Abstract
Context: Euphorbia hirta L. (Euphorbiaceae) (E. hirta) is a tree locally used as a traditional medicine in Africa and Australia to treat numerous diseases such as hypertension, respiratory ailments, tumors, and wounds, and it has reported antiallergic, antipyretic, anti-inflammatory activities, etc.
Objective: This study evaluated the ability of fresh leaves of E. hirta ethanol extract to inhibit the intracellular tumor necrosis factor α (TNF-α) level in the synovial fluid and neutrophils in lipopolysaccharide (LPS)-induced inflamed rat knees.
Materials and methods: Female Wister albino rats 140–160 g were used. E. hirta ethanol extract was given orally at 25, 50, 100, and 200 mg/kg, 2 h before an intra-articular (i.a.) injection of LPS. Two and three hours later, synovial fluid and neutrophils levels of intracellular TNF-α production were measured.
Results: In the time course of the experiment, E. hirta maximum inhibition at 100 and 200 mg/kg (p.o.) dose showed 16.5 ± 1.34 and 14.4 ± 1.30% of synovial fluid, 4.26 ± 0.36 and 3.78 ± 0.29% of neutrophils levels of intracellular TNF-α productions at 2 h after LPS injection. LPS control displayed 22.97 ± 1.61 and 6.78 ± 0.34% of synovial fluid and neutrophils levels of intracellular TNF-α at 2 h after LPS injection. Intracellular TNF-α was also estimated at 3 h after LPS injection.
Discussion and conclusion: The LPS-injected rat knee model gives a comparative study of acute anti-inflammatory responses. E. hirta inhibition of proinflammatory intracellular cytokine TNF-α production with LPS-induced inflamed rat knee is of great importance in defining the anti-arthritic potential of E. hirta.
Introduction
Euphorbia hirta L. (Euphorbiaceae) (common name Euphorbia) is a small annual herb common to tropical countries (CitationSoforowa, 1982). The stem and leaves produce white or milky juice (CitationLind & Tallantire, 1971). In East and West Africa, extracts of the plant are used in treatment of asthma and respiratory tract inflammation (CitationKokwaro, 1993). It is also used for coughs, chronic bronchitis, and other pulmonary disorders in Malagasy (CitationWong Ting Fook, 1980). E. hirta is known to have antiallergic, antipyretic, anti-inflammatory properties (CitationLanhers et al., 1991; CitationSingh et al., 2006; CitationShih et al., 2010). In addition, low dosages of E. hirta seem to be beneficial in reducing cartilage degeneration and expressions of matrix metalloproteinase in arthritic rats (CitationLee et al., 2008). In Nigeria, extracts or exudates of the plant are used as ear drops and in the treatment of boils, sore and wound healing promotion (CitationIgoli et al., 2005; CitationAnononymous, 2005).
Rheumatoid arthritis (RA) is an autoimmune disease characterized by chronic inflammation of synovial membranes and proliferation of the synovial lining, leading to cartilage damage and ultimately joint destruction (CitationGeorganas et al., 2000). Proinflammatory cytokines, especially tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), are thought to play a critical role in the pathobiologic properties of RA, inducing and propagating a chronic inflammatory process in the synovial membrane (CitationIvashkiv, 1996; CitationBrennan et al., 1998; CitationWong et al., 2003). These cytokines and their receptors are over expressed in synovial tissue of RA patients compared with that of patients with degenerative joint disease (CitationWendling et al., 1993; CitationMatsuno et al., 2002; CitationChoy et al., 2002; CitationNakahara & Nishimoto, 2006). These cytokines play an important role in attracting and activating inflammatory cells and in the degradation of cartilage and bone. In arthritis, pain and tissue destruction are associated with elevated synovial levels of TNF-α (CitationWatkins et al., 1994; CitationBrennan et al., 1998; CitationNordahl et al., 2000; CitationAlstergren & Kopp, 2006). TNF-α mRNA is unregulated in monoarthritis cartilage as compared with normal cartilage (CitationAmin, 1999). Consequently, several publications have focused on the synovial fluid levels of various inflammatory cytokines in patients with RA, psoriatic arthritis, and ankylosing spondylitis, with the clinical utility of such measurements being advocated (CitationAmital et al., 2007; CitationRomero et al., 2008).
Neutrophils are the most abundant cells present in the synovial fluid of the affected joints and they are also abundant at the pannus/cartilage interface, where most tissue damage occurs (CitationMohr et al., 1981). Neutrophils isolated from RA joints show an activated (primed) phenotype indicating that they have undergone an activation process and have likely released important inflammatory mediators Furthermore, during acute and chronic joint inflammation such as RA, T cells interact with monocytes and macrophages to generate abundant proinflammatory cytokines, and B cells play multiple pathologic roles both in antibody-dependent and antibody-independent ways (CitationLeandro, 2009; CitationMarston et al., 2010).
Materials and methods
Extraction of test material
Fresh leaves of E. hirta were obtained from the botanical garden of Indian Institute of Integrative Medicine formerly known as Regional Research Laboratory (CSIR), Jammu and Kashmir, India, correctly identified and authenticated by routine pharmacognostic procedures. A voucher sample is retained and deposited at IIIM, Herbarium, Jammu, J&K State, India. The material was ensured to be free from pathogens, aflatoxins, pesticidal residues and heavy metals to meet WHO guidelines of purity and safety (CitationWorld Health Organization, 1998). The test material was prepared as fresh suspensions using 1% sterile gum acacia.
Test material was ground to coarse powder; 500 g of the powdered material was extracted with 95% ethyl alcohol (2 L) at room temperature by mechanical stirring for 2 h. The extraction process was repeated another three times under similar conditions. Pooled extract was concentrated under reduced pressure and gummy residue (44 g) was stored in desiccating conditions until further use.
Markers
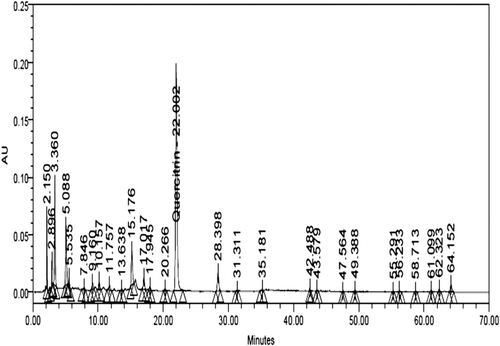
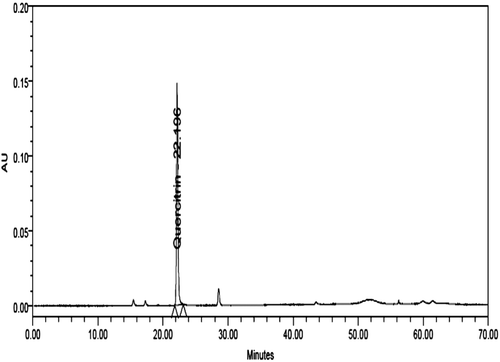
The marker was isolated from ethanol extract by column chromatography. The extract (20 g) was subjected to silicagel (100–200 mesh) chromatography. The column was eluted using a gradient of CHCl3-CH3OH (100:0–0:100) to afford 20 fractions. All the 20 fractions were checked on TLC (run in n-butanol: acetic acid: water: 4:1:5), and spots were visualized by freshly prepared borinate-PEG solution (2-aminoethyldiphenylborinate, 1% in CH3OH: polyethylene glycol-4000, 5% in C2H5OH, 1:1, vol/vol). Out of 20 fractions, fraction-8 (eluted in 15% CH3OH in CHCl3) showed one major spot in TLC. The fraction was subjected to repeated column chromatography on silica gel to obtain a compound. The compound was identified as quercitrin (CitationEldahshan, 2011). It was finally purified by crystallization and identified by 1H, 13C NMR and in comparison with data reported in the literature.
Chemoprofiling
Equipment
The Waters HPLC system comprising of two Waters 515 HPLC pumps, automatic sampling unit (Waters 717 plus auto sampler), column oven, photodiode array detector (Waters 2996), Merck RP-18 column (5 μm, 250 × 4.00 mm ID), temperature control module II and Waters Empower software was used for data analysis and data processing.
Experimental conditions
Quercitrin was quantified in the extract at 30°C using HPLC. The analysis was performed at a flow rate of 1.0 ml/min using a mobile phase consisting of can (B):1.5% acetic acid in water (A) [gradient:time in minute (B%): 0 (12), 25 (21), 30 (25), 40 (50), 50 (75), 60 (90), 70 (12)]. The photodiode array detector was set at wavelength of 340 nm for quantification.
Sample preparation and quantification
The accurately weighed quantity of the dried extract (21 mg) was dissolved in 2 ml methanol: water (1:1 vol/vol mixture) HPLC grade. The sample was centrifuged and filtered through Millipore micro filter (0.45 μm) and was used for analysis. The filtrate 10 µL was injected in the HPLC system (). Quercitrin (1.2 mg) was dissolved in 5 ml methanol:water (1:1 vol/vol mixture) HPLC grade. From the solution 2, 4, 6, 8, 10 µL was injected in the HPLC system for plotting of calibration curve ().The linearity was observed in the concentration range of 480 to 2400 μg/ml. The marker compounds in the extract were quantified using the calibration curve. It was found that the extract contained 0.55% quercitrin.
Animals
Female Wistar albino rats 140–160 g obtained from the animal house of the Indian Institute of Integrative Medicine formerly known as Regional Research Laboratory, Jammu and Kashmir, India. Animals were employed in groups of eight for the study. All the animals were maintained in transparent polycarbonate filter top cages in animal isolator cabins at 22 ± 2°C with 12-h light/dark cycle and free access to pellet food (Ashirwad Pvt Ltd., Ghaziabad, Uttar Pradesh, India) and autoclaved water. According to ethical regulations on animal research all animals used in experimental work received human care.
Standard drug
Prednisolone 5 mg/kg per oral (p.o.) dose was used as immunosuppressive control. Prednisolone was freshly prepared and administered orally during the study.
Effect on general behavior and acute toxicity
Graded doses of E. hirta ethanol extract (maximum single oral dose 2000 mg/kg) were orally administered once to groups of three animals after approval of Institutional Animal Ethics Committee following OECD guidelines No. 423 (CitationOECD, 1996). The animals were observed continuously for 2 h and then at half hourly intervals for the next 6 h for changes in gross general behavior, i.e., spontaneous motor activity, reactivity, gait, respiration, ptosis, appearance of writhing, piloerection, etc., and daily for 1 week for any possible drug induced mortality.
LPS-induced arthritis
Female Wistar albino rats weighing 140–160 g were used. Animals were fasted for 18 h to set an experiment. E. hirta ethanol extract was administrated at 25, 50, 100, and 200 mg/kg, 2 h before an intra-articular (i.a.) injection of LPS (Sigma lipopolysaccharide (LPS) E. coli). Rats were injected intra-artricularly right and left knees from each animal with 50 µl/knee of saline containing 10 µg LPS. Synovial fluid and neutrophils levels of intracellular TNF-α production were measured at 2 and 3 h after an i.a. injection of LPS. Prednisolone p.o. was given at 5 mg/kg. Synovial fluid extracted out from the inflamed rat knee (CitationSingh et al., 1997) was processed for intracellular TNF-α production by flow cytometry.
Estimation of synovial fluid levels of TNF-α production by flow cytometry
Synovial fluid samples were obtained from LPS-induced rat knees. Extracted synovial fluid (200 µl) was pipetted directly into a 12 × 75 mm fluorescence-activated cell sorting tube. RBCs were lysed using 2 ml of 1 × lysing solution (BD Biosciences, CA) for 10 min. After centrifugation at 300g for 5 min, the supernatant was aspirated and 1× permeabilizing solution (500 µl; BD Biosciences) was added into the pellet and incubated for 10 min at room temperature in the dark. The tubes were centrifuged and the supernatant was removed without disturbing the pellet. After washing with 3 ml buffer (1% bovine serum albumin, 0.1% NaN3, 1× PBS), cytokine-specific antibodies (20 µl, TNF-α; BD Biosciences) were added to the cells and incubated for 30 min at room temperature in the dark. After one final wash, cells were resuspended in 1% paraformaldehyde (500 µl) and stored at 4°C until flow cytometry analysis. Cells were acquired using a (LSR; BD Biosciences) flow cytometer and data were analyzed using Cell Quest software. A minimum of 10,000 cells was counted from each sample.
Neutrophils isolation
Neutrophils were separated from whole blood by the histopaque gradient method (Histopaque-Sigma procedures No. 1077): 200 μl of the sample was run. The analysis of cell count and event percentages was carried out in a BD LSR II flow cytometer after the removal of any traces of the red blood cells by FACS lysing solution. The gating of the required cell population was carried out according to the standard procedures of the flow cytometer (CitationKhan et al., 2006).
Estimation of neutrophils levels of intracellular TNF-α production by flow cytometry
Neutrophils were taken in Falcon tubes followed up with the addition of Golgi plug. Erythrocytes were lysed for 10-min incubation at room temperature with 2 ml 1 × FACS lysing solutions (BD Biosciences) and centrifuged at 300 g for 10 min at room temperature after the cells were washed once with 1 ml phosphate buffer solution by centrifugation at 300 g for 5 min. Thereafter, 500 µl of 1× permeabilization was added, followed by 10 min of incubation at room temperature. After incubation, the cells were washed once with 500 µl phosphate buffer saline solutions (PBS)/0.5% BSA by centrifugation at 300 g for 5 min supernatant were removed. Monoclonal anti-TNF-α PE (20 µl) was added to separate the tubes containing the cell pellets. The samples were vortexed and incubated for another 30 min at room temperature. The permeabilized cells were finally washed in 1 ml PBS/0.5% BSA by centrifugation at 300 g for 5 min. The supernatant were removed and the cells were resuspended in 500 µl 1% PFA, and stored at 2–8°C in the dark. The fixed cells were then analyzed within 24 h in a flow cytometric analyzer using Cell Quest software (LSR; BD Biosciences). Leukocyte subpopulations were distinguished by their different light-scattering properties. Forward scatter reflects the cell size and side scatter reflects the complexity/granularity.
Statistical analysis
Data represents mean ± SEM of eight animals. **p < 0.01; ***p < 0.001 compared to sensitized control (analysis of variance, ANOVA followed by Tukey–Kramer for multiple comparisons).
Results
Effect of E. hirta ethanol extract and prednisolone on synovial fluid levels of intracellular cytokine TNF-α
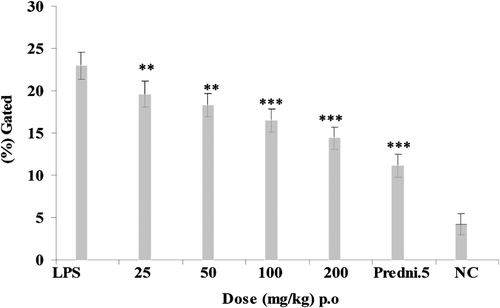
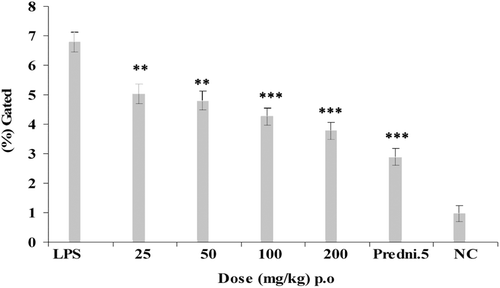
Animals were treated with E. hirta ethanol extract (p.o.) at graded doses of 25, 50, 100, and 200 mg/kg, 2 h before an i.a. injection of LPS. The maximum significant down regulation was showed at 100 and 200 mg/kg dose that showed 16.5 ± 1.34% and 14.4 ± 1.3% of synovial fluid levels of intracellular TNF-α production against the LPS control group showing 22.97 ± 1.6% of intracellular TNF-α production at 2 h after LPS injection, respectively (). Intracellular TNF-α production was also estimated at 3 h after LPS injection that showed the maximum inhibition of intracellular TNF-α at 100 and 200 mg/kg that showed 17.5 ± 1.34% and 16.01 ± 1.3% decrease of intracellular TNF-α production against LPS control group showing 20.8 ± 1.65% (). Prednisolone (5 mg/kg) was used as standard drug for 2 and 3 h of experiments that showed 11.15 ± 1.33% and 13.98 ± 1.31% of intracellular TNF-α inhibition. Comparative analysis of the effects of the E. hirta ethanol extract and prednisolone 5 mg/kg of intracellular TNF-α production at 2 and 3 h that showed the maximum decrease of intracellular TNF-α productions at 2 h.
Effect of E. hirta ethanol extract and prednisolone on neutrophil levels of intracellular cytokine TNF-α
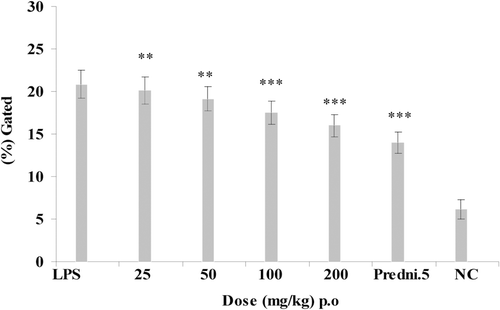
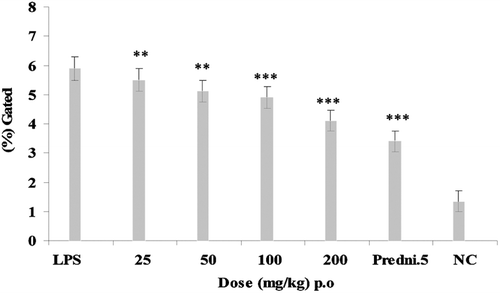
Animals were treated with E. hirta ethanol extract (p.o.) at graded doses of 25, 50, 100, and 200 mg/kg, 2 h before an i.a. injection of LPS. However, the maximum effect at 100 and 200 mg/kg dose that showed 4.26 ± 0.3% and 3.78 ± 0.29% decreased of neutrophils level of intracellular TNF-α production against LPS control group showing 6.78 ± 0.34% of intracellular TNF-α production at 2 h after injection of LPS (). Intracellular TNF-α production was also estimated at 3 h after LPS injection that showed inhibition of intracellular TNF-α at 100 and 200 mg/kg that showed 4.89 ± 0.37% and 4.1 ± 0.36% decrease of intracellular TNF-α production against LPS control group showed 5.9% ± 0.41% of intracellular TNF-α inhibition (). Prednisolone 5 mg/kg was used as standard drug for both 2 and 3 h of LPS challenged experiments which showed 2.89 ± 0.28% and 3.4 ± 0.35% intracellular TNF-α inhibition. Comparative analysis of the effects of E. hirta and prednisolone 5 mg/kg on neutrophils levels of intracellular TNF-α production at 2 and 3 h showed the maximum decrease of intracellular TNF-α production of E. hirta at 2 h.
Discussion
The use of plants and their extracts in treatment of diseases dates back to 460–370 BC when Hypocrites practiced the art of healing by the use of plant-based drugs (CitationSoforowa, 1982). Traditionally, extracts of the plant are used in sore and wound healing, as ear drops for boils in the ear and treatment of boils. They are also used in the control of diarrhea and dysentery (CitationKokwaro, 1993; CitationIgoli et al., 2005). Rats were injected intra-artricular (i.a.) with 50 µl of saline containing 10 μg/knee LPS and synovial fluid levels of intracellular TNF-α were measured at 2 and 3 h after an i.a. injection of LPS (CitationSing et al., 1997). In this study, the results obtained indicated that the E. hirta ethanol extract of the plant inhibited significantly synovial fluid levels of intracellular TNF-α at 100 and 200 mg/kg, 2 h after injection of LPS. Therefore, the extract contains substances that can inhibit the intracellular TNF-α after injection of LPS at 2 and 3 h course of experiment ( and ). The graphical representation clearly showed that the maximum significant inhibition of synovial fluid levels of intracellular TNF-α levels at 2 h after injection of LPS.
Neutrophils are polymorphonuclear leukocytes that constitute the first line of defense against bacterial and fungal infections. They are important for resolving inflammatory processes and regulating immune reactions. Neutrophils have a very short half-life in the circulation (8–20) h, and are able to enter infected or inflamed tissues (CitationEdwards, 1994). Recruitment to tissues occurs because the cells contact and interact with endothelial molecules that promote neutrophil rolling, tight adhesion, and diapedesis to damaged sites. With the onset of phagocytosis of microorganisms by neutrophils, various cellular processes are triggered, including a respiratory burst and the secretion of proteolytic enzymes and immunomodulatory products. Most forms of treatment for arthritis are mainly symptomatic and aim to alleviate symptoms such as pain and swelling. Common forms of treatment include nonsteroidal anti-inflammatory drugs and painkillers.
As the LPS-injected rat knees model gives a comparative study of acute anti-inflammatory responses, we choose to analyze intracellular TNF-α level in neutrophils by this acute condition. E. hirta showed inhibition of neutrophil levels of intracellular TNF-α production at 2 and 3 h after LPS challenge ( and ). Therefore, E. hirta showed dose-dependent significant inhibition of intracellular TNF-α levels at 2 h after injection of LPS. The chronically inflamed synovial membrane is characterized by leucocyte infiltration where T cells, monocytes and macrophages are more abundant. In patients with RA, inflammatory mediators such as cytokines, chemokines, and chemokine receptors are currently under intensive investigation.
RA, a devastating disease, afflicts ~1% of the general population and 3 times more women than men (CitationAlamanos & Drosos, 2005). The proinflammatory cytokine TNF-α has been shown to play a pivotal role in RA and many other serious diseases such as Crohn’s disease and psoriasis. Although substantial progress has been made in the treatment of these diseases with the use of anti-TNF-α biological agents (CitationAckermann & Kavanaugh 2007) including etanercept, infliximab, and adalimumab, there still remains a sizable percentage (30 to 40%) of patients who are unresponsive to all currently used and approved medications (CitationDe Vries & Tak, 2005).
Therefore, the discovery of new compounds that can be administered orally and for which the mechanism of action differs from that of the biological TNF-α inhibitors would significantly add to the available for treating RA and other immunological disorders. Several cytokines, chemokines, and matrix metalloproteinase with serum levels that correlate with the disease activity of RA have been reported. Furthermore, a favorable clinical response following the administration of TNF-α inhibitors was shown to be accompanied by the reduced serum levels of these inflammatory mediators (CitationHyrich et al., 2006).
This animal model is well characterized and has a number of pathological similarities to human RA (CitationAnthony & Haqqi, 1999). Like human RA, synovitis and erosions of cartilage and bone are hallmarks of the CIA rat model. The aim of this study was to determine whether known TNF-α inhibitors of different types are active in blocking TNF-α release in the inflamed rat knee. This cytokine act both directly and indirectly. TNF-α act directly by promoting the release of metalloproteinases and leukotrienes which are responsible for tissue damage. LPS is a known widely accepted TNF-α stimulator and it’s a choice of stimulant in TNF-α inhibitor studies. Hence, we used LPS of E. coli (CitationFeldmann et al., 1996) for acute release of TNF-α in synovial fluid.
Conclusion
Thus, using this model we demonstrate the reduction of synovial fluid and neutrophils levels of intracellular TNF-α production by E. hirta. The elevation of intracellular TNF-α production into the LPS-injected rat knee provides an animal model to compare the potency, oral activity and duration of action of E. hirta on the modulation of intracellular TNF-α levels in the joints. Therefore, E. hirta will be chosen to evaluate for future experiments.
Acknowledgments
The authors are grateful for the Research Center of Pharmacy, King Saud University, Riyadh, Saudi Arabia.
Declaration of interest
The authors declared no conflict of interest.
References
- Anthony DD, Haqqi TM. (1999). Collagen-induced arthritis in mice: An animal model to study the pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol, 17, 240–244.
- Amin AR. (1999). Regulation of tumor necrosis factor-alpha and tumor necrosis factor converting enzyme in human osteoarthritis. Osteoarthr Cartil, 7, 392–394.
- Alamanos Y, Drosos AA. (2005). Epidemiology of adult rheumatoid arthritis. Autoimmun Rev, 4, 130–136.
- Anononymous. (2005). The use of Euphorbia hirta in the treatment of sores, boils and wounds. Personal communication with the chairman, Imo state Traditional Medicine Practioners: Owerri Nigeri.
- Ackermann C, Kavanaugh A. (2007). Tumor necrosis factor as a therapeutic target of rheumatologic disease. Expert Opin Ther Targets, 11, 1369–1384.
- Alstergren P, Kopp S. (2006). Insufficient endogenous control of tumor necrosis factor-alpha contributes to temporomandibular joint pain and tissue destruction in rheumatoid arthritis. J Rheumatol, 33, 1734–1739.
- Amital H, Gilburd B, Shoenfeld Y. (2007). Probiotic supplementation with Lactobacillus casei (Actimel) induces a Th1 response in an animal model of antiphospholipid syndrome. Ann N Y Acad Sci, 1110, 661–669.
- Brennan FM, Maini RN, Feldmann M. (1998). Role of pro-inflammatory cytokines in rheumatoid arthritis. Springer Semin Immunopathol, 20, 133–147.
- Khan B, Ahmad SF, Bani S, Kaul A, Suri KA, Satti NK, Athar M, Qazi GN. (2006). Augmentation and proliferation of T lymphocytes and Th-1 cytokines by Withania somnifera in stressed mice. Int Immunopharmacol, 6, 1394–1403.
- Choy EH, Isenberg DA, Garrood T, Farrow S, Ioannou Y, Bird H, Cheung N, Williams B, Hazleman B, Price R, Yoshizaki K, Nishimoto N, Kishimoto T, Panayi GS. (2002). Therapeutic benefit of blocking interleukin-6 activity with an anti-interleukin-6 receptor monoclonal antibody in rheumatoid arthritis: A randomized, double-blind, placebo-controlled, dose-escalation trial. Arthritis Rheum, 46, 3143–3150.
- de Vries N, Tak PP. (2005). The response to anti-TNF-alpha treatment: Gene regulation at the bedside. Rheumatology (Oxford), 44, 705–707.
- Edwards SW. (1994). Biochemistry and Physiology of the Neutrophil. Cambridge University Press: London, p 299.
- Eldahshan OA. (2011). Isolation and structure elucidation of phenolic compounds of Carob leaves grown in Egypt. Curr Res J Biol Sci, 3, 52–55.
- Feldmann M, Brennan FM, Maini RN. (1996). Role of cytokines in rheumatoid arthritis. Annu Rev Immunol, 14, 397–440.
- Georganas C, Liu H, Perlman H, Hoffmann A, Thimmapaya B, Pope RM. (2000). Regulation of IL-6 and IL-8 expression in rheumatoid arthritis synovial fibroblasts: The dominant role for NF-kappa B but not C/EBP beta or c-Jun. J Immunol, 165, 7199–7206.
- Hyrich KL, Watson KD, Silman AJ, Symmons DP; British Society for Rheumatology Biologics Register. (2006). Predictors of response to anti-TNF-alpha therapy among patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Rheumatology (Oxford), 45, 1558–1565.
- Ivashkiv LB. (1996). Cytokine expression and cell activation in inflammatory arthritis. Adv Immunol, 63, 337–376.
- Igoli JO, TA Ogaji, Anyiin Tor, Igoli NP. (2005). Traditional medicine practice amongst the Igede people of Nigeria. Part II. Afri J Tra Comp Alt Med, 2, 134–152.
- Kokwaro JO. (1993). Medicinal Plants in East Africa. 2nd edn. East African Literature Bureau: Nairobi, Kenya.
- Lind EM, Tallantire AC. (1971). Some Common Flowering Plants of Uganda. Oxford University Press: Nairobi, p 182.
- Lanhers MC, Fleurentin J, Dorfman P, Mortier F, Pelt JM. (1991). Analgesic, antipyretic and anti-inflammatory properties of Euphorbia hirta. Planta Med, 57, 225–231.
- Lee KH, Chen YS, Judson JP, Chakravarthi S, Sim YM, Er HM. (2008). The effect of water extracts of Euphorbia hirta on cartilage degeneration in arthritic rats. Malays J Pathol, 30, 95–102.
- Leandro MJ. (2009). Anti-tumour necrosis factor therapy and B cells in rheumatoid arthritis. Arthritis Res Ther, 11, 128.
- Mohr W, Westerhellweg H, Wessinghage D. (1981). Polymorphonuclear granulocytes in rheumatic tissue destruction. III. an electron microscopic study of PMNs at the pannus-cartilage junction in rheumatoid arthritis. Ann Rheum Dis, 40, 396–399.
- Matsuno H, Yudoh K, Katayama R, Nakazawa F, Uzuki M, Sawai T, Yonezawa T, Saeki Y, Panayi GS, Pitzalis C, Kimura T. (2002). The role of TNF-alpha in the pathogenesis of inflammation and joint destruction in rheumatoid arthritis (RA): A study using a human RA/SCID mouse chimera. Rheumatology (Oxford), 41, 329–337.
- Marston B, Palanichamy A, Anolik JH. (2010). B cells in the pathogenesis and treatment of rheumatoid arthritis. Curr Opin Rheumatol, 22, 307–315.
- Nordahl S, Alstergren P, Kopp S. (2000). Tumor necrosis factor-alpha in synovial fluid and plasma from patients with chronic connective tissue disease and its relation to temporomandibular joint pain. J Oral Maxillofac Surg, 58, 525–530.
- Nakahara H, Nishimoto N. (2006). Anti-interleukin-6 receptor antibody therapy in rheumatic diseases. Endocr Metab Immune Disord Drug Targets, 6, 373–381.
- OECD (1996). (Organization for Economic Cooperation and Development). Guidelines for testing of Chemicals. Guideline 423, Acute Oral Toxicity - Acute Toxic Class Method, Adopted, March 22.
- Romero C, Anchez S, Robinson WH, Tomooka BH. (2008). Identification of acute phase reactants and cytokines useful for monitoring infliximab therapy in ankylosing spondylitis. Clin Rheumatol, 27, 1429–1435.
- Soforowa EA. (1982). Medicinal Plant and Traditional Medicine in Africa. John Wiley and Sons: Chichester, p 198.
- Singh HN, Blancuzzi V, Greenwood S, Skiles JW, O’Byrne EM. (1997). Synovial fluid levels of tumor necrosis factor-alpha in the inflamed rat knee: Modulation by dexamethasone and inhibitors of matrix metalloproteinase and phosphodiesterase. Inflamm Res, 46 Suppl 2, S153–S154.
- Singh GD, Kaiser P, Youssouf MS, Singh S, Khajuria A, Koul A, Bani S, Kapahi BK, Satti NK, Suri KA, Johri RK. (2006). Inhibition of early and late phase allergic reactions by Euphorbia hirta L. Phytother Res, 20, 316–321.
- Shih MF, Cheng YD, Shen CR, Cherng JY. (2010). A molecular pharmacology study into the anti-inflammatory actions of Euphorbia hirta L. on the LPS-induced RAW 264.7 cells through selective iNOS protein inhibition. J Nat Med, 64, 330–335.
- WHO. (1998). Quality Control Guidelines for Medicinal Plant Materials. World Health Organization: Geneva, p 111.
- Wong Ting Fook, WTH. (1980). The Medicinal Plants of Mauritius. ENDA Publication No. 10, Dakar.
- Wendling D, Racadot E, Wijdenes J. (1993). Treatment of severe rheumatoid arthritis by anti-interleukin 6 monoclonal antibody. J Rheumatol, 20, 259–262.
- Watkins LR, Wiertelak EP, Goehler LE, Smith KP, Martin D, Maier SF. (1994). Characterization of cytokine-induced hyperalgesia. Brain Res, 654, 15–26.
- Wong PK, Campbell IK, Egan PJ, Ernst M, Wicks IP. (2003). The role of the interleukin-6 family of cytokines in inflammatory arthritis and bone turnover. Arthritis Rheum, 48, 1177–1189.