Abstract
Context: Podophyllotoxin is a natural product that inhibits the polymerization of tubulin and has served as a prototype for the development of diverse antitumor agents in clinical use, such as etoposide, teniposide and etopophos. Reumacon, another semisynthetic derivative, reached its clinical phase for the treatment of rheumatoid arthritis.
Objective: This study investigated the analgesic and anti-inflammatory properties of three compound derivatives from podophyllotoxin.
Materials and methods: During a phytochemical study performed on Juniperus thurifera Linne (Cupressaceae) leaves, among other products, several cyclolignans, such as podophyllotoxin, deoxypodophyllotoxin, deoxypicropodophyllotoxin and thuriferic acid were isolated. These compounds, obtained afterwards through semisynthesis, were assayed as analgesic and anti-inflammatory agents. Additionally, the cytotoxic activity of thuriferic acid was evaluated in three cancer cell lines, P-388, A-549 and HT-29, and these data were compared with previous cytotoxicity results obtained for the other three compounds.
Results: Analgesic activity results showed that deoxypicropodophyllin is as effective as deoxypodophyllotoxin to inhibit nociceptive perception induced by acetic acid in mice (77.8% ± 4.1% and 71.3% ± 6.5%, respectively), while its cytotoxicity [1.01 × 10−7 (GI50 M)] is 100-fold less. Other set of experiments showed that thuriferic acid, a derivative of podophyllotoxin a thousand times less citotoxic [1.21 × 10−5 (GI50 M)] than deoxypodophyllotoxin, caused significant inhibition of paw edema development in the carrageenan-induced inflammation test (63.4% ± 3.3%), effect comparable to those of deoxypodophyllotoxin (66.3% ± 4.4%), and the standard drug indomethacin (61.5% ± 2.5%).
Conclusion: We conclude that deoxypicropodophyllotoxin and thuriferic acid are effective in reducing edema formation. However, deoxypicropodophyllin is more related with analgesic activity than anti-inflammatory effect.
Introduction
Podophyllotoxin (1a) is a naturally occurring lignan abundant in the rhizomes of Podophyllum emodi L. (Berberidaceae) and other related species, which has served as a starting point for the development of different anticancer agents, such as etoposide (2a), teniposide (2b; Schacter, Citation1996; Stahelin et al., Citation1989) and etopophos (2c; Kluza et al., Citation2006). These drugs are currently used for the treatment of lung and testicle cancers and certain leukemias. Beside these marketed drugs, a considerable number of derivatives, such as NK 611 (2e; Rassmann et al., Citation1998) and GL 331 (2f; Chang et al., Citation2003), among others, have reached different clinical phase development levels, improving some pharmacological aspects. Diacylation of etopophos has yielded tafluposide (2d; Kluza et al., Citation2006), currently studied in clinical trials.
While podophyllotoxin is an inhibitor of the polymerization of tubulin (Dumontet & Jordan, Citation2010), drugs deriving from epipodophyllotoxin (1b) – such as 2a, 2b or 2c – act by inhibiting topoisomerase II (Hande, Citation1998; Jordan et al., Citation1998; Meresse et al., Citation2004; You, Citation2005). Tafluposide is a dual inhibitor of topoisomerases I and II. Additionally, podophyllotoxin is the precursor of other derivatives used for the treatment of psoriasis and malaria (Moraes et al., Citation2002). Some podophyllotoxin preparations are used for the treatment of condyloma acuminatum and other warts (Hartwell, Citation1958). Another podophyllotoxin-related compound is Reumacon (2g; Svensson & Pettersson, Citation2003), which just reached a clinical phase for the treatment of rheumatoid arthritis. Patients with rheumatoid arthritis treated with reumacon presented an important reduction of inflammation during clinical trials; however, this substance showed important gastrointestinal adverse effects. Other substances inhibiting topoisomerase II, such as doxorubicin, or inhibiting topoisomerase I, such as captothecin, have also served as antiarthritic agents (Jackson et al., Citation2008).
With this background, assays were performed on the analgesic and anti-inflammatory activity of deoxypodophyllotoxin (1c), deoxypicropodophyllin (1d) and thuriferic acid (3), three cyclolignans isolated during a phytochemical study carried out on Juniperus thurifera L. (Cupressaceae) leaves (San Feliciano et al., Citation1989). Due to the nonavailability of sufficient amounts of products 1d and 3 to carry out the assays described in this publication, their semisynthetic preparation was performed from deoxypodophyllotoxin (1c) and podophyllotoxin (1a), respectively.
Materials and methods
Isolation of compounds 1c, 1d and 3
Through the phytochemical study of Juniperus thurifera leaves, deoxypodophyllotoxin (1c), deoxypicropodophyllin (1d) and thuriferic acid (3) among other compounds were isolated. The plant collection and the phytochemical study carried out were described previously (San Feliciano et al., Citation1988). Due to the small amount of sample available for compounds 1d and 3, their semisynthetic preparation was carried out in order to perform the assays described.
Preparation of compounds 1d and 3
Preparation of 1d
Compound 1c (400 mg) was dissolved in 25 mL of a 5% KOH solution in MeOH. After keeping it at room temperature and shaking it during 2 h, it was extracted with EtOAc and allowed to crystallize, obtaining 390 mg of 1d (m.p. = 171–172 °C). Its physical and spectroscopic properties were identical to those described for the natural product (San Feliciano et al., Citation1989).
Preparation of 3
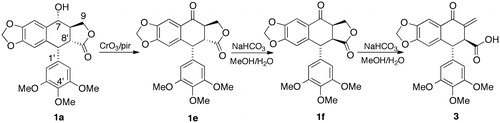
Dissolved 1a (400 mg) in 15 mL of pyridine were treated with 250 mg of CrO3 in 5 mL of pyridine by a previously described procedure (San Feliciano et al., Citation1988). The suspension was shaken overnight at room temperature. After the extraction of the reaction crude, it was subjected to flash chromatography (Et2O:CH2Cl2 6:1), obtaining 350 mg of 1e (m.p. = 190–192 °C). Its spectroscopic properties were identical to those described for this product (Dewick & Jackson, Citation1981).
The same procedure previously described (San Feliciano et al., Citation1988) was used to treat 350 mg of 1e with 10 mL of a saturated NaHCO3 solution in MeOH/H2O (). The mixture was kept for 60 min at room temperature with constant shaking. After the usual extraction process, it was subjected to flash chromatography in silica gel (CH2Cl2:MeOH 98:2), obtaining 310 mg of 3 (m.p. = 93–96 °C). Its physical and spectroscopic properties were identical to those described for the natural product 3 (López-Pérez et al., 1995). The 13C NMR spectra for compound 3 and hundreds of other related compounds can be found in the free database NAPROC-13 (López-Pérez et al., 2007).
Compounds 1c, 1d and 3 were purified by successive recrystallizations before the assays were performed.
Molecular modeling
Molecular modeling studies of 1c, 1d and 3 were performed in a Silicon Graphics Indigo working station (Silicon Graphics, Inc., Sunnyvale, CA). Models were built using the Macromodel v.5.5 facilities (Mohamadi et al., Citation1990). A conformational analysis of the three compounds was carried out using the Montecarlo method and MM2 (Allinger, Citation1977) as force field. In order to reproduce the experimental conditions in which the NMR spectra were registered, the calculations were performed considering chloroform implicitly as the solvent. For each of the compounds, 1000 conformations were generated, and those presenting up to 10 kJ/mol above the overall minimum were selected. With the aim of keeping a higher precision degree on their energies, they were subjected afterwards to a Hartee–Fock calculation, with the 6-31+G* base in Spartan 8.1 (Wavefunction, Inc., Irvine, CA).
Cytotoxicity
A screening procedure was used to assess the antitumoral activity of thuriferic acid (3) against the following: P-388 (lymphoid neoplasm from DBA/2 mouse), A-549 (human lung carcinoma), and HT-29 (human colon carcinoma) cell lines. Cells were seeded into 16 mm wells [multidishes (NUNC 42 001)] at concentrations of 1 × 104 (P-388) and 2 × 104 (A-549) (HT-29) cells/well, respectively, in 1 mL aliquots of MEM 1OFCS medium containing the compound to be evaluated at the concentrations tested. In each case, a set of control wells was incubated in the absence of the sample and counted daily to ensure the exponential growth of cells. After 4 d at 37 °C, 10% CO2 and 98% humid atmosphere, P-388 cells were observed through an inverted microscopy and the degree of inhibition was determined by comparison with the controls, whereas A-549 and HT-29 cells were stained with crystal violet before examination. The cytotoxicity values for deoxypodophyllotoxin (1c) and deoxypicropodophyllin (1d) have been previously reported (San Feliciano et al., Citation1993).
In vivo assays
As mentioned previously, we obtained the initial cytotoxicity data for all products assessed and these were compared to podophyllotoxin cytotoxicity. Due to the small amount of products available for testing antinociceptive and anti-inflammatory activities, it was not possible to make a dose-response curve. Hence, we based our dose selection on the cytotoxicity results and the sublethal doses used by other authors (Baker et al., Citation1950) to evaluate podophyllotoxin activity in rats.
Since the sublethal doses reported for the podophyllotoxin in rats is 20 mg/kg, and deoxypicropodophyllotoxin and thuriferic acid are less cytotoxic than podophyllotoxin, we used the doses of 50 mg/kg in rats and 10 mg/kg in mice. In both models, survival was 100% for all podophyllotoxin derivatives tested.
Analgesic activity
In the antinociceptive activity assessment of the compounds, male Swiss mice (25–30 g) divided in five groups (n = 6 each) were used. To each group, one of the following treatments was administered orally: acetylsalicylic acid (ASA; 200 mg/kg), deoxypodophyllotoxin (1c; 50 mg/kg), deoxypicropodophyllin (1d; 50 mg/kg) and thuriferic acid (3; 50 mg/kg). One group of mice received morphine (4 mg/kg, s.c.) as a reference opioid. The three podophyllotoxin related products were suspended in 2% carboxymethylcellulose (CMC). A control group received only the vehicle (2% CMC; 0.1 mL/100 g). One hour after receiving the treatment, 1% acetic acid (AcH) was administered intraperitoneally and the animals were immediately placed in transparent recipients, appropriate to determine the number of writhes (characterized by contraction of the abdominal musculature and extension of the hind limbs) in a 20 min observation period (Collier et al., Citation1968).
The writhing inhibition percentage was calculated using the following equation:
Where Ct is the number of writhes in the group treated and Cc is the number of writhes in the control group.
Anti-inflammatory activity
In order to assess the anti-inflammatory activity, the carrageenan plantar edema test in Sprague Dawley rats (150–200 g) was performed (Winter et al., Citation1962). Six animal groups were used, each of which received indomethacin (10 mg/kg), deoxypodophyllotoxin (1c; 10 mg/kg), deoxypicropodophyllin (1d; 10 mg/kg) or thuriferic acid (3; 10 mg/kg). A control group was kept, receiving the vehicle used for the product suspension (2% CMC; 0.1 mL/10 g). The administration of the different treatments was performed orally and 1 h after the administration of the treatments; edema was induced by injection of carrageenan (0.1 mL, 1%, w/v in saline) into the subplantar tissue of the right hind paw.
Inflammation was quantified determining the difference of volume displacement in the rear right foot before the administration of the treatments and 1, 3 and 6 h after the injection of carrageenan, using a plethysmometer 7150 (Ugo Basile, Italy) as the oncometric model.
The inhibition percentage of the inflammatory reaction was determined for each rat by comparison with control and calculated by the following formula:
All the experimental procedures were in accordance with the Guidelines on Ethical Standards for Investigation of Experimental Pain in Animals (Zimmermann, Citation1983), after protocol approval by the Bioethics Committee of Pharmacology Department.
Statistical analysis
Data are shown as mean ± SE. The percentage of analgesia was calculated considering the number of writhes of the CMC control group as 100% algesia (previously described). Furthermore, the inflammation test results are expressed as the percentage change compared to the control (before the treatment) and the values of the displacement volume for each of the observation times. The results were statistically analyzed through one-way ANOVA, followed by a Turkey test for multiple comparisons; a probability value of <0.05 was considered statistically significant.
Results and discussion
Deoxypodophyllotoxin (1c) assayed throughout this research was isolated during a phytochemical study carried out on J. thurifera leaves (San Feliciano et al., Citation1989). Deoxypicropodophyllin (1d) and thuriferic acid (3) were prepared from 1c and 1a, respectively. In basic medium, 1c is transformed into 1d as a consequence of epimerization in C-8′. The yield of the reaction is quantitative and the structure of 1d was determined by comparison with an authentic sample. Compound 3 was prepared from natural podophyllotoxin 1a by oxidation to podophyllotoxone 1e, followed by treatment with NaHCO3 in MeOH/H2O (). Once the alcohol was oxidized to a ketone (1e), it was treated with bases, producing the epimerization of the trans-lactone 1e into the cis-lactone picropodophyllone 1f, followed by the opening of the lactone, yielding thuriferic acid (San Feliciano et al., Citation1988). This product showed identical spectroscopic characteristics to those of the natural product 3 (). Thuriferic acid is different from the other two lignans due to the absence of the characteristic γ-lactone; instead it has a free carboxylic acid and a conjugated ketone. The three compounds were characterized from the spectroscopic point of view and purified by successive recrystallizations before the assays were performed.
Deoxydophyllotoxin 1c and deoxypicropodophyllin 1d are epimers in C-8′. The inversion of this stereocenter presents important changes in the space disposition and in the rigidity/flexibility between both molecules, as shown by the molecular modeling studies (). While deoxypodophyllotoxin 1c presents a single main conformation with the trimethoxyphenyl group in pseudoaxial disposition (, 1c), deoxypicropodophyllin 1d is distributed in four different conformational dispositions (I–IV) with an energy difference between them of less than 0.5 kcal/mol. This small difference in the stability between the four different conformations allows the exchange among them and so it yields a conformational flexibility facing the rigidity shown by deoxypodophyllotoxin, which presents only one conformation. The minimum-energy conformation of deoxypicropodophyllin shows a pseudoequatorial disposition for the trimethoxyphenyl group (1d-I), different to that found for this same group in deoxypodophyllotoxin 1c. This difference in space disposition of the trimethoxyphenyl group and in flexibility/rigidity of the tetracyclic system is due to the disposition of the lactone, trans-lactone in 1a and cis-lactone in 1d, as has been previously stated.
Figure 2. Molecular models of deoxypodophyllotoxin (1d), deoxypicropodophyllotoxin (1d-I–IV), and thuriferic acid (3). In parentheses, the relative electronic energies obtained through RHF/6-31+G* calculation.
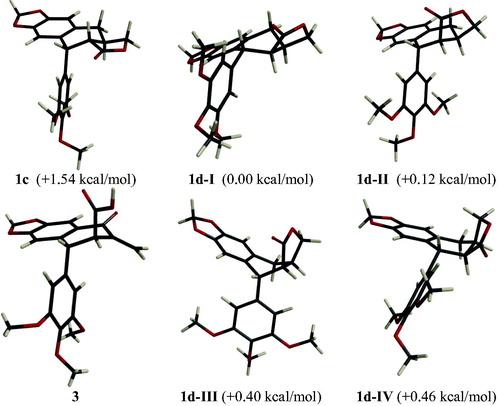
Thuriferic acid (3) has two conformations, one with the trimethoxyphenyl group and the carboxylic acid in pseudoaxial disposition () and another with higher energy with both groups in pseudoequatorial disposition.
The results obtained through molecular modeling for these three compounds are in agreement with the RMN 1H data; as a matter of fact, the coupling constants obtained experimentally present a great similarity in their values deriving from the theoretical models (Haasnoot et al., Citation1980).
The results obtained in the cytotoxicity assays are shown in , including literature values for compounds 1c and 1d and the reference podophyllotoxin (1a). According to the results obtained, thuriferic acid is significantly less cytotoxic than podophyllotoxin and the rest of the analogues tested. These data, in agreement with previously reported data, shows that the trans-lactones lignans are about one order of magnitude more cytotoxic than the corresponding cis-lactones analogues (San Feliciano et al., 1993).
Table 1. Cytotoxicity of podophyllotoxin and derivates: deoxypodophyllotoxin (1c), deoxypicropodophyllin (1d) and thuriferic acid (3).
The writhing test has long been used as a screening tool for the assessment of analgesic properties of new substances. It is accepted that AcH acts by releasing endogenous substances, such as bradykinin and serotonin, as well as cytokines, which stimulate peripheral nociceptive fibers (Ribeiro et al., Citation2000). Additionally, prostaglandins generated by cyclooxygenase 1 (COX-1) in the injection site have an important role in nociceptive transmission, which explains the effectiveness of nonsteroidal anti-inflammatory drugs in this experimental model (Collier et al., Citation1968; Deraedt et al., Citation1980). It is important to note that other types of analgesics, such as the opioids, are also effective in this test without inhibiting COX. In accordance with these observations, in the present work, it was observed that morphine and AAS caused a significant inhibition on the writhing responses induced by AcH (59% and 88%, respectively) when compared to the control group ().
Figure 3. Percentage of inhibition of abdominal writhes as a parameter of the analgesic activity of acetylsalicylic acid (AAS), morphine, deoxypicropodophyllin (1d), deoxypodophyllotoxin (1c) and thuriferic acid (3). *p < 0.05 versus AAS; §p < 0.05 versus morphine; n = 6.
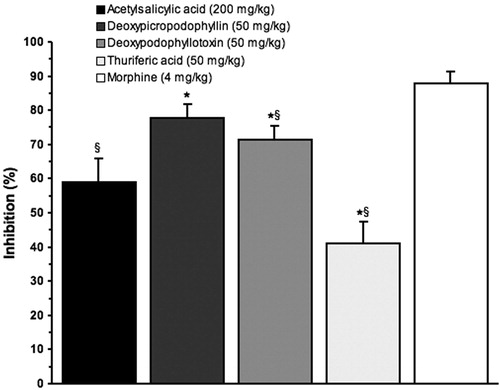
In the abdominal writhing models used to assess the antinociceptive activity of deoxypodophyllotoxin 1c, deoxypicropodophyllin 1d and thuriferic acid 3, it was observed that the two first compounds generate a greater protective effect for the chemical algesic stimulus than the group receiving ASA, achieving analgesia of 77.8% and 71.3% for the two lactonic lignans deoxypodophyllotoxin 1c and deoxypicropodophyllin 1d (). Our results, suggest a peripherally mediated analgesic activity based on the association of the model with stimulation of peripheral receptors (Zakaria et al., Citation2008).
It is known that the mediators previously described (bradykinin, serotonin and prostaglandins) not only mediate pain (Guay et al., Citation2004), but also produce an acute inflammation in the peritoneal zone and hence the analgesic activity results were complemented with inflammation studies using a carrageenan-induced plantar edema model. In these assays, it was observed that the inflammatory agent produced evident redness and it was determined that it induced a pronounced edema that was visibly developed during the first 6 h and persisted for more than 24 h.
As it can be observed in , the anti-inflammatory activity results obtained with deoxypodophyllotoxin 1c and thuriferic acid 3 (66.3% ± 4.4% and 63.4% ± 3.3%, respectively) at the first hour of assessment were similar to those observed in the group receiving indomethacin, used as the anti-inflammatory control (61.5% ± 2.5%). On the other hand, even when it showed anti-inflammatory activity, deoxypicropodophyllin 1d was statistically inferior to the rest of the treatments assayed.
Figure 4. Percentage of plantar edema inhibition due to the administration of indomethacin, deoxypicropodophyllin (1d), deoxypodophyllotoxin (1c) and thuriferic acid (3). *p < 0.05 versus indomethacin; n = 6.
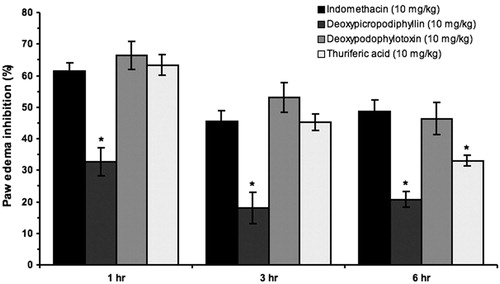
It is worth mentioning that for all the groups, the maximum anti-inflammatory effect was observed at the first hour after the administration of carrageenan.
If the activity profile of each treatment is compared to indomethacin, it is observed that compound 3 shows very similar anti-inflammatory characteristics to the nonsteroidal anti-inflammatory agent (). Indomethacin and thuriferic acid 3 share the presence of a free carboxylic acid, a structural similarity that could be related to their analogy in the anti-inflammatory activity profile. On the other hand, deoxypodophyllotoxin 1c, which also developed a significant anti-inflammatory effect, possesses a higher potency profile for a longer period of time: even 6 h after the administration of carrageenan, the plantar edema inhibition effect was superior to 55%.
These results show a significant change in the analgesic and anti-inflammatory profile in deoxypicropodophyllin 1d with respect to its epimer at C-8′ deoxypodophyllotoxin 1c, which also implies a significant decrease of cytotoxicity. An important change is also observed in the model when the opening of the lactone takes place to yield thuriferic acid 3, in which the presence of a free carboxylic acid makes its pharmacological profile similar to indomethacin in the inhibition of the plantar edema.
Conclusions
Podophyllotoxin, a prototype for the development of diverse antitumor agents, could not be used in clinical practice given its high toxicity; however, a simple modification, such as epimerization in C-8′ of the molecule produces a considerable diminishing of its cytotoxicity and an increase of analgesic activity. This improvement can be attributed to important changes in its space disposition and to the decreasing reactivity of the γ-lactone which is less tensioned in the C-8′ epimer than in the podophyllotoxin. Concomitantly, a decrease in the anti-inflammatory effect is observed. In addition, the opening of the γ-lactone in podophyllotoxin to yield thuriferic acid improves its anti-inflamatory properties while significantly reduces its cytotoxicity. In consequence, deoxypicropodophyllin and thuriferic acid can be proposed as prototypes for the development of, respectively, new analgesic and antiinflamtarory drugs, free from cytotoxic effects, contrary to the patented compound reumacon.
Acknowledgements
The authors are grateful to Humberto López Castillo, MD, MEd, MSc for his review of the manuscript.
Declaration of interest
The authors wish to thank SENACYT-Panama (Secretaría Nacional de Ciencia, Tecnología e Innovación) for the grants numbers SNI-167, INF 11-054 and FID 11-090, under which this research was conducted. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Allinger NL. (1977). Conformational-analysis.130. MM2 – hydrocarbon force-field utilizing V1 and V2 torsional terms. J Am Chem Soc 99:8127–34
- Baker Z, Davison C, Smith P. (1950). The effect of podophyllotoxin, colchicines, urethane, and nitrogen mustard on the respiration of normal and suprarenalectomized rat lymphatic tissue. J Exp Med 92:113–19
- Chang H, Shyu KG, Lee CC, et al. (2003). GL331 inhibits HIF-1alpha expression in a lung cancer model. Biochem Biophys Res Commun 302:95–100
- Collier HO, Dinneen LC, Johnson CA, Schneider C. (1968). The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br J Pharmacol Chemother 32:295–310
- Deraedt R, Jouquey S, Delevallee F, Flahaut M. (1980). Release of prostaglandins E and F in an algogenic reaction and its inhibition. Eur J Pharmacol 61:17–24
- Dewick PM, Jackson DE. (1981). Cytotoxic lignans from podophyllum, and the nomenclature of aryltetralin lignans. Phytochemistry 20:2277–80
- Dumontet C, Jordan MA. (2010). Microtubule-binding agents: A dynamic field of cancer therapeutics. Nat Rev Drug Discov 9:790–803
- Guay J, Bateman K, Gordon R, et al. (2004). Carrageenan-induced paw edema in rat elicits a predominant prostaglandin E2 (PGE2) response in the central nervous system associated with the induction of microsomal PGE2 synthase-1. J Biol Chem 279:24866–72
- Haasnoot CAG, Deleeuw F, Altona C. (1980). The relationship between proton-proton NMR coupling-constants and substituent electronegativities. 1: an empirical generalization of the Karplus equation. Tetrahedron 36:2783–92
- Hande KR. (1998). Etoposide: Four decades of development of a topoisomerase II inhibitor. Eur J Cancer 34:1514–21
- Hartwell JL, Schrecker AW. (1958). The chemistry of podophyllum. Fortschr Chem Org Naturst 15:83–166
- Jackson JK, Higo T, Hunter WL, Burt HM. (2008). Topoisomerase inhibitors as anti-arthritic agents. Inflamm Res 57:126–34
- Jordan A, Hadfield JA, Lawrence NJ, McGown AT. (1998). Tubulin as a target for anticancer drugs: agents which interact with the mitotic spindle. Med Res Rev 18:259–96
- Kluza J, Mazinghien R, Irwin H, et al. (2006). Relationships between DNA strand breakage and apoptotic progression upon treatment of HL-60 leukemia cells with tafluposide or etoposide. Anticancer Drugs 17:155–64
- López-Pérez JL, del Olmo E, de Pascual-Teresa B, et al. (1995). Unambiguous configurational and conformational determination of thuriferic acid. Tetrahedron 51:6343–8
- López-Pérez JL, Therón R, del Olmo E, Díaz D. (2007). NAPROC-13: a database for the dereplication of natural product mixtures in bioassay-guided protocols. Bioinformatics 23:3256–7
- Meresse P, Dechaux E, Monneret C, Bertounesque E. (2004). Etoposide: Discovery and medicinal chemistry. Curr Med Chem 11:2443–66
- Mohamadi F, Richards NGJ, Guida WC, et al. (1990). Macro-model – An integrated software system for modeling organic and bioorganic molecules using molecular mechanics. J Comput Chem 11:440–67
- Moraes RM, Lata H, Bedir E, et al. (2002) The American mayapple and its potential for podophyllotoxin production. In: Janick J, Whipkey A, eds. Trends in New Crops and New Uses. Alexandria, Virginia: ASHS Press, 527–32
- Rassmann I, Thodtmann R, Mross M, et al. (1998). Phase I clinical and pharmacokinetic trial of the podophyllotoxin derivative NK611 administered as intravenous short infusion. Invest New Drugs 16:319–24
- Ribeiro RA, Vale ML, Thomazzi SM, et al. (2000). Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur J Pharmacol 387:111–18
- San Feliciano A, Gordaliza M, Miguel del Corral, JM, et al. (1993). Antineoplastic and antiviral activities of some cyclolignans. Planta Med 59:246–9
- San Feliciano A, López JL, Medarde M, et al. (1988). Thuriferic acid. A novel lignan type from Juniperus thurifera L. Tetrahedron 44:7255–60
- San Feliciano A, Medarde M, Lopez JL, et al. (1989). Lignans from Juniperus thurifera L. Phytochemistry 28:2863–6
- Schacter L. (1996). Etoposide phosphate: What, why, where, and how? Semin Oncol 23:1–7.
- Stahelin H, von Wartburg A. (1989). From podophyllotoxin glucoside to etoposide. Prog Drug Res 33:169–266
- Svensson B, Pettersson H. (2003). Reumacon (CPH82) showed similar X-ray progression and clinical effects as methotrexate in a two years comparative study on patients with early rheumatoid arthritis. Scand J Rheumatol 32:83–8
- Winter CA, Risley EA, Nuss GW. (1962). Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med 111:544–7
- You Y. (2005). Podophyllotoxin derivatives: Current synthetic approaches for new anticancer agents. Curr Pharm Des 11:1695–717
- Zakaria ZA, Ghani ZD, Nor RN, et al. (2008). Antinociceptive, anti-inflammatory, and antipyretic properties of an aqueous extract of Dicranopteris linearis leaves in experimental animal models. J Nat Med 62:179–87
- Zimmermann M. (1983). Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16:109–10