Abstract
Context: The ethanol extracts and their fractions of three Indian medicinal plants, Ervatamia coronaria (Jacq.) Stapf, (Apocynaceae), Mimosa pudica L. (Mimosaceae) and Caesalpinia bonduc (L.) Roxb. (Caesalpiniaceae) were tested for their cytotoxic activity in the brine shrimp lethality (BSL) bioassay and in various cancer cell lines. The plants were selected based on their traditional use in the treatment of cancer/tumors.
Objectives: To investigate the in vitro cytotoxicity of Ervatamia coronaria, Mimosa pudica and Caesalpinia bonduc.
Materials and methods: Ethanolic extracts and their fractions of E. coronaria, M. pudica and C. bonduc were subjected to cytotoxicity studies using BSL bioassay method with concentrations of 10, 50, 100, 500 and 1000 µg/ml. The alkaloid fraction of E. coronaria with significant cytotoxicity in BSL bioassay was subjected to in vitro cytotoxicity studies with HT-29, A-549, HepG-2, MCF-7 and L-6 cell lines at concentrations of 12.5, 25, 50, 100 and 200 µg/ml and a DNA fragmentation study using the HT-29 cell line.
Results: The alkaloid fractions of E. coronaria and M. pudica showed significant cytotoxicity with LC50 values of 65.83 and 85.10 µg/ml in the BSL bioassay, respectively. The purified alkaloid fraction of E. coronaria exhibited highest cytotoxicity in HT-29, A-549 and MCF-7 cell lines with IC50 values of 32.5, 47.5 and 72.5 µg/ml, respectively, and induced DNA fragmentation in the HT-29 cell line at a concentration of 65 µg/ml.
Conclusion: The alkaloid fraction of E. coronaria exhibited significant cytotoxicity. Alkaloids such as ervatamine, apparicine and coronaridine that were earlier reported may be responsible for this activity.
Introduction
Cancer is a group of diseases where a group of cells display uncontrolled growth, invasion and metastasis. According to WHO, cancer is a leading cause of death worldwide. In 2007, around 13% of all deaths were due to cancer (Jemal et al., Citation2011). It has been estimated that by 2030 deaths due to cancer may be around 12 million. In India, the International Agency for Research on Cancer estimated indirectly that more than half a million people died from cancer in 2008, representing about 8% of all estimated global cancer deaths and about 6% of all deaths in India (Ferlay et al., Citation2008). The number of cancer deaths in India is projected to increase because of population growth and increasing life expectancy (Jha, Citation2009). The estimated cancer mortality in 2000 was over 1.5 million, which is 3.61% of all deaths.
Anticancer agents from plant sources have various modes of action that inhibit various stages of the cancer cell growth. These phytoconstituents may act as anticancer agents either by inhibiting the cell growth or by killing the cancer cells (cytotoxic). The United States National Cancer Institute (NCI) played a major role in screening a large number of anticancer compounds from natural source. In 1955, the NCI set up the Cancer Chemotherapy National Service Center to act as a public screening center for anti-cancer activity of compounds (Goodman & Walsh, Citation2001). Many of these phytoconstituents have proved to be highly potent anticancer agents such as Vinca alkaloids, irinotecon, camptothecins, etoposides and paclitaxel (taxol). The NCI collected about 35 000 plants from 20 countries and has screened around 114 000 extracts for their anticancer activity (Shoeb, Citation2006). Of the 92 anticancer drugs commercially available prior to 1983 in the US and among worldwide-approved anticancer drugs between 1983 and 1994, 60% are of natural origin (Cragg et al., Citation1997).
In this context, the present study investigated the aerial parts of Ervatamia coronaria (syn. Tabernaemontana coronaria Jacq.) Willd. (Apocynaceae), whole plant of Mimosa pudica Linn. (Mimosaceae) and root bark of Caesalpinia bonduc (L.) Roxb. Dandy & Exell. (syn. Caesalpinia bonducella Flem.) (Caesalpiniaceae) for their cytotoxic activity. The extraction and fractionation method was a slight modification of the method proposed by Cos et al. (Citation2006). The leaves and aerial parts of E. coronaria have been reported to have anticancer property (Khare, Citation2007). The whole plant of M. pudica is used in the treatment of uterine tumors and root bark of C. bonduc is used in the treatment of various tumors in the traditional systems of medicine in India (Kirtikar & Basu, Citation1999).
Hence, the ethanol extract and their various fractions of these three plants were subjected for cytotoxicity evaluation using the brine shrimp lethality (BSL) bioassay and the fractions with promising results were subjected for in vitro cell line studies using MTT assay followed by DNA fragmentation studies.
Materials and methods
Plant material
The aerial parts of E. coronaria were collected from Belgaum surroundings in the month of June 2010; whole plant of M. pudica was collected from the vicinity of ICMR Belgaum in the month of June 2010; root bark of C. bonduc was collected from Jath, Sangli, Maharastra in the month of August 2010. Taxonomic identification and authentication was done by Dr. Harsha Hegde (Research Scientist), Indian Council of Medical Research, Belgaum, Karnataka. The voucher specimens (RMRC-546; RMRC-547 and RMRC-556, respectively) are deposited at the Herbarium and crude drug museum of Indian Council of Medical Research, Belgaum, Karnataka along with a sample of crude drug. All the plant materials were washed under running tap water and shade-dried. The shade-dried plant materials were powdered using a dry grinder to obtain coarse powder. The powdered crude drugs were stored in airtight containers separately until further use.
Cell lines and culture media
HT-29 (Human, colon cancer), A-549 (Human, small cell lung carcinoma), HepG-2 (Human, hepatic cancer), MCF-7 (Human, breast cancer) and L-6 (Rat, normal skeletal muscle) cell cultures were procured from National Centre for Cell Sciences, Pune, India. Stock cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% inactivated fetal bovine serum (FBS), penicillin (100 IU/ml), streptomycin (100 µg/ml) and amphotericin B (5 µg/ml) in an humidified atmosphere of 5% CO2 at 37 °C until confluent. The cells were dissociated with trypsin phosphate versene glucose solution (0.2% trypsin, 0.02% EDTA, 0.05% glucose in PBS). The stock cultures were grown in 25 cm2 culture flasks (Tarsons India Pvt. Ltd, Kolkata, India).
Extraction and fractionation
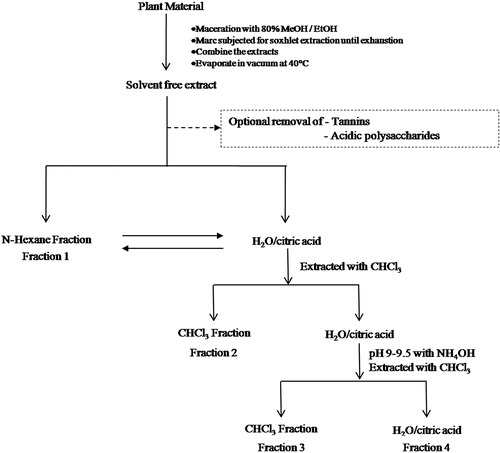
The air-dried powdered plant materials were subjected to maceration using ethanol for 48 h. The extract was filtered and the marc was subjected to Soxhlet extraction using ethanol as a solvent for 10 h. The extract was filtered at elevated temperature and mixed with the extract obtained by maceration. The combined extract was concentrated under reduced pressure at 40 °C using a rotary evaporator. The solvent free extract was subjected to fractionation using the protocol given by Cos et al. (Citation2006) with slight modifications. The detailed method is given in the form of a flow chart (). This procedure was employed to avoid any possible destruction of chemical constituents due to higher temperature during repeated extraction.
BSL bioassay
The brine shrimp (Artemia salina Lich.) eggs were obtained from Department of Pharmacology, Manipal College of Pharmacy, Manipal, India. The assay was a test for determination of the cytotoxicity of the extracts/fractions. The procedure and method was as described by McLaughlin et al. (Citation1998). The chamber was divided into two equal parts. Aeration was given in both the compartments. One part was illuminated with a bulb (60 W), while the other was darkened. Surface sterilized brine shrimp eggs were sprinkled in the dark side and incubated at room temperature for 48 h. As the hatching occurred, the nauplii were swum towards the illuminated side, where they were collected by a Pasteur pipette. Samples of the extracts/fractions were prepared by dissolving 50 mg of extract in 5 ml of DMSO to get 5000 µg/ml stock solution and further diluted with seawater to get the required concentrations (10, 50, 100, 500 and 1000 µg/ml). Dried vials were taken and 10 shrimps were transferred in each vial and then the volume was made up to 5 ml with seawater. A drop of dry yeast suspension (3 mg in 5 ml seawater) was added to each vial as food for shrimps. For each concentration tests were carried out in triplicate. Control vials were prepared by adding equal volumes of distilled water. The vials were maintained under illumination. After 24 h survivors were counted, by using 3× magnifying glass and the percentage of mortality and LC50 values were calculated by Probit analysis using SPSS-10.0.5 software (Armonk, NY).
MTT assay
For cytotoxicity studies, a weighed quantity of sample was dissolved in DMSO and the volume was made up with DMEM supplemented with 2% inactivated FBS to obtain a stock solution of 1 mg/ml concentration and sterilized by filtration. Serial two-fold dilutions were prepared from this for carrying out cytotoxic studies.
The monolayer cell culture was trypsinized and the cell count was adjusted to 1.0 × 105 cells/ml using DMEM medium containing 10% FBS. To each well of the 96-well microtiter plate, 0.1 ml of the diluted cell suspension (approximately 10 000 cells) was added. After 24 h, when a partial monolayer was formed, the supernatant was flicked off, the monolayer was washed once with medium and 100 µl of different test concentrations of fraction was added on to the partial monolayer in microtiter plates. The plates were incubated at 37 °C for 3 d in 5% CO2 atmosphere, and microscopical examination was carried out and observations were recorded at 24 h intervals. After 72 h, drug solutions in the wells were discarded and 50 µl of MTT in PBS were added to each well. The plates were gently shaken and incubated for 3 h at 37 °C in a 5% CO2 atmosphere. The supernatant was removed and 100 µl of propanol were added and the plates were gently shaken to solubilize the formed formazan. The absorbance was measured using a microplate reader at a wavelength of 540 nm. The percentage growth inhibition was calculated using the following formula and concentration of test drug needed to inhibit cell growth by 50% (IC50) values is generated from the dose-response curves for each cell line (Danizot & Lang, Citation1986).
DNA fragmentation studies
HT-29 cells (3 × 106/ml) were seeded into 60 mm plates and incubated at 37 °C with a 5% CO2 atmosphere for 24 h. The cells were washed with medium and treated with double the IC50 value of fraction (65 µg/ml), standard drug (30 ng/ml) and incubated at 37 °C, 5% CO2 for 24 h. As the incubation time ended, the chromosomal DNA of cancer cells was prepared with Roche apoptotic DNA ladder kit. Briefly, cells were harvested and lysed with lysis buffer for 10 min. The samples were mixed with isopropanol before passing through the filter and washed. The DNA was eluted from the filter and treated with RNAs at 37 °C for 30 min before loading onto 2% agarose gel electrophoresis and run 50 V/cm for 3 h. The gel was visualized under an UV trans-illuminator and photographed (Wan et al., Citation2005).
Data analysis
The LC50 values were calculated by Probit analysis at 95% confidence level using SPSS-10.0.5 statistical software.
Results
Phytochemical screening
All the extracts and the fractions were subjected to preliminary phytochemical investigations. Fractions I and II of all the extracts were found to contain highly non-polar constituents such as steroids and triterpenoids. Fraction III of both M. pudica and E. coronaria extract showed the presence of alkaloids. Fraction IV of all the extracts contained polyphenols and flavonoids. Saponins were found in fraction IV of E. coronaria ().
Table 1. Phytochemical screening of ethanol extracts and fractions of Ervatamia coronaria, Mimosa pudica and Caesalpinia bonduc.
BSL bioassay
All the three extracts and their fractions were subjected to the BSL bioassay at concentrations of 10, 50, 100, 500 and 1000 µg/ml. The alkaloidal fractions (Fraction III) of E. coronaria and M. pudica exhibited cytotoxicity with a LC50 values of 65.83 and 85.10 µg/ml, whereas other extracts and fractions showed moderate to no cytotoxicity in the BSL bioassay (). With promising results from the BSL assay, the E. coronaria alkaloidal fraction (Fraction III) was further purified and tested for cytotoxic activity in different in vitro models.
Table 2. Effect of different extracts/fractions with the BSL bioassay.
MTT assay
Since the alkaloidal fraction of E. coronaria extract revealed potent cytotoxicity in the BSL bioassay it was further purified and subjected for in vitro cytotoxicity studies with various cancer cell lines using the MTT assay (). The percentage cell death was tested for each cell line after 72 h of incubation. The fraction inhibited the proliferation of cells in a dose-dependent manner in the concentration range of 12.5–200 µg/ml with significant cytotoxicity at all the five tested cell lines. The activity was more prominent in HT-29 (human, colon cancer) and A-549 (human, small cell lung carcinoma) cell lines with IC50 (concentration required to inhibit 50% of cell growth) values of 32.5 and 47.5 µg/ml, respectively. In other cell lines it showed moderate cytotoxicity ().
Figure 2. Nonlinear regression pattern against probability scale of MTT assay of E. coronaria alkaloidal fraction.
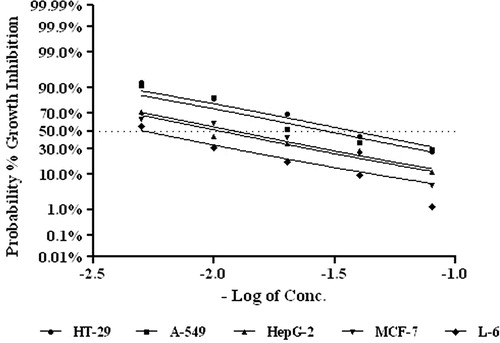
Table 3. Cytotoxic properties of Ervatamia coronaria alkaloidal fraction in different cell lines by MTT assay after 72 h of exposure.
DNA fragmentation study
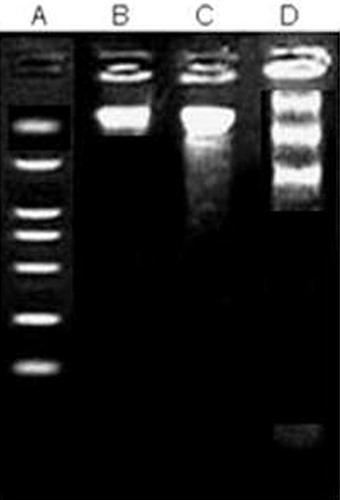
The alkaloidal fraction of E. coronaria was tested for DNA fragmentation pattern to confirm its cytotoxic activity in the HT-29 cell line using paclitaxol as standard molecule. The fraction showed clear fragmentation of the DNA ladder proving further its toxic effect on the tested cell line ().
Discussion
The major objective of the present study was to isolate the cytotoxic constituents from E. coronaria, M. pudica and C. bonduc through bioactivity-guided isolation. The study revealed that the alkaloidal fraction of M. pudica and E. coronaria showed promising cytotoxicity in the BSL bioassay. The BSL bioassay gives preliminary information about the toxic nature of compounds in rapidly multiplying cells, and supports their cytotoxic nature (Genupur et al., Citation2006; Silva et al., Citation2007). The alkaloidal fraction of E. coronaria was selected for further confirmation of cytotoxic activity using five different cancerous cell lines. The total alkaloidal fraction of E. coronaria showed significant cytotoxicity in human colon cancer (HT-29). Human small cell lung carcinoma (A-549) and human breast cancer cell lines (MCF-7) were also found sensitive to E. coronaria alkaloids. Two other cell lines, human hepatic cancer (HepG-2) and rat normal skeletal muscle (L-6), were comparatively less sensitive to these alkaloids (). The different parts of E. coronaria are used traditionally for the treatment of cancer (Khare, Citation2007). The results of our study provides scientific validation for the traditional claim.
Based on the cell line study data, we further proceeded with DNA fragmentation studies using HT-29 cell lines. The study was conducted simultaneously with paclitaxol which is a clinically proven anticancer agent. The results were again encouraging showing the clear fragmentation of the HT-29 DNA.
Various authors have reported a number of alkaloids from E. coronaria. A bisindole alkaloid, 19, 20-dihydroervahanine A was isolated from the stems of E. coronaria grown in Brazil (Henriques et al., Citation1996). Knox and Slobbe (Citation1971) isolated ervatamine an α-acyl-indolic alkaloid for the first time. Another indole alkaloid, apparicine, was isolated from the leaves of E. coronaria (Atta-ur-Rahaman et al., Citation1984). Coronaridine, an ibogamine related alkaloid, has been isolated from Ervatamia sp. (Gormam et al., Citation1960). A major indole alkaloid, vovacristine, has been isolated from E. coronaria and it has exhibited a cytostatic, cytotoxic and mutagenic effect in wild-type and repair-deficient yeasts (Melo et al., Citation1986).
Most of the clinically proved cytotoxic compounds are indole alkaloids, including Vinca alkaloids (Feng et al., Citation2010). The ibogan alkaloid, coronaridine, showed appreciable cytotoxicity toward sensitive (KB/S) as well as vincristine-resistant (KB/VJ300) cells (Kam et al., Citation2004). Six vobasinyl ibogan type bisindole alkaloids including four new ones, ervachinines A-D, were isolated from whole Ervatamia chinensis plant together with 10 known monoterpenoid indole alkaloids and found to be quite potent in terms of cytotoxic activity (Guo et al., Citation2012). These data indicate that the alkaloids of E. coronaria may contain a useful cytotoxic component that needs further study regarding anticancer properties.
Conclusions
The results of studied parameters confirm the potential of these three taxa for the production of cytotoxic components. This report provides very important data regarding the alkaloids of E. coronaria as a potent cytotoxic component. Further isolation, identification and antineoplastic studies of these compounds comprise ongoing investigations.
Declaration of interest
The authors report no conflicts of interest.
Acknowledgements
Authors are thankful to the Principal, KLE University’ College of Pharmacy, Belgaum for providing facilities to carry out this work.
References
- Atta-ur-Rahman, Daulatabadi N, Muzaffar A. (1984). Isolation of apparicine from the leaves of Ervatamia coronaria. J Nat Prod 47:900
- Cos P, Vlietinck AJ, Berghe DV, Maes L. (2006). Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J Ethnopharmacol 106:290–302
- Cragg GM, Newman DJ, Sander KM. (1997). Natural products in drug discovery and development. J Nat Prod 60:52–60
- Danizot F, Lang R. (1986). Rapid colorimetric assay for cell growth and survival modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods 89:271–7
- Feng T, Li Y, Wang YY, et al. (2010). Cytotoxic indole alkaloids from Melodinus tenuicaudatus. J Nat Prod 73:1075–9
- Ferlay J, Shin HR, Bray F, et al. (2008). Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127:2893–917
- Genupur A, Jesu JL, Srinivasan N, et al. (2006). Synthesis and cytotoxicity of novel isomeric C-seco limonoids. Eur J Med Chem 41:997–1002
- Goodman J, Walsh V. (2001). The Story of Taxol: Nature and Politics in the Pursuit of an Anti-cancer Drug. Cambridge, UK: Cambridge University Press
- Gormam M, Neuss N, Cone NJ, Deyrup JA. (1960). Alkaloids from apocynaceae III. Alkaloids of Tabernaemontana and Ervatamia. The structure of coronaridine, a new alkaloid related to ibogamine. J Am Chem Soc 82:1142–5
- Guo LL, He HP, Di YT, et al. (2012). Indole alkaloids from Ervatamia chinensis. Phytochemistry 74:140–5
- Henriques AT, Melo AA, Moreno PRH, et al. (1996). Ervatamia coronaria: Chemical constituents and some pharmacological activities. J Ethnopharmacol 50:19–25
- Jemal A, Bray F, Center MM, et al. (2011). Global cancer statistics. CA Cancer J Clin 61:69–90
- Jha P. (2009). Avoidable global cancer deaths and total deaths from smoking. Nat Rev Cancer 9:655–64
- Kam TS, Sim KM, Pang HS, et al. (2004). Cytotoxic effects and reversal of multidrug resistance by ibogan and related indole alkaloids. Bioorg Med Chem Lett 14:4487–9
- Khare CP. (2007). Indian Medicinal Plants An Illustrated Dictionary. Berlin/Heidelberg: Springer-Verlag
- Kirtikar KR, Basu BD. (1999) Indian Medicinal Plants, 2nd ed. Dehra Dun: International Book Distributor
- Knox JR, Slobbe J. (1971). Three novel alkaloids from Ervatamia orientalis. Tetrahedron Lett 24:2149–51
- McLaughlin JL, Rogers LL, Anderson JE. (1998). The use of biological assays to evaluate botanicals. Drug Inf J 32:513–24
- Melo AA, Querol CB, Henriques AT, Henriques JA. (1986). Cytostatic, cytotoxic and mutagenic effects of voacristine, an indole alkaloid in wild-type and repair-deficient yeasts. Mutat Res 171:17–24
- Shoeb M. (2006). Anticancer agents from medicinal plants. Bangladesh J Pharmacol 1:35–41
- Silva DJK, Sousa PJ, Andrade EH, Maia JG. (2007). Antioxidant capacity and cytotoxicity of essential oil and methanol extract of Aniba canelilla (H.B.K.) Mez. J Agr Food Chem 55:9422–6
- Wan CK, Wang C, Cheung HY, et al. (2005). Triptolide induces Bcl-2 cleavage and mitochondria dependent apoptosis in p53-dependent HL-60 cells. Cancer Lett 241:1–11