Abstract
Context: Neuropsychiatric disorders, like anxiety and depression, are global problems for clinical researchers in neurology. Recently, some authors have shown neuroprotective and anti-inflammatory effects of Scrophularia striata Boiss (Scrophulariaceae) extract in rodents.
Objective: The purpose of the current study was to investigate the effects of S. striata extract on anxiety and depressant-like behaviors and find a possible mechanism for these impacts.
Materials and methods: In this study, the elevated plus-maze (EPM) and forced swimming test (FST), which are useful models for selective identification of anxiolytic and antidepressant drug effects in rodents, were used. We investigated the effects of S. striata ethanol extract at different doses (20, 50, 100, 160 and 220 mg/kg) on anxiety and depression behaviors in the EPM and FST, and then we assessed the role of γ-aminobutyric acid (GABA)A receptor in modulation of the effects of S. striata extract in the brain.
Results: Our results showed that effective doses of S. striata (100 and 160 mg/kg) increased the percentages of open arm time and entries in the EPM and decreased immobility time in the FST in comparison with control group, indicating anxiolytic and antidepressant effects, respectively. Moreover, intracerebroventricular administration of GABAA receptor agonist (muscimol; 1 µg/rat) enhanced the impact of S. striata, and GABAA receptor antagonist (bicuculline; 1 µg/rat) blocked these effects in rats, indicating that significant interactions existed between S. striata and the GABAergic system in the brain.
Discussion and conclusion: Findings of this study suggest that anxiolytic and antidepressant effects of S. striata may be modulated via the GABAergic system.
Introduction
Neuropsychiatric disorders, like anxiety and depression, can influence any aspects of life. These behavioral disorders are common causes of reduced quality of life and impaired functioning in late life (van't Veer-Tazelaar et al., Citation2009). Recent studies have showed that inflammation in the brain is associated with increased levels and severity of anxiety and depression in humans and rodents (Pitsavos et al., Citation2006; Raison et al., Citation2006). In this respect, previous studies have indicated neuroprotective and anti-inflammatory effects of Scrophularia striata Boiss (Scrophulariaceae) extract in experimental studies (Azadmehr et al., Citation2009; Ostad et al., Citation2010). On the other hand, Monsef–Esfahani and his colleagues (Citation2010) have isolated quercetin from S. striata compounds. In line with this subject, several previous studies have indicated the neuroprotective, anxiolytic and antidepressant effects of this flavonoid in laboratory animals (Aguirre-Hernández et al., Citation2010; Bhutada et al., Citation2010; Cho et al., Citation2006). Nowadays, many researches have focused on the study of the effects of natural products for the treatment of psychiatric disorders in humans using animal models.
It is well-known that various neurotransmitter systems such as serotonin, γ-aminobutyric acid (GABA), dopamine and glutamate as central elements are involved in regulation and modulation of anxiety and depression behaviors at molecular levels of neural systems of brain (Bergink et al., Citation2004; Graeff et al., Citation1996; Kalueff & Nutt, Citation2007; Kano et al., Citation2011). Moreover, recent studies have provided substantial evidence that the pharmacological effects of herbal medicines can be modulated by different neurotransmitter systems in rodents (Hsieh et al., Citation2010). GABA is an amino acid which acts as the main inhibitory neurotransmitter in the central nervous system (CNS) through different receptor sites, classified as GABAA, GABAB and GABAC (Möhler, Citation2012). The distinct mechanisms in the etiology of anxiety and depression are unclear; however, the involvement of GABAA receptor in the regulation of anxiety and depression has been the subject of extensive laboratory and clinical studies. It has long been known that GABAergic agents and drugs have strong anxiolytic and antidepressant effects in humans and animals. In line with this discussion, a number of clinical studies relate functioning of the GABAA receptor to anxiety and depression disorders, resulting in successful use of this receptor protein as a molecular target for anxiolytic and antidepressant drugs (Möhler, Citation2012).
In the current study, we focused on one of the best characterized of these receptors, the GABAA receptor, and evaluated the role of GABAA receptor in mediating the effects of S. striata extract in the brain.
Materials and methods
Subjects
Male Wistar rats weighing 240–280 g were purchased from the Pasteur Institute of Iran. The animals were individually housed in standard rat polycarbonate cages. The colony room was maintained under a 12:12 h light/dark cycle (light on 07:00 h) in a temperature (23 ± 1 °C) and humidity (50% ± 5%) controlled room and were allowed unlimited access to food and water, except during the behavioral tests. All experiments were performed between 12:00 and 17:00 h and each rat was tested only once. In addition, all stages of the present study were performed using protocols approved by the Research and Ethics Committee of the Tabriz University of Medical Sciences and were conducted under the recommended conditions of the Guide for the Care and Use of Laboratory Animals of the National Institute of Health (NIH; Publication No. 85-23, revised 1985). Eight animals were used in each group of experiments. All efforts were made to minimize animal suffering or discomfort, and reduce the number of animals used.
Plant collection and total extract
The aerial parts of S. striata were collected from the northwestern part of Iran, in the Ilam region, in January 2011, and were dried at 30 ± 1 °C. A sample was authenticated by M. Kosari-Nasab and a voucher specimen of this plant is deposited at the Herbarium of Tehran University (No: 36501). Total extract was obtained as previously described by Azadmehr et al. (Citation2009). The plant extract was dissolved in water and used at appropriate concentrations.
Intracerebroventricular (Intra-CV) injection
Animals were allowed to adapt to the laboratory conditions for at least 1 week before surgery and were anesthetized intraperitoneally with ketamine hydrochloride (50 mg/kg; Alfasan, Woerden-Holland) plus xylazine (4 mg/kg; Alfasan, Woerden-Holland) and placed in a Stoelting stereotaxic instrument (Stoelting Co., Wood Dale, IL). Stainless steel guide cannulas (21-gauge) were stereotaxically implanted with coordinates from Paxinos and Watson (Citation2007) [AP-0.82 mm; ML + 1.5 mm and DV + 2.0 mm; related to bregma]. The cannula was fixed to the skull with acrylic dental cement. The animals were then allowed 7 d before the test to recover from surgery, and were handled about 4 min each day prior to behavioral testing to minimize nonspecific stress. Intra-CV treatments were performed with an internal cannula (27-gauge), terminating 1 mm below the tip of the guides; it was fitted into the guide cannula and connected by polyethylene tubing to a 1 μL Hamilton syringe. Drugs (as mentioned in below sections) were administered in a volume of 1 µL in the right lateral cerebral ventricle over a 60 s period. The inner cannula was left in place for an additional 60 s to allow diffusion of the solution and to reduce the possibility of reflux. Intra-CV injection was made 5 min before testing (Solati et al., Citation2011).
Elevated plus-maze
One of the most popular tests of anxiety-like behavior in rats is the elevated plus-maze (EPM), in which the reduced amount of time spent or entries in open arms of the EPM suggests the operation of anxiety-like processes. The EPM was a plus-shaped apparatus, constructed from wood, elevated to a height of 50 cm above the floor. This apparatus consisted of a central platform (10 cm × 10 cm); two open arms (50 cm × 10 cm) and two equalized closed (50 cm × 10 cm × 50 cm) arms opposite to each other with an open roof. The EPM was placed in the center of a quiet and dimly lit room. The behavior of rats was directly observed using a mirror, suspended at an angle above the EPM. Behavioral data were collected by a “blind” observer who quietly sat at 1 m behind of the one of the closed arms of the EPM, using a chronometer. Five minutes following their respective drug treatment, rats were placed individually at the center of the EPM, facing one of the open arms. The observer measured (1) time spent in the open arms, (2) time spent in the closed arms, (3) number of entries into the open arms, and (4) number of entries into the closed arms during the 5 min test period. An entry was defined as all four paws inside the arm. The EPM was cleaned with distilled water after each test performing on a rat. For the purpose of analysis, open-arm activity was quantified as the amount of time that the rat spent in the open arms relative to the total amount of time spent in any arm (open/total × 100), and the number of entries into the open arms was quantified relative to the total number of entries into any arm (open/total × 100). Total arm entries (open arm plus close arm spending times) used as an index of locomotor activity (LMA) (Solati et al., Citation2010, Citation2011).
Forced swimming test
The forced swimming test (FST) remains one of the most widely used tools for assessing antidepressant activity. To describe this behavioral model in rats, the following procedure was adopted. Rats were individually placed into the transparent glass cylinders (height: 80 cm, diameter: 30 cm), filled with water to a height of 40 cm and maintained at 23 ± 1 °C. The water was replaced by fresh water between each test. For the first exposure, rats were placed in the water for 15 min (pre-test session). Twenty-four hours later, animals were placed in the water cylinder again for a 5 min session (test session), and the total duration of immobility were recorded during this time. Each rat was judged to be immobile when it ceased struggling and remained floating motionless in the water and making only those movements necessary to keep their head above water. A decrease in the duration of immobility is an indicative of an antidepressant-like effect (Garcia et al., Citation2008).
Drugs
The following drugs were used in the experiments: muscimol and bicuculline (Sigma Chemical Co., St. Louis, MO). Muscimol was dissolved in sterile 0.9% saline and bicuculline which was dissolved in a minimal volume of diluted acetic acid (1 drop; 5 µL by Hamilton micro-syringe 10 µL) and made up to a volume of 5 mL with saline and was then diluted to the required volume with vehicle. Control animals received saline or vehicle. Drug solutions were freshly prepared before administration.
Effects of S. striata extract on anxiety behavior
Six groups of rats received oral administration of various doses of S. striata extract (20, 50, 100, 160 and 220 mg/kg) or vehicle consecutively for 12 days. The control animals received the same amount of the vehicle control. The test session was performed 60 min after the last treatment. Percentage of open arm time (OAT %) and open arm entries (OAE %), and LMA variables were measured as described in the method section in all EPM experiments.
Effects of intra-CV injections of GABAA receptor agonist and antagonist on anxiety behavior
Three groups of rats received intra-CV microinjection of GABAA receptor agonist, muscimol (0.5, 0.75 and 1 µg/rat). The other three groups received GABAA receptor antagonist, bicuculline (0.5, 1 and 2 µg/rat) and were compared with the vehicle control group. The test session was performed 5 min after intra-CV injections.
Effects of S. striata extract alone or in combination with intra-CV injection of muscimol or bicuculline on anxiety behavior
Nine groups of rats received orally the S. striata extract (effective doses; 100 and 160 mg/kg) or vehicle, consecutively, for 12 days. (I) Three groups of rats received intra-CV injection of saline (1 μL/rat) 60 min after the last treatment. (II) Another six groups of rats received intra-CV injection of muscimol (1 µg/rat) or bicuculline (1 μg/rat) 60 min following the last treatment. The test session was performed 5 min after intra-CV administration.
Effects of S. striata extract on depression behavior
Six groups of rats received oral administration of various doses of S. striata extract (20, 50, 100, 160 and 220 mg/kg) or vehicle consecutively for 12 days. The control animals received the same amount of the vehicle control. The test session was performed 60 min after the last treatment. The total duration of immobility was measured as described in the method section in all experiments.
Effects of intra-CV injections of GABAA receptor agonist and antagonist on depression behavior
Three groups of rats received intra-CV microinjection of GABAA receptor agonist, muscimol (0.5, 0.75 and 1 µg/rat). The other three groups received GABAA receptor antagonist, bicuculline (0.5, 1 and 2 µg/rat) and were compared with the vehicle control group. The test session was performed 5 min after intra-CV administration.
Effects of S. striata extract alone or in combination with intra-CV injection of muscimol or bicuculline on depression behavior
Nine groups of rats received orally the S. striata extract (effective doses; 100 and 160 mg/kg) or vehicle, consecutively, for 12 days. (I) Three groups of rats received intra-CV injection of saline (1 μL/rat) 60 min after the last treatment. (II) Another six groups of rats received intra-CV injection of muscimol (1 µg/rat) or bicuculline (1 μg/rat) 60 min following the last treatment. The test session was performed 5 min after intra-CV administration.
Verification of cannula placements
At the end of behavioral tests, each rat was killed by chloroform overdose and then 1 µL/rat of a 1% methylene-blue solution was injected into the intra-CV as a marker of the injection sites. Brains were removed after decapitation and fixed in a 10% formalin solution at least for 10 days. The brains were sliced and the sites of injection were verified according to the atlas of Paxinos and Watson (Citation2007). Data from animals with injection sites located outside the intra-CV region were not used in the analysis.
Statistics
The statistical analyses were performed using the Statistical Package for Social Sciences (SPSS, Version 20, IBM Co., Chicago, IL). Since data displayed normal distribution and homogeneity of variance, one-way ANOVA was used for comparison between the effects of different doses of drugs with vehicle. Two-way ANOVA was used for evaluation of interactions between drugs. Following a significant F-value, post hoc analysis (Tukey’s-test) was performed for assessing specific group comparisons. Differences with p < 0.05 between experimental groups at each point were considered statistically significant.
Results
Effects of S. striata extract on anxiety behavior
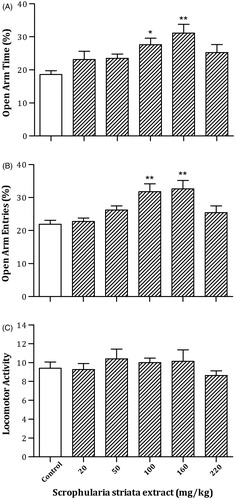
shows the effects of oral administration of the different doses of S. striata extract or vehicle consecutively for 12 days on anxiety behaviors in the EPM. A one-way ANOVA and post hoc analysis revealed that S. striata at doses of 100 and 160 mg/kg significantly increased OAT % [F(5,42) = 4.17, p < 0.005] and OAE % [F(5,42) = 5.89, p < 0.001], but no remarkable change in the LMA [F(5,42) = 0.65, p > 0.05] was observed. The data indicate induction of the anxiolytic response following the administration of S. striata extract.
Figure 2. Effects of oral administration of the different doses of S. striata extract or vehicle consecutively for 12 days on rat behavior in the EPM. Rats were treated with different doses of S. striata extract (20, 50, 100, 160 and 220 mg/kg). Each bar represents mean ± S.E.M. (n = 8) of OAT % (A), OAE % (B) or LMA (C). Significant differences: *p < 0.05 and **p < 0.01 compared to the control group.
Effects of intra-CV injections of GABAA receptor agonist and antagonist on anxiety behavior
(left panel) indicates the effects of intra-CV injections of the different doses of muscimol on anxiety behaviors in the EPM. A one-way ANOVA and post hoc analysis revealed that muscimol at dose of 1 µg/rat significantly increased OAT % [F(3,28) = 5.69, p < 0.005] and OAE % [F(3,28) = 3.31, p < 0.04], but no significant change in the LMA [F(3,28) = 0.31, p > 0.05] was observed. The data indicate the induction of an anxiolytic response in this brain site following the administration of GABAA receptor agonist.
Figure 3. Effects of intra-CV injection of GABAA receptor agonist or antagonist on rat behavior in the EPM. Rats were treated with saline (1 μL/rat); different doses of muscimol (0.5, 0.75 and 1 µg/rat) or bicuculline (0.5, 1 and 2 µg/rat) 5 min before EPM test in left and right panels. Each bar represents mean ± S.E.M. (n = 8) of OAT % (A), OAE % (B) or LMA (C). Significant differences: *p < 0.05 and **p < 0.01 compared to the control group.
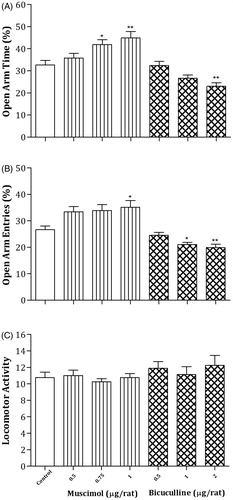
However, rats injected with different doses of bicuculline showed a significant decrease in OAT % [F(3,28) = 6.93, p < 0.002] and OAE % [F(3,28) = 6.91, p < 0.002] at a dose of 2 µg/rat, but no significant change in LMA [F(3,28) = 0.53, p > 0.05] was observed. The data show the induction of an anxiogenic response following the injection of GABAA receptor antagonist (, right panel).
Effects of S. striata extract alone or in combination with intra-CV injection of muscimol or bicuculline on anxiety behavior
indicates the effects of oral administration of effective doses of S. striata extract or vehicle consecutively for 12 days alone or in combination with intra-CV injection of muscimol (1 µg/rat) or bicuculline (1 µg/rat) on anxiety parameters in the EPM. In the GABAA agonist treatment groups, one-way ANOVA revealed that muscimol significantly increased the OAT % and OAE % at a dose of 160 mg/kg of S. striata extract, indicating lower levels of anxiety in the S. striata/muscimol group in comparison with water/muscimol (OAT %: [F(2,21) = 7.49, p < 0.005] and OAE % [F(2,21) = 4.25, p < 0.03]) or water/saline (OAT %: [F(3,28) = 21.43, p < 0.001] and OAE % [F(3,28) = 12.08, p < 0.001]) control group. In addition, in the GABAA antagonist treatment groups, one-way ANOVA revealed that bicuculline did not alter OAT % [F(2,21) = 1.47, p > 0.05] and OAE % [F(2,21) = 1.43, p > 0.05] at all doses of S. striata in comparison with water/muscimol control group, while significantly decreased OAT % [F(3,28) = 5.96, p < 0.004] and but not significant OAE % [F(3,28) = 1.71, p > 0.05] at all doses of S. striata in comparison with the water/saline control group. Also, no significant change was observed in the LMA of all groups.
Figure 4. Effects of S. striata extract alone or in combination with intra-CV injection of muscimol or bicuculline on anxiety behavior in the EPM. The rats treated with oral administration of the effective doses of S. striata extract or vehicle consecutively for 12 days, then rats received saline (1 μL/rat), muscimol (1 µg/rat) or bicuculline (1 μg/rat) intra-CV 60 min after the last treatment of extract and 5 min before testing. Each bar represents mean ± S.E.M. (n = 8) of OAT % (A), OAE % (B) or LMA (C). Significant differences: *p < 0.05 and **p < 0.01 compared to the control group; + p < 0.05 and ++p < 0.01 compared to the water/muscimol control group.
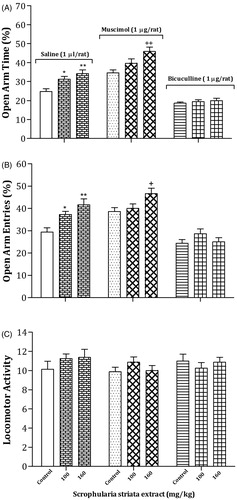
Two-way analyses revealed that there were not any significant interactions between most effective doses of S. striata (Factor A) and muscimol (Factor B) on OAT % [Factor A; F(2,42) = 15.17, p < 0.001, Factor B; F(1,42) = 42.62, p < 0.001, Factor (A × B); F(2,42) = 0.4, p > 0.05], OAE % [Factor A; F(2,42) = 11.99, p < 0.001, Factor B; F(1,42) = 11.24, p < 0.003, Factor (A × B); F(2,42) = 1.27, p > 0.05] and LMA [Factor A; F(2,42) = 1.39, p > 0.05, Factor B; F(1,42) = 1.6, p > 0.05, Factor (A × B); F(2,42) = 0.45, p > 0.05]. However, two-way analyses revealed that significant interactions existed between most effective doses of S. striata (Factor A) and bicuculline (Factor B) on OAT % [Factor A; F(2,42) = 7.92, p < 0.002, Factor B; F(1,42) = 90.63, p < 0.001, Factor (A × B); F(2,42) = 4.48, p < 0.02], OAE % [Factor A; F(2,42) = 6.58, p < 0.004, Factor B; F(1,42) = 38.44, p < 0.001, Factor (A × B); F(2,42) = 4.45, p < 0.02]. No significant interaction was indicated on LMA [Factor A; F(2,42) = 0.35, p > 0.05, Factor B; F(1,42) = 0.14, p > 0.05, Factor (A × B); F(2,42) = 0.37, p > 0.05].
Effects of S. striata extract on depression behavior
shows the effects of oral administration of the different doses of S. striata extract or vehicle consecutively for 12 days on depression behavior in the FST. A one-way ANOVA and post hoc analysis revealed that S. striata at a dose of 100 and 160 mg/kg considerably decreased the total duration of immobility time [F(5,42) = 5.73, p < 0.001] in the FST test. The results indicate the induction of antidepressant effects following the administration of S. striata extract.
Figure 5. Effects of oral administration of the different doses of S. striata extract or vehicle consecutively for 12 days (A); effects of intra-CV injection of GABAA receptor agonist or antagonist (B); effects of S. striata extract alone or in combination with intra-CV injection of muscimol or bicuculline on depression behaviors in the FST. More details of the results of statistical analysis and post hoc comparisons are provided in the Results section. Each bar represents mean ± S.E.M. (n = 8) of total duration of immobility. Significant differences: *p < 0.05 and **p < 0.01 compared to the control or saline group; +p < 0.05 compared to the water/muscimol control group.
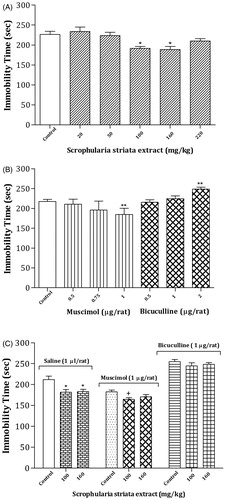
Effects of intra-CV injections of GABAA receptor agonist and antagonist on depression behavior
(B; left panel) indicates the effects of intra-CV injections of the different doses of muscimol on depression behavior in the FST. A one-way ANOVA and post hoc analysis revealed that muscimol at dose of 1 µg/rat markedly decreased the total duration of immobility time [F(3,28) = 5.92, p < 0.004] in FST test. The data show the induction of antidepressant effects in this brain site following the administration of GABAA receptor agonist.
However, rats injected with different doses of bicuculline showed a significant increase in the total duration of immobility time [F(3,28) = 6.04, p < 0.004] at a dose of 2 µg/rat. The findings show the induction of depression response following the injection of GABAA receptor antagonist (, right panel).
Effects of S. striata extract alone or in combination with intra-CV injection of muscimol or bicuculline on depression behavior
indicates the effects of oral administration of effective doses of S. striata extract or vehicle consecutively for 12 days alone or in combination with intra-CV injection of muscimol (1 µg/rat) or bicuculline (1 µg/rat) on the depression parameter in the FST. In the GABAA agonist treatment groups, one-way ANOVA revealed that muscimol significantly decreased the total duration of immobility time at a dose of 100 mg/kg of S. striata, indicating lower levels of depressant in the S. striata/muscimol group in comparison with the water/muscimol [F(2,21) = 3.68, p < 0.04] or water/saline [F(3,28) = 12.48, p < 0.001] control group. In addition, in the GABAA antagonist treatment groups, one-way ANOVA revealed that bicuculline did not alter the total duration of immobility time [F(2,21) = 0.75, p > 0.05] at all doses of S. striata in comparison with water/muscimol control group, but significantly increased the total duration of immobility time [F(3,28) = 8.30, p < 0.001] at all doses of S. striata in comparison with the water/saline control group. Also, no significant change was observed in the LMA.
Two-way analyses revealed there were not any significant interactions between most effective doses of S. striata (Factor A) and muscimol (Factor B) on the total duration of immobility time [Factor A; F(2,42) = 9.1, p < 0.002, Factor B; F(1,42) = 15.43, p < 0.001, Factor (A × B); F(2,42) = 1.05, p > 0.05]. Also, two-way analyses revealed that there were not any significant interactions between most effective doses of S. striata (Factor A) and bicuculline (Factor B) on this parameter [Factor A; F(2,42) = 5.65, p < 0.008, Factor B; F(1,42) = 114.21, p < 0.001, Factor (A × B); F(2,42) = 0.17, p > 0.05] in the FST test.
Discussion
To our knowledge, this is the first report of pharmacological effects of this plant on neuropsychiatric disorders. In the current study, our results indicated that administration of S. striata extract decreased levels of anxiety and depression-like behaviors in rats. In line with these findings, a recently published study conducted by Monsef-Esfahani et al. (Citation2010) showed the presence of quercetin as an effective compound in this plant. On the other hand, some authors have reported that quercetin can decrease anxiety and depression (Aguirre-Hernández et al., Citation2010; Bhutada et al., Citation2010) and there is also a relationship between quercetin function and neurotransmitter systems in the CNS (Goutman & Calvo, Citation2004; Kambe et al., Citation2010; Rotelli et al., Citation2009) which may alter psychiatric behaviors through indirect mechanisms. To explore this issue further, we have investigated interaction between the effects of S. striata and the GABAergic system in the brain. Our results showed that intra-CV microinjection of muscimol decreased anxiety and depression, whereas administration of bicuculline in this brain site increased levels of these disorders. The exact role of the GABAergic network in the various areas of brain in the regulation of anxiety and depression is not clearly recognized. However, in agreement with our data, previous studies have shown that GABAA receptor is largely distributed throughout the CNS and the GABAergic system plays an important role in the regulation and control of anxiety and depression, so that GABAergic agents or drugs are known for their powerful anxiolytic and antidepressant effects in humans and animals (Möhler, Citation2012). Moreover, another part of our study showed that co-administration of the bicuculline with S. striata extract decreased anxiolytic and antidepressant effects, while co-administration of the muscimol with S. striata increased these effects relative to the control group. These findings demonstrate that GABAA agonist can enhance the anxiolytic and antidepressant impacts of S. striata extract, while GABAA antagonist can block these effects.
Furthermore, it is well known that stress activates the hypothalamic–pituitary–adrenal axis with the stimulation of corticotrophin releasing factor (CRF) release from the hypothalamus. In the next step, CRF stimulates secretion of adrenocorticotropin hormone from the anterior pituitary and the stress hormone such as cortisol in humans and corticosterone in rodents (Bhutada et al., Citation2010). As a consequence, it should be emphasized that the CRF system is responsible for the regulation of anxiety and depression behaviors. In line with this, a recent report demonstrated that quercetin attenuated stress-induced behavioral effects. It also decreased levels of CRF expression in the brain (Bhutada et al., Citation2010).
On the one hand, numerous studies in recent years have reported anti-inflammatory effects of S. striata extract in rodents and found the presence of quercetin in this plant (Azadmehr et al., Citation2009; Monsef-Esfahani et al., Citation2010). On the other hand, several studies have indicated antioxidant, anti-inflammatory, neuroprotective, anxiolytic and antidepressant effects of quercetin in experimental models (Aguirre-Hernández et al., Citation2010; Bhutada et al., Citation2010). Collectively, in the light of presented evidence, it is conceivable that the effects of S. striata could be related to the presence of quercetin as a component of S. striata.
Conclusions
The pharmacological results of these experimental models show that S. striata decreases levels of anxiety and depression in rats. The mechanisms that lead to decrease the disorders in these conditions are likely complex. However, the above evidence suggests that the GABAergic system may modulate the effects of S. striata extract in rats. More studies are needed to investigate these pharmacological effects, including the investigation of different neurotransmitter systems.
Acknowledgements
We are very grateful to Mr. Ali-Akbar Salari for his help in statistical analysis of the data and English editing of the manuscript.
Declaration of interest
The authors declare there are no financial and commercial conflicts of interest in the present study.
This study was supported by the Neurosciences Research Center, Tabriz University of Medical Sciences (Project Number: 90-61-5).
References
- Aguirre-Hernández E, González-Trujano ME, Martínez AL, et al. (2010). HPLC/MS analysis and anxiolytic-like effect of quercetin and kaempferol flavonoids from Tilia americana var. mexicana. J Ethnopharmacol 127:91–7
- Azadmehr A, Afshari A, Baradaran B, et al. (2009). Suppression of nitric oxide production in activated murine peritoneal macrophages in vitro and ex vivo by Scrophularia striata ethanolic extract. J Ethnopharmacol 124:166–9
- Bergink V, Van Megen HJG, Westenberg HGM. (2004). Glutamate and anxiety. Eur Neuropsychopharm 14:175–83
- Bhutada P, Mundhada Y, Bansod K, et al. (2010). Reversal by quercetin of corticotrophin releasing factor induced anxiety-and depression-like effect in mice. Prog Neuro-Psychoph 34:955–60
- Cho JY, Kim IS, Jang YH, et al. (2006). Protective effect of quercetin, a natural flavonoid against neuronal damage after transient global cerebral ischemia. Neurosci Lett 404:330–5
- Garcia LSB, Comim CM, Valvassori SS, et al. (2008). Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog Neuro-Psychoph 32:140–4
- Goutman JD, Calvo DJ. (2004). Studies on the mechanisms of action of picrotoxin, quercetin and pregnanolone at the GABAρ1 receptor. Brit J Pharmacol 141:717–27
- Graeff FG, Guimarães FS, De Andrade TGCS, Deakin JFW. (1996). Role of 5-HT in stress, anxiety, and depression. Pharmacol Biochem Behav 54:129–41
- Hsieh MT, Peng WH, Wu CR, et al. (2010). Review on experimental research of herbal medicines with anti-amnesic activity. Planta Med 76:203–17
- Kalueff AV, Nutt DJ. (2007). Role of GABA in anxiety and depression. Depress Anxiety 24:495–517
- Kambe D, Kotani M, Yoshimoto M, et al. (2010). Effects of quercetin on the sleep–wake cycle in rats: Involvement of gamma-aminobutyric acid receptor type A in regulation of rapid eye movement sleep. Brain Res 1330:83–8
- Kano O, Ikeda K, Cridebring D, et al. (2011). Neurobiology of depression and anxiety in Parkinson’s disease. Parkinson's Disease 2011:5pp. Article ID 143547
- Möhler H. (2012). The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology 62:42–53
- Monsef-Esfahani HR, Hajiaghaee R, Shahverdi AR, et al. (2010). Flavonoids, cinnamic acid and phenyl propanoid from aerial parts of Scrophularia striata. Pharm Biol 48:333–6
- Ostad S, Salavati P, Ramezani M. (2010). Neuroprotective extract prepared from the aerial parts of Scrophularia striata Boiss against glutamate-induced neurotoxicity in primary cultures of rat pups cortical neurons. 9th International ISSX Meeting; 2010 Sep 4–8; Istanbul, Turkey
- Paxinos G, Watson C. (2007). The Rat Brain in Stereotaxic Coordinates. San Diego: Elsevier Academic Press
- Pitsavos C, Panagiotakos D, Papageorgiou C, et al. (2006). Anxiety in relation to inflammation and coagulation markers, among healthy adults: The ATTICA study. Atherosclerosis 185:320–6
- Raison CL, Capuron L, Miller AH. (2006). Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trend Immunol 27:24–31
- Rotelli AE, Aguilar CF, Pelzer LE. (2009). Structural basis of the anti-inflammatory activity of quercetin: Inhibition of the 5-hydroxytryptamine type 2 receptor. Eur Biophys J 38:865–71
- Solati J, Salari AA, Bakhtiari A. (2011). 5HT1A and 5HT1B receptors of medial prefrontal cortex modulate anxiogenic-like behaviors in rats. Neurosci Lett 504:325–9
- Solati J, Zarrindast MR, Salari AA. (2010). Dorsal hippocampal opioidergic system modulates anxiety-like behaviors in adult male Wistar rats. Psychiatry Clin Neurosci 64:634–41
- Van't Veer-Tazelaar PJ, Van Marwijk HWJ, Van Oppen P, et al. (2009). Stepped-care prevention of anxiety and depression in late life: A randomized controlled trial. Arch Gen Psychiatry 66:297–304