Abstract
Context: Angiogenesis is an essential factor for cancer progression. Although more attention is paid in angiogenesis on its role in cancer biology, many other non-neoplastic diseases are also angiogenic-dependent. Recently, there is motivation to control cancer via inhibition of angiogenesis.
Objective: Quercus infectoria Olivier var (Fagaceae) (oak) is a plant whose different parts, such as its fruit shell, have been used extensively as a traditional drug in the west part of Iran. Although some biological properties of oak are determined, its effects on angiogenesis are unclear. So, we investigated the antiangiogenic effects of oak acorn shell.
Materials and methods: Fresh oak acorns were collected, and after authentication; hydroalcoholic extract of acorn shells (5, 10, 20, 30, 40, 60, 80, and 100 μg/ml) was used for evaluation of its cytotoxicity, antiproliferative, and antiangiogenic effects in vitro. Also, effects of the extract on vascular endothelial growth factor (VEGF), matrix metalloproteinase-2 (MMP-2) and MMP-9 secretion were assayed using enzyme-linked immunosorbent assay (ELISA) and gelatin zymography. Results: Treatment with hydroalcoholic extract in eight doses resulted in a significant decrease of endothelial cell proliferation and angiogenesis with an IC50 value of ~20 μg/ml, without any toxic effect. At 40 μg/ml, the extract inhibited MMP-9 activity; however, a dose-dependent reduction (60–80 µg/ml) in MMP-2 activity was seen. VEGF secretion was decreased with increase in the concentration of the extract from 5 to 100 μg/ml.
Discussion and conclusion: This study indicated that hydroalcoholic extract of oak acorn shell acts as a potent antiangiogenic agent which exerts its inhibitory effect mainly through downregulation of essential mediators such as VEGF and MMPs.
Introduction
Angiogenesis is formation of new capillaries from pre-existing vessels and plays a critical role in physiological processes such as development and wound healing (CitationFolkman, 1971) as well as pathologic processes (CitationBauer et al., 2005; CitationYancopoulos et al., 1998). In malignant diseases, angiogenesis helps rapid tumor growth and eventual metastasis (CitationMakrilia et al., 2009) by supplying oxygen, nutrition, and providing a fast way for tumor cells to reach the other parts of the body. Angiogenesis is regulated by the balance between variety of inducer and inhibitor factors of angiogenesis. Thus, decrease in the activity of proangiogenic factors and increase in the activity of antiangiogenic factors may provide a proper approach for managing various cancers (CitationCarmeliet, 2000; CitationRibatti, 2009; CitationRisau, 1997). Antiangiogenic therapy involves suppressing the angiogenic factors and promotion of antiangiogenic factors. Two of the most important angiogenic factors are vascular endothelial growth factor (VEGF) and matrix metalloproteinase (MMP). VEGF is one of the potent mediators of vascular permeability (CitationFerrara et al., 2003), whereas MMPs include 23 secreted or cell surface proteases which act together and with other protease classes to turn over the extracellular matrix (ECM) (CitationNewby, 2012). After identification of endostatin as an inhibitor of angiogenesis (CitationFolkman, 2006), a variety of antiangiogenic compounds such as withaferin, derived from Withania somnifera (L.) (Solanaceae) (CitationMohan et al., 2004), aqueous extract of shallot (CitationMohammadi et al., 2009), soybean trypsin inhibitor (CitationShakiba et al., 2007), green tea catechin (CitationPark et al., 2003; CitationTang et al., 2007) and a peptide from shark cartilage (CitationHassan et al., 2005) have been isolated from natural sources, which most of them suppress angiogenesis through inhibition of VEGF and MMPs. Beside synthetic drugs, drug development from natural products has become a promising strategy for identification of new antiangiogenic and antitumor compounds. Natural products contain a variety of complex organic chemicals and some derived compounds which may have synergistic activity for suppressing angiogenesis (CitationRamawat & Goyal, 2009). Thus, natural products with antiangiogenic properties can be effective in prevention of malignant tumors progression and metastasis. According to different experimental studies, antioxidants including phytochemicals can reduce the painful side-effects of radiotherapy and chemotherapy in cancer treatment, and inhibits tumor angiogenesis and metastatic growth, These compounds kill cancer cells selectively by apoptosis induction, while preventing apoptosis in normal cells, in vitro and in vivo (CitationBorek, 2004). Tannins and other polyphenols were reported to be very potent antioxidants (CitationKhennouf et al., 2003). Different parts of Quercus (Fagaceae) species such as nutgall, bark, leaf, fruit, and acorn shell have been used in folk remedy by the Iranian people as astringent, tonic, antiseptic, hemorrhage inhibitor, and a wound healing agent. Quercus infectoria Olivier var is known in the Persian and Kurdish vernacular as baloot and baroo, respectively. In the western part of Iran, oak acorn shell flour have been used traditionally for curing wounds. Different pharmaceutical applications of various species of Quercus such as wound healing effects (CitationUmachigi et al., 2008), free radical scavenging (CitationAlmeida et al., 2008), anti-inflammatory activity (CitationAroonrerk & Kamkaen, 2009), antibacterial property (CitationBasri & Fan, 2004; CitationGüllüce et al., 2004; CitationVoravuthicunchia et al., 2006), antifungal activity (CitationGüllüce et al., 2004; CitationSoon et al., 2007), antiviral and larvicidal potentials, being as an astringent, antidiabetic and antitermorine agent and as a local anesthetic, have been investigated (CitationSoon et al., 2007), but there is no report in the literature concerning the antiangiogenic effects of Quercus. The aim of the present study was to examine the direct effects of hydroalcoholic extract of oak acorn shell on migration of endothelial cells (ECs) for formation of capillaries and to elucidate the extract inhibitory effects on VEGF and MMPs as critical mediators of angiogenesis.
Materials and methods
Materials
Dulbecco’s minimum essential medium (DMEM), 10× minimum essential medium (10× MEM) were supplied from Gibco Invitrogen (Carlsbad, CA, USA). Fetal bovine serum (FBS) was from Gibco (Grand Island, NY, USA). Rat tail collagen type I (2 mg/ml in 0.5 M acetic acid) was from Sigma (St Louis, MO, USA). Enzyme-linked immunosorbent assay (ELISA) kit for assay of VEGF was supplied from R&D Systems (Minneapolis, MN, USA). Acetic acid and ethanol were obtained from Merck (Darmstadt, Germany). Dextran-coated cytodex 3-microcarriers were from Amersham Pharmasia Biotech (Piscataway, NJ, USA). Lactate dehydrogenase (LDH) and cytotoxicity assay kits were purchased from Roche Diagnostics (Mannheim, Germany). Human umbilical vein endothelial cells (HUVEC) obtained from the National Cell Bank (Pasteur Institute, Iran). Sterile double distilled water was used to prepare all solutions.
Plant materials
Fresh oak (Q. infectoria) acorn shells were collected from Kermanshah province of Iran in March 2010 and authenticated by Dr. S.M. Maassoumi (voucher number 1135) in the Faculty of Agriculture, Razi University (Kermanshah, Iran).
Preparation of hydroalcoholic extract of oak acorn shell
Fresh acorn shells were fragmented and dried at room temperature (25 ± 2°C) in the shade for 7 days. Dried materials were ground to powder and stored in closed containers. Hydroalcoholic extract was obtained by dissolving 4 g of raw material in 100 ml of 50% (vol/vol) ethanol and shaken for 48 h at 4°C. The mixture was filtered and the clear filtrate was concentrated, vacuum dried and kept at –20°C.
Cytotoxicity assay
To determine maximum nontoxic and cytotoxic concentrations of hydroalcoholic extract of oak acorn shell, several concentrations of extract were prepared and added to medium containing confluent HUVEC cell line. For this purpose, cells were cultured in 96-well culture plates at a density of 1 × 104 cells/well in DMEM supplemented with 2% FBS and incubated at 37°C and 5% CO2 for 24 h. Thereafter, different concentrations of hydroalcoholic extract (5, 10, 20, 40, 80, 160, 320, and 640 μg/ml) were added to the wells and the plates were incubated for additional 72 h. To obtain cell-free medium, the culture medium was centrifuged at 1500g for 5 min at 4°C. Afterwards, 100 µl of supernatant of the wells were transferred to a new 96-well culture plate and LDH release was assessed by LDH Kit at 490 nm with background subtraction at 630 nm, according to the manufacturer instruction. The LD50 value, which represents the concentration of extract causing 50% death in the cells, was calculated.
Antiproliferative assay
The antiproliferative effect of the hydroalcoholic extract of oak acorn shell on HUVECs was assessed in medium supplemented with 10% FBS. Exponentially growing cells were seeded in round-bottomed 25 cm3 flasks and allowed to attach overnight. HUVECs were cultured for 24 h at 37°C and 5% CO2. The cells were divided into control and test groups, and then cultured in 24-well culture plate at a density of 5 × 104 cells/well in DMEM supplemented with 10% FBS, 100 U/ml penicillin, and 100 µg/ml streptomycin. Test group of cells were incubated in medium supplemented with increasing concentrations of hydroalcoholic extract of oak acorn shell, (5, 10, 20, 30, 40, 60, 80, and 100 μg/ml) for an additional 72 h. Thereafter, the cells were harvested by trypsinization and counted with a Coulter Counter (KX-21 Sysmex Co.) against control wells. This assay was carried out in triplicate for each fraction. The IC50 value, which represents the concentration of extract causing 50% inhibition in cell proliferation, was calculated.
Wound repair assay by ECs
Cell migration was evaluated by a wound repair assay. HUVECs were cultured in 24-well culture plates. When the cells were confluent, identical straight strip wounds were created using sterile plastic yellow tips (scratch test). Then the cells were washed with phosphate-buffered saline (PBS) and incubated with DMEM containing 2% FBS (FBS concentration which allows cell survival with no cell proliferation) and different concentrations of hydroalcoholic extract of oak acorn shell (5, 10, 20, 30, 40, 60, 80, and 100 μg/ml). After 24-h of incubation, the HUVECs were washed twice with PBS and fixed in 4% paraformaldehyde (in PBS) at room temperature. Following cell staining with Giemsa, the cells were photographed using a camera connected to an inverted microscope at 10× magnifications.
Preparation of collagen gels
For collagen gel formation, seven volumes of cold type I collagen solution with one volume of 10× minimal essential medium and two volume of sodium bicarbonate solution (11.76 mg/ml) were mixed in a sterile flask, kept on ice to prevent immediate gelation.
HUVEC capillary tube formation in collagen matrix and evaluation of angiogenesis in vitro
HUVECs which were grown in DMEM supplemented with 10% FBS at 37°C and 5% CO2 were used after 3–5 passages for this experiment. Afterwards, the cells were mixed with sterilized cytodex-3 microcarriers coated beads with gelatin at a ratio of 30 cells per bead in 1 ml of DMEM medium (CitationAuerbach et al., 2003) supplemented with 10% heat-inactivated FBS. The mixture was shaken gently every 20 min for 4 h at 37°C and 5% CO2. Thereafter, the mixture was transferred to a 24-well tissue culture plate and left for 12–16 h in 1 ml of DMEM at 37°C and 5% CO2. On the following day, beads with cells were resuspended in type 1 collagen gel and 50 μl of collagen/bead mixture was added to each well of a 96-well tissue culture plate and allowed to clot for 20 min at 37°C, 5% CO2. Then, 250 μl of DMEM medium was added to each well. In order to study the antiangiogenic effect of the hydroalcoholic extract of oak acorn shell, different concentrations of the extract (5, 10, 20, 30, 40, 60, 80, and 100 μg/ml) were added to the wells. After 3–5 days of treatment, the anti-tubulogenesis effect of hydroalcoholic extract of oak acorn shell was monitored microscopically. All the angiogenic cells and capillary-like structures were photographed using a digital camera.
Gelatin zymography
The effect of hydroalcoholic extract of oak acorn shell on the enzymatic activities of MMP-2 and MMP-9 was assessed using gelatin zymography. Confluent HUVECs were isolated and immediately incubated in the absence of serum (FBS) with different concentrations of the extract for 16 h. The protein content of the serum-free supernatant media from HUVECs which were treated with hydroalcoholic extract was measured according to the method of Bradford using bovine serum albumin as the standard. Then, the supernatants of the treated and control wells were mixed with sample buffer and loaded onto a 7.5% polyacrylamide gel containing 1% SDS and 1 mg/ml gelatin under nonreducing condition. After electrophoresis, gels were washed twice with washing buffer containing 2.5% Triton X-100 for 1 h at room temperature (to remove SDS) followed by a brief rinsing in washing buffer without Triton X-100. Then the gels were incubated at 37°C for 24 h in substrate buffer containing 25 mM Tris, pH 7.5 and 5 mM CaCl2 for the development of enzyme activity bands. Afterwards, the gels were stained with coomassie brilliant blue R-250 in 50% methanol and 10% glacial acetic acid for 30 min and destained subsequently in a 4% methanol and 8% acetic acid destaining solution. The gelatinolytic activity of MMPS was detected as bright clear bands against the background of coomassie brilliant blue stained gelatin.
Determination of VEGF level with ELISA
The HUVEC cell line in logarithmic growth phase was cultured in 25 cm3 flasks at the density of 2 × 105 in DMEM medium supplemented with 10% FBS for 24 h. For the measurement of VEGF secretion, the cells were treated with different concentrations of hydroalcoholic extract of oak acorn shell in serum-free medium for another 24 h. Cell-free culture supernatants were collected and VEGF concentrations were assessed using a quantitative ELISA kit (R&D Systems), according to the manufacturer’s instructions.
Statistical analysis
The data was analyzed by SPSS (version 16) and the significance of values was determined with the two-tailed Student’s t-test and the levels of p ≤ 0.05 were used as a criterion of the statistical significance. All results are expressed as the mean ± SD. The p ≤ 0.05 are considered statistically significant.
Results
To examine the direct effect of oak acorn shell hydroalcoholic extract on vascular formation by ECs, the effect of the extract on cell viability and each step of the angiogenesis process including EC proliferation, migration, and tubulogenesis were examined.
Effect of hydroalcoholic extract of oak acorn shell on EC proliferation
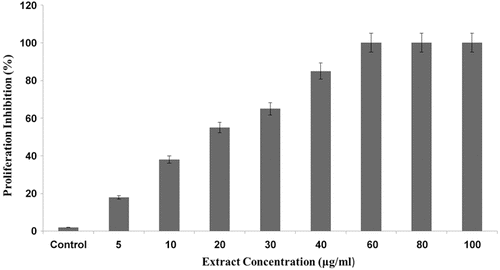
In order to investigate the antiangiogenic behavior of the hydroalcoholic extract of oak acorn shell, normal HUVECs were incubated with the extract in a concentration range of 5, 10, 20, 30, 40, 60, 80, and 100 μg/ml, below its cytotoxic concentration (150 μg/ml). As indicated in , the extract strongly inhibits EC growth proliferation, in a dose-dependent manner, with a half-maximal inhibition (IC50) of ~20 μg/ml. Furthermore, significant inhibition of proliferation was observed when the extract was administered at the concentration of 40 μg/ml. To determine whether or not the reduced cell growth was due to cell death, a LDH assay was performed (data not shown). The LDH release from hydroalcoholic extract-treated cells was increased slightly compared to untreated control cells, confirming the specific inhibitory effects of hydroalcoholic extract on EC proliferation without affecting cell viability. However, increasing concentration of hydroalcoholic extract >40–100 μg/ml had no more inhibitory effect.
Effect of hydroalcoholic extract of oak acorn shell on EC migration
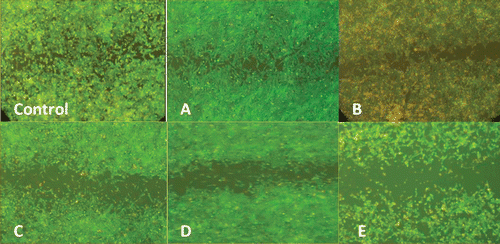
Migration of ECs represents another critical step in angiogenesis, which allows cells to separate from pre-existing vessels and to form new ones. Therefore, the effect of hydroalcoholic extract on the extent of HUVEC motility was investigated using in vitro wound healing assay. As shown in , in the absence of the extract, (untreated control) HUVECs migrated to the center of initial wound (gap zone) and made that mostly filled at 18 h. When HUVECs were treated with increasing concentrations of the hydroalcoholic extract (5, 10, 20, 30, 40, 60, 80, and 100 μg/ml), the width of wound area increased concentration-dependently suggesting that the extract potently inhibits the ability of ECs to migrate (from the concentration of 10 μg/ml) without considerable toxic effects.
Figure 2. The inhibitory effect of hydroalcoholic extract of oak acorn shell on the human umbilical vein endothelial cells (HUVECs) migration. These pictures (×10 magnification) show the effect of various concentrations of hydroalcoholic extract of oak acorn shell (0; control, A: 5 μg/ml; B: 10 μg/ml; C: 20 μg/ml; D: 30 μg/ml; and E: 40–100 μg/ml) of the extract on endothelial cell (EC) migration in the wound-healing model after 48 h.
Effect of hydroalcoholic extract of oak acorn shell on capillary tube formation
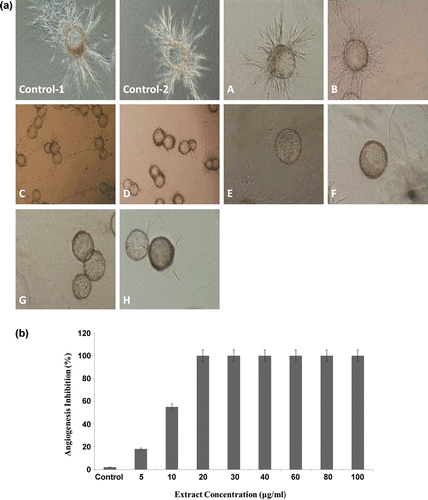
The effect of the hydroalcoholic extract of oak acorn shell on the morphological differentiation of ECs into capillary-like structures and their ability for tube formation was investigated. After 3 days of culture in a collagen matrix, untreated control wells showed a branching pattern of capillary tube structures. As shown in , the hydroalcoholic extract of oak acorn shell at concentration of 5 μg/ml showed partial antiangiogenic effects, but at higher doses it perfectly prevented the formation of endothelial tubular structures and inhibited branching of ECs from Cytodex microcarriers. Totally, the hydroalcoholic extract of oak acorn shell showed a strong anti-tubulogenesis effect, in which tubulogenesis was completely inhibited at the concentration of 20 μg/ml (,).
Figure 3. (a) The inhibitory effect of hydroalcoholic extract on in vitro endothelial cell (EC) tube formation in the collagen gel. Spontaneous formation of capillary-like structures by human umbilical vein endothelial cells (HUVECs) on “dextran-coated cytodex microcarriers” was used to assess antiangiogenic potential of hydroalcoholic extract of oak acorn shell. Angiogenesis of ECs in the untreated wells (control–1, 2). The EC attached to particles has been migrated through the collagen matrix. (A–H) Inhibition of angiogenesis of the ECs treated by different (5, 10, 20, 30, 40, 60, 80, and 100 µg/ml) concentrations of the extract. After the cells were treated with the extract for the indicated time, the cells were photographed. Pictures are representative example of three independent experiments (×10 magnification). (b) Hydroalcoholic extract of oak acorn shell inhibited angiogenesis on three-dimensional model of human umbilical ECs.
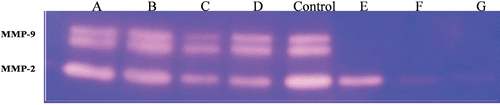
Effect of hydroalcoholic extract of oak acorn shell on MMP-2 and MMP-9 gelatinolytic activity
Since MMPs secreted by EC play a key role in the process of ECM remodeling and ECs tube formation, the effect of the extract on the activity as well as the level of MMPs were examined. In order to study the effect of hydroalcoholic extract on MMP-2 and MMP-9 secretions, equal volumes of conditioned media of serum-starved HUVECS were treated with hydroalcoholic extract and their controls were subjected to gelatin zymography. The zymogram showed major bands with gelatinolytic activity corresponding to MMP-2 and MMP-9. According to , considerable decrease in MMP-2 and MMP-9 secretion in test groups in comparison to control group is evident. Hydroalcoholic extract at the concentration of 40 μg/ml inhibited MMP-9 activity completely. However, a dose-dependent reduction in MMP-2 activity was seen ().
Figure 4. Gelatin zymography assay of extracellular matrix metalloproteinase-9 (MMP-9) and MMP-2 expression of human umbilical vein endothelial cells (HUVECs) treated with increasing concentrations of hydroalcoholic extract 5 µg/ml (lane A), 10 μg/ml (lane B), 20 μg/ml (lane C), 30 μg/ml (lane D), control (lane Control), 40 μg/ml (lane E), 60 μg/ml (lane F), 80 μg/ml (lane G). MMP-9 was inhibited by concentrations between 40 and 80 µg/ml, see lane E–F, the inhibition of MMP-2 is shown with an excess amount (60–80 µg/ml, lane F–G) of it. Data shown are the representative zymography from three independent experiments.
Effect of hydroalcoholic extract of oak acorn shell on VEGF
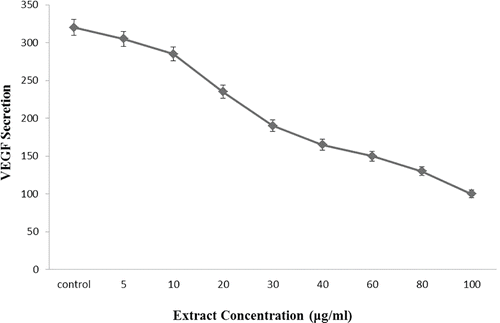
Tumor angiogenesis is controlled by positive and negative modulators produced by cancer, stromal (including ECs, smooth muscle cells, and fibroblasts) and infiltrating cells. Many of these modulators are polypeptide growth factors such as fibroblast growth factor-2 (FGF-2 or basic FGF) and VEGF. Indeed, VEGF which is the most critical angiogenic promoter, initiates EC proliferation, elongation and reorientation, transforming their morphology to the highly ordered, elongated phenotype of cell lining the inner surface of blood vessels. Therefore, we examined the inhibitory effects of hydroalcoholic extract on the VEGF secretion in HUVEC. As indicated in , a VEGF-specific ELISA assay demonstrated that the treatment of HUVEC with hydroalcoholic extract of oak acorn shell decreased the VEGF secretion. This reduction was significant in a dose-dependent manner.
Discussion
Nowadays, many studies have focused on plants, their extracts and the natural compounds which may have fewer side-effects than synthetic compounds for treatment of diseases (CitationHeber, 2004). There is also increasing evidence that the chemopreventive properties of fruits and vegetables originates from the additive and synergistic effects of several phytochemicals present in these foods (CitationHerbst, 2006). Various plant species have compounds that can be used for prevention and treatment of various diseases such as cancers or prevention of cancer angiogenesis (CitationLiu, 2008), and also for treatment of some other angiogenic-dependent diseases such as corneal neovascularization (CitationOner et al., 2007) psoriasis, arthritis, and macular degeneration.
Angiogenesis is an essential process for rapid tumor growth because it supplies enough nutrition and oxygen for tumors and also has a key role in tumor metastasis (CitationMakrilia et al., 2009). Since proliferation, growth and progression of most tumors are promoted by angiogenesis, malignant tumor promotion, expansion and metastasis are not achievable without an adequate blood supply. This process can be a good target for antiangiogenic compounds beside conventional therapies such as surgery, chemotherapy and radiotherapy.
Some therapeutic and biological effects of Q. infectoria were examined previously (CitationAlmeida et al., 2008; CitationAroonrerk & Kamkaen, 2009; CitationBasri & Fan, 2004; CitationBhat et al., 1998; CitationBorek, 2004; CitationGüllüce et al., 2004; CitationPithayanukul et al., 2009; CitationSoon et al., 2007; CitationUmachigi et al., 2008; CitationVoravuthicunchia et al., 2006), but until now, antiangiogenic properties of different parts of this plant have remained unknown.
Quercus species have been used for medicinal purposes for many centuries as traditional medicines through the world. Q. infectoria has been used traditionally as a part of postpartum care by Iranians, Arabs, Indians, Malays, and Chinese after child birth to treat vaginal discharge and related postpartum infections (CitationSoon et al., 2007). In Iranian traditional medicine, different parts of Quercus species (bark, leaf, nutgall, fruit, and acorn shell) have been used as astringent, tonic, antiseptic, hemorrhage inhibitor, wound healing agent and for treating cancerous ulcers, impetigo, tonsillitis, tuberculosis, edema, diarrhea, dermal diseases such as eczema, and some other diseases. Also, in Algerian folk medicine, Quercus ilex L. (leaves, roots, and bark) has been used to treat gastritis, gastric ulcers and the tannins of this plant are thought to be responsible for the medicinal effects (CitationKhennouf et al., 2003). Oral administration of 70% acetone extracts of Quercus suber L. and Quercus coccifera L. leaves to mice, prevented the formation of ethanol-induced lesions in the stomach, and it is believed that tannin constituents of these plants is responsible for their effect (CitationKhennouf et al., 2003). Hydrolysable tannins from nutgalls of Quercus ilex are implied to be the major contributors in the prevention of free-radical-mediated disorders including inflammation and hepatotoxicity (CitationPithayanukul et al., 2009). Furthermore, antimicrobial activity of ethanol extract of Quercus ilex, mainly against Gram negative bacteria and Candida albicans, and the anti-Helicobacter pylori activity of the crude and semi-purified extracts of Q. infectoria nutgalls demonstrated the antimicrobial contents of Quercus species (CitationGüllüce et al., 2004; CitationSoon et al., 2007). Other research showed a positive effect of ethanol extract of Q. infectoria nutgalls on wound healing by increasing the levels of superoxide dismutase and catalase enzymes of the body which quench the superoxide radicals in rats (CitationUmachigi et al., 2008). In addition, inhibitory effects of Q. infectoria nutgalls on mitogene-activating protein kinase (MAPK), interleukin-1β (IL-1β) and COX-2 expression, prostaglandin E2 (PGE2), anti-IL-6, and anti-PGE2 effects, anti-inflammatory activity has been demonstrated (CitationAroonrerk & Kamkaen, 2009). Since the hydroalcoholic solubility (and bioavailability) of plant extracts can be considered as an advantage for their effective antiangiogenic or antitumor potential, we used the hydroalcoholic extract of oak acorn shell in our research. In the present study, it has been shown that hydroalcoholic extract of acorn shell of Q. infectoria is an effective inhibitor of angiogenesis in vitro. Angiogenesis process includes degradation of basement membrane and surrounding ECM, proliferation, migration, and tubulogenesis of ECs, which depends on proangiogenic factors like VEGF and matrix degrading enzymes like MMP-2 and MMP-9. Cytotoxic effect of hydroalcoholic extract was tested in vitro and below the lethal concentration (150 μg/ml) antiangiogenic activity of the extract was examined on HUVECs in the 3-microcarrier collagen-cytodex model. It is shown that the minimal concentration of the hydroalcoholic extract was needed for complete inhibition of angiogenesis with no toxic effects on the cells is 20 μg/ml. One of the early stages in angiogenesis is degradation of basement membrane and surrounding ECM which afterwards results in proliferation, migration, and tubulogenesis of ECs. This stage depends on proangiogenic factors like VEGF and matrix degrading enzymes like MMP-2 and MMP-9 (CitationVu & Werb, 2000). Expression of MMP-2 and MMP-9 are strongly associated with invasion and metastasis in cancers (CitationMendes et al., 2005; CitationZheng et al., 2006). Our results indicated that the hydroalcoholic extract of oak acorn shell decreases expression of both MMP-2 and MMP-9 with a stronger inhibitory effect on MMP-9 expression at the concentration of 40 μg/ml, whereas MMP-2 expression was inhibited at 60 μg/ml. Decline in MMP-2 and MMP-9 expression by hydroalcoholic extract of oak acorn shell, inhibits ECs to degrade the surrounding ECM and therefore prevents the subsequent migration and proliferation and finally inhibits formation of new vessels. VEGF is a potent mitotic and migratory factor for ECs and also has cellular effects on both the ECs proliferation and behavior. VEGFs are expressed and secreted by ECs (autocrine) and many tumor cells. They contribute to tumor expansion associated with neovascularization. Based on these facts; there is this possibility that the inhibition of proliferation, migration and tubulogenesis of ECs in collagen matrix is a result, in part, of the reducing of VEGF secretion from ECs as well as tumor cells. In our study, it is shown that VEGF secretion was inhibited in a dose-dependent manner. Therefore, we suggest that hydroalcoholic extract of oak acorn shell plant has antiangiogenic and antitumor effects by inhibiting proliferation and migration and also reducing MMP-2 and MMP-9 gene expression, besides prevention of VEGF secretion.
Conclusion
Although properties like antibacterial (CitationBasri & Fan, 2004), wound healing (CitationUmachigi et al., 2008), anti-inflammatory (CitationAroonrerk & Kamkaen, 2009), and others of Q. infectoria had been investigated previously, there is no report in the literature concerning the possible antiangiogenesis properties of oak acorn shell. The results of the present study revealed that the hydroalcoholic extract of oak acorn shell inhibits angiogenesis through the prevention of VEGF and MMP-2 and MMP-9 secretion, besides inhibition of ECs proliferation and migration. Although more in vivo and in vitro studies in animal models and lab based angiogenic systems are needed to clarify the curative and preventive role of oak acorn shell extract in cancer onset and progression, these results can be considered as a starting point for better evaluation of antiangiogenesis and anticancer potential of Quercus.
Acknowledgments
The authors thank colleagues and technicians in Medical Biology Research Center of Kermanshah University of Medical Sciences for their valuable help, especially Professor Ali Mostafaie for his valuable scientific help and Dr. Sirous Ghobadi for reviewing this article.
Declaration of interest
The authors appreciate the financial support of this work by the Research Council of Kermanshah University of Medical Sciences, Kermanshah, Iran. The authors report no conflict of interest.
References
- Almeida IF, Fernandes E, Lima JL, Costa PC, Bahia MF. (2008). Protective effect of Castanea sativa and Quercus robur leaf extracts against oxygen and nitrogen reactive species. J Photochem Photobiol B, Biol, 91, 87–95.
- Aroonrerk N, Kamkaen N. (2009). Anti-inflamatory activity of Quercus infectoria, Glycyrrhiza uralensis, Kaemperia galanga and Coptis chinensis, the main components of thai herbal remedies for aphthous ulcer. J Health Res, 23, 17–22.
- Auerbach R, Lewis R, Shinners B, Kubai L, Akhtar N. (2003). Angiogenesis assays: a critical overview. Clin Chem, 49, 32–40.
- Basri DF, Fan SH. (2004). The potential of aqueous and acetone extracts of galls of Quercus infectoria as antibacterial agents. Indian J Pharmacol, 37, 26–29.
- Bauer SM, Bauer RJ, Velazquez OC. (2005). Angiogenesis, vasculogenesis, and induction of healing in chronic wounds. Vasc Endovascular Surg, 39, 293–306.
- Bhat TK, Singh B, Sharma OP. (1998). Microbial degradation of tannins–a current perspective. Biodegradation, 9, 343–357.
- Borek C. (2004). Dietary antioxidants and human cancer. Integr Cancer Ther, 3, 333–341.
- Carmeliet P. (2000). Mechanisms of angiogenesis and arteriogenesis. Nat Med, 6, 389–395.
- Ferrara N, Gerber HP, LeCouter J. (2003). The biology of VEGF and its receptors. Nat Med, 9, 669–676.
- Folkman J. (1971). Tumor angiogenesis: Therapeutic implications. N Engl J Med, 285, 1182–1186.
- Folkman J. (2006). Angiogenesis. Annu Rev Med, 57, 1–18.
- Güllüce M, Adigüzel A, Ogütçü H, Sengül M, Karaman I, Sahin F. (2004). Antimicrobial effects of Quercus ilex L. extract. Phytother Res, 18, 208–211.
- Hassan ZM, Feyzi R, Sheikhian A, Bargahi A, Mostafaie A, Mansouri K, Shahrokhi S, Ghazanfari T, Shahabi S. (2005). Low molecular weight fraction of shark cartilage can modulate immune responses and abolish angiogenesis. Int Immunopharmacol, 5, 961–970.
- Heber D. (2004). Vegetables, fruits and phytoestrogens in the prevention of diseases. J Postgrad Med, 50, 145–149.
- Herbst RS. (2006). Therapeutic options to target angiogenesis in human malignancies. Expert Opin Emerg Drugs, 11, 635–650.
- Khennouf S, Benabdallah H, Gharzouli K, Amira S, Ito H, Kim TH, Yoshida T, Gharzouli A. (2003). Effect of tannins from Quercus suber and Quercus coccifera leaves on ethanol-induced gastric lesions in mice. J Agric Food Chem, 51, 1469–1473.
- Liu DW. (2008). [Mass casualty critical care for surgical patients during a disaster]. Zhonghua Wai Ke Za Zhi, 46, 1843–1844.
- Makrilia N, Lappa T, Xyla V, Nikolaidis I, Syrigos K. (2009). The role of angiogenesis in solid tumours: An overview. Eur J Intern Med, 20, 663–671.
- Mendes O, Kim HT, Stoica G. (2005). Expression of MMP2, MMP9 and MMP3 in breast cancer brain metastasis in a rat model. Clin Exp Metastasis, 22, 237–246.
- Mohammadi K, Motlagh HR, Mansouri Shakiba Y, Keshavarz M, Khodarahmi R, Siami A. (2009). Anti-angiogenic effect of aqueous extract of shallot (Allium ascalonicum) bulbs in rat aorta ring model. Yakhteh Med J, 11, 190–195.
- Mohan R, Hammers HJ, Bargagna-Mohan P, Zhan XH, Herbstritt CJ, Ruiz A, Zhang L, Hanson AD, Conner BP, Rougas J, Pribluda VS. (2004). Withaferin A is a potent inhibitor of angiogenesis. Angiogenesis, 7, 115–122.
- Newby AC. (2012). Matrix metalloproteinase inhibition therapy for vascular diseases. Vascul Pharmacol, 56, 232–244.
- Oner FH, Bezerra Y, Peyman GA, Conway MD, Lewis JM, Liu Z, Greenway F, Woltering EA. (2007). Antiangiogenic effect of a Chinese sweet leaf tea extract in experimental corneal neovascularization. Pharm Biol, 45, 44–47.
- Park EH, Joo MH, Kim SH, Lim CJ. (2003). Antiangiogenic activity of Gardenia jasminoides fruit. Phytother Res, 17, 961–962.
- Pithayanukul P, Nithitanakool S, Bavovada R. (2009). Hepatoprotective potential of extracts from seeds of Areca catechu and nutgalls of Quercus infectoria. Molecules, 14, 4987–5000.
- Ramawat KG, Goyal S. (2009)). Natural products in cancer chemoprevention and chemotherapy. In:Ramawat KG, ed. Herbal Drugs: Ethnomedicine to Modern Medicine. Berlin; Heidelberg:Springer, 153–172.
- Ribatti D. (2009). Endogenous inhibitors of angiogenesis: A historical review. Leuk Res, 33, 638–644.
- Risau W. (1997). Mechanisms of angiogenesis. Nature, 386, 671–674.
- Shakiba Y, Mansouri K, Mostafaie A. (2007). Anti-angiogenic effect of soybean kunitz trypsin inhibitor on human umbilical vein endothelial cells. Fitoterapia, 78, 587–589.
- Soon LK, Hasni E, Law KS, Walliullah SS, Fardi CG, Syed Mohsin SSJ. (2007). Ultrastructural findings and elemental analysis of Quercus infectoria Olive. Ann Microscopy, 7, 32–37.
- Tang FY, Chiang EP, Shih CJ. (2007). Green tea catechin inhibits ephrin-A1-mediated cell migration and angiogenesis of human umbilical vein endothelial cells. J Nutr Biochem, 18, 391–399.
- Umachigi SP, Jayaveera KN, Ashok Kumar CK, Kumar GS, Vrushabendra Swamey BM, Kishore Kumar DV. (2008). Studies on wound healing properties of Quercus infectoria. J Pharm Res, 7, 913–919.
- Voravuthicunchia SP, Limsuwan S, Michell H. (2006). Effects of Punica granatum pericarpus and Quercus infectoria nutgalls on cell surface hydrophobicity and cell survival of Helicobacter pylori. Health Sci, 52, 154–159.
- Vu TH, Werb Z. (2000). Matrix metalloproteinases: Effectors of development and normal physiology. Genes Dev, 14, 2123–2133.
- Yancopoulos GD, Klagsbrun M, Folkman J. (1998). Vasculogenesis, angiogenesis, and growth factors: Ephrins enter the fray at the border. Cell, 93, 661–664.
- Zheng H, Takahashi H, Murai Y, Cui Z, Nomoto K, Niwa H, Tsuneyama K, Takano Y. (2006). Expressions of MMP-2, MMP-9 and VEGF are closely linked to growth, invasion, metastasis and angiogenesis of gastric carcinoma. Anticancer Res, 26, 3579–3583.