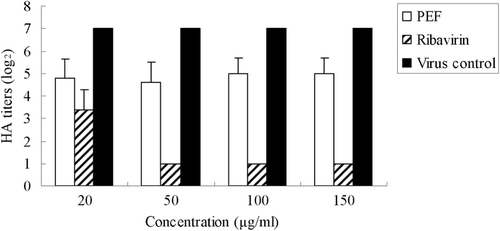
Protective effect of PEF on MDCK cells
To determine whether pre-treatment of cells with PEF or ribavirin induced changes that would have resulted in the reduction of the virus infectivity of the supernatant, we pre-incubated MDCK cell monolayers with different concentrations of PEF or ribavirin for 2 h before virus infection. The results showed that both PEF and ribavirin pretreated cells exhibit little decrease in the HA titer compared with the virus control (). This indicated that MDCK cells did not acquire durable resistance to influenza virus infection after pretreatment with PEF and ribavirin.
Figure 4. HA titers of influenza virus in the culture supernatant treated with PEF or ribavirin on cells alone. The virus virions released to the supernatant were titered by the hemagglutinin (HA) test. Values are means ± S.D. from three independent experiments. The bars indicate standard deviations. Where there is no bar, there is no standard deviation.
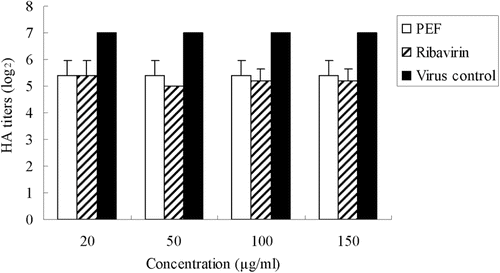
Discussion
Insects encounter a wide range of pathogenic organisms in their environment, of which viruses are but one component. More evidence revealed that antiviral activities were related to immune function in animals (CitationPierson et al., 2000; CitationZanetti, 2005). Probably as a consequence of their short lifespans, insects have never evolved an equivalent to the vertebrate antibody-based humoral defense system (CitationClarke & Clem, 2003). But they developed a special way to defend against viral infection. For example, silkworm, budworm and blow fly were verified to produce antiviral factors when they infected by virus (CitationChernysh et al., 2002; CitationPonnuvel et al., 2003; CitationOurth, 2004; CitationNakazawa et al., 2004; CitationPopham et al., 2004; CitationShelby & Popham, 2006; CitationYao et al., 2008). However, the antiviral factor of housefly remains practically untapped.
Housefly carries many kinds of viruses which are unhealthy for human and animal. However, there have been no reports that housefly larvae have any serious diseases, indicating they have a strong immune system (CitationWang et al., 2010). Moreover, the larvae of housefly have also been used to study immune-induced molecules because they can survive in a pathogenic environment. The antibacterial (CitationHao et al., 2008; CitationFu et al., 2009; CitationJin et al., 2010), antitumor (CitationHou et al., 2007; CitationCao et al., 2010b), antiproliferative (CitationCao et al., 2010a), burn wound-healing properties (CitationFeng et al., 2010) and anti-pro-inflammatory properties (CitationChu et al., 2011) from the larvae of housefly have already been characterized, but the antiviral component in the housefly is still unclear. In the present study, a protein enriched fraction was isolated from the larvae of the housefly and showed an excellent antiviral effect against human influenza virus H1N1 in vitro. These results would help us understand insect immune mechanisms against viral pathogens and promote the potential value of an antiviral substance from the larvae of housefly.
Ribavirin (1-β-d-ribofuranosy-l,2,4-triazole-3-carboxamide), discovered over 30 years ago, is the first synthetic, broad spectrum antiviral nucleoside which exerts antiviral activity against a wide range of DNA and RNA viruses including influenza viruses (CitationSnell, 2001). Ribavirin was used as positive control in the experiment and four different modified protocols were applied to investigate the antiviral activity of PEF and ribavirin against human influenza virus in vitro. When PEF treated before or during virus adsorption/fusion, the virus yields of the supernatant were decreased greatly. Ribavirin was performed in parallel under the same conditions and its antiviral effect is much lower than that of PEF. PEF did not show significant antiviral activity when treated after virus adsorption/fusion, whereas ribavirin exhibited its excellent antiviral effect under this condition. Ribavirin is phosphorylated by cellular adenosine kinase into ribavirin mono-, di-, and triphosphate (RMP, RDP and RTP, respectively) and exhibits its antiviral effect via direct and indirect mechanisms (CitationGu et al., 2006). RMP affects viral protein synthesis and limits replication of genome and RTP inhibits directly some viral RNA-dependent RNA polymerase activity (CitationGu et al., 2006). It acts at a stage in the influenza-infected cell, possibly by inhibiting the production of essential nucleotides and hence RNA synthesis. However, the dominant effect of PEF was direct virucidal activity and the interference on the adsorption of cell and virus. These results indicated that both PEF and ribavirin have an inhibitory effect on influenza virus, but their antiviral mechanism is different.
Influenza A virus has two major membrane associated surface glycoproteins, HA and neuraminidase (NA). It has been established that the influenza virus NA is a key target for antiviral intervention (CitationPalese & Compans, 1976). The enzymatic activity of viral NA is the target of a new class of anti-influenza drugs (CitationGubareva et al., 2002). Influenza virus HA binds to the sialic acid moiety of glycoproteins and glycolipids on cell-surface receptors, thereby initiating the process of attachment of the virus to a cell and subsequent infection (CitationHonda et al., 2002). Therefore, both HA and NA are attractive potential targets in the search for anti-influenza drugs. The present primary results demonstrated that the antiviral effect of PEF might be partially due to a direct interaction with virus particles or their entry into the cell. So the possible mechanism of PEF is to inhibit the enzymatic activity of viral NA or to destroy viral HA.
Conclusions
In this work, we demonstrated for the first time that a protein enriched fraction from the larvae of housefly has strong antiviral activity against influenza virus H1N1. In conclusion, the present results indicated that PEF has the potential as a natural ingredient for antiviral therapeutic drug. Also it is important to investigate the non-specific immune response mechanism of housefly against virus. In addition, studies on purification and antiviral activity of the PEF from larvae of housefly will be further examined and investigated. These studies are important in the utilization of housefly larvae and exploitation of their commercial value.
Declaration of interest
This work was supported by the Chinese National Natural Science Foundation (31172162) and National Higher-education Institution General Research and Development Funding (120002040214). There is no conflict of interest to report. The authors alone are responsible for the content and writing of the paper.
Refernces
- Ai H, Wang F, Lei C. (2008). Antioxidant activities of protein-enriched fraction from the larvae of housefly, Musca domestica. Nat Prod Res, 22, 507–515.
- Belaid A, Aouni M, Khelifa R, Trabelsi A, Jemmali M, Hani K. (2002). In vitro antiviral activity of dermaseptins against herpes simplex virus type 1. J Med Virol, 66, 229–234.
- Cao X, Huo Z, Lu M, Mao D, Zhao Q, Xu C, Wang C, Zeng B. (2010). Purification of lectin from larvae of the fly, Musca domestica, and in vitro anti-tumor activity in MCF-7 cells. J Insect Sci, 10, 164.
- Cao X, Sun Y, Wang C, Zeng B. (2010). Purification and characterization of a new d-galactose-specific lectin from the housefly, Musca domestica, and its antiproliferative effect on human K562 and MCF-7 tumor cells. J Insect Sci, 10, 79.
- Chernysh S, Kim SI, Bekker G, Pleskach VA, Filatova NA, Anikin VB, Platonov VG, Bulet P. (2002). Antiviral and antitumor peptides from insects. Proc Natl Acad Sci USA, 99, 12628–12632.
- Chu FJ, Jin XB, Zhu JY. (2011). Housefly maggots (Musca domestica) protein-enriched fraction/extracts (PE) inhibit lipopolysaccharide-induced atherosclerosis pro-inflammatory responses. J Atheroscler Thromb, 18, 282–290.
- Clarke TE, Clem RJ. (2003). Insect defenses against virus infection, the role of apoptosis. Int Rev Immunol, 22, 401–424.
- Feng X, Cheng G, Chen SY, Yang H, Huang W. (2010). Evaluation of the burn healing properties of oil extraction from housefly larva in mice. J Ethnopharmacol, 130, 586–592.
- Fu P, Wu J, Guo G. (2009). Purification and molecular identification of an antifungal peptide from the hemolymph of Musca domestica (housefly). Cell Mol Immunol, 6, 245–251.
- Gu CJ, Zheng CY, Zhang Q, Shi LL, Li Y, Qu SF. (2006). An antiviral mechanism investigated with ribavirin as an RNA virus mutagen for foot-and-mouth disease virus. J Biochem Mol Biol, 39, 9–15.
- Gubareva LV, Webster RG, Hayden FG. (2002). Detection of influenza virus resistance to neuraminidase inhibitors by an enzyme inhibition assay. Antiviral Res, 53, 47–61.
- Hao YJ, Jing YJ, Qu H, Li DS, Du RQ. (2008). Purification and characterization of a thermal stable antimicrobial protein from housefly larvae, Musca domestica, induced by ultrasonic wave. Acta Biol Hung, 59, 289–304.
- Honda T, Yoshida S, Arai M, Masuda T, Yamashita M. (2002). Synthesis and anti-influenza evaluation of polyvalent sialidase inhibitors bearing 4-guanidino-Neu5Ac2en derivatives. Bioorg Med Chem Lett, 12, 1929–1932.
- Hou L, Shi Y, Zhai P, Le G. (2007). Antibacterial activity and in vitro anti-tumor activity of the extract of the larvae of the housefly (Musca domestica). J Ethnopharmacol, 111, 227–231.
- Jin X, Mei H, Li X, Ma Y, Zeng AH, Wang Y, Lu X, Chu F, Wu Q, Zhu J. (2010). Apoptosis-inducing activity of the antimicrobial peptide cecropin of Musca domestica in human hepatocellular carcinoma cell line BEL-7402 and the possible mechanism. Acta Biochim Biophys Sin (Shanghai), 42, 259–265.
- Kimura K, Mori S, Tomita K, Ohno K, Takahashi K, Shigeta S, Terada M. (2000). Antiviral activity of NMSO3 against respiratory syncytial virus infection in vitro and in vivo. Antiviral Res, 47, 41–51.
- Nakazawa H, Tsuneishi E, Ponnuvel KM, Furukawa S, Asaoka A, Tanaka H, Ishibashi J, Yamakawa M. (2004). Antiviral activity of a serine protease from the digestive juice of Bombyx mori larvae against nucleopolyhedrovirus. Virology, 321, 154–162.
- Ourth DD. (2004). Antiviral activity against human immunodeficiency virus-1 in vitro by myristoylated-peptide from Heliothis virescens. Biochem Biophys Res Commun, 320, 190–196.
- Oxford JS. (1975). Inhibition of the replication of influenza A and B viruses by a nucleoside analogue (ribavirin). J Gen Virol, 28, 409–414.
- Palese P, Compans RW. (1976). Inhibition of influenza virus replication in tissue culture by 2-deoxy-2,3-dehydro-N-trifluoroacetylneuraminic acid (FANA), mechanism of action. J Gen Virol, 33, 159–163.
- Pierson T, McArthur J, Siliciano RF. (2000). Reservoirs for HIV-1: Mechanisms for viral persistence in the presence of antiviral immune responses and antiretroviral therapy. Annu Rev Immunol, 18, 665–708.
- Ponnuvel KM, Nakazawa H, Furukawa S, Asaoka A, Ishibashi J, Tanaka H, Yamakawa M. (2003). A lipase isolated from the silkworm Bombyx mori shows antiviral activity against nucleopolyhedrovirus. J Virol, 77, 10725–10729.
- Popham HJ, Shelby KS, Brandt SL, Coudron TA. (2004). Potent virucidal activity in larval Heliothis virescens plasma against Helicoverpa zea single capsid nucleopolyhedrovirus. J Gen Virol, 85, 2255–2261.
- Rimmelzwaan GF, Baars M, Claas EC, Osterhaus AD. (1998). Comparison of RNA hybridization, hemagglutination assay, titration of infectious virus and immunofluorescence as methods for monitoring influenza virus replication in vitro. J Virol Methods, 74, 57–66.
- Shelby KS, Popham HJ. (2006). Plasma phenoloxidase of the larval tobacco budworm, Heliothis virescens, is virucidal. J Insect Sci, 6, 1–12.
- Snell NJ. (2001). Ribavirin–current status of a broad spectrum antiviral agent. Expert Opin Pharmacother, 2, 1317–1324.
- Wanaratana S, Panyim S, Pakpinyo S. (2011). The potential of house flies to act as a vector of avian influenza subtype H5N1 under experimental conditions. Med Vet Entomol, 25, 58–63.
- Wang FR, Ai H, Lei CL, Huang W. (2006). Studies on the antiviral activity of the homogenate of Musca domestic larvae. Chinese Bull Entomol, 43, 82–85.
- Wang Y, Dang X, Zheng X, Wang J, Zhang W. (2010). Effect of extracted housefly pupae peptide mixture on chilled pork preservation. J Food Sci, 75, M383–M388.
- Yao H, He F, Guo A, Cao C, Lu X, Wu X. (2008). Gene analysis of an antiviral protein SP-2 from Chinese wild silkworm, Bombyx mandarina Moore and its bioactivity assay. Sci China, C, Life Sci, 51, 879–884.
- Zanetti M. (2005). T for two: when helpers need help. Autoimmun Rev, 4, 571–578.