Abstract
Context: It is now well established that the surface of nanocarriers with specific ligands defines a new biological identity, which assist in targeting and internalization of the nanocarriers to specific cell populations, such as cancers and disease organs.
Objective: The aim of this study is to develop systemically administrable dual ligands modified nano-system which could both target cancer cells and macrophages in the liver.
Methods: Transferrin (Tf) and mannan (M) were linked onto polyethylene glycol-phosphatidylethanolamine (PEG-PE) and PE separately to get transferrin-PEG-PE (T-PEG-PE) and mannan-PE (M-PE) ligands for the surface modification of carriers. The in vivo transfection efficiency of the novel dual ligands modified (D-modified) vectors were evaluated in tumor bearing animal models.
Results: D-modified solid lipid nanoparticles/enhanced green fluorescence protein plasmid (D-SLN/pEGFP) has a particle size of 198 nm and a gene loading quantity of 89%. D-SLN/pEGFP displayed over 25% higher transfection efficiency than M-PE modified SLN/pEGFP (M-SLN/pEGFP) in HepG2 cells and T-PEG-PE modified SLN/pEGFP (T-SLN/pEGFP) in Kupffer cells (KCs) isolated from mice.
Conclusion: It could be concluded that T-PEG-PE and M-PE could function as excellent active targeting ligands to improve the cell targeting ability of the carriers and the dual ligands modified vectors could be applied as a promising active targeting gene delivery system.
Introduction
The efficiency of nanomedicine used for gene therapy is relying on the efficient targeted gene delivery systems. Non-viral gene delivery systems such as polymeric nanoparticles (Fields et al., Citation2012), liposomes (Kong et al., Citation2012), and solid lipid nanoparticles (SLN) (Jiang et al., Citation2012) have been widely developed for they have advantages such as less toxicity, low immunogenicity, and ease of modification. Much attention has been paid to the use of cationic SLN as gene carriers, which may offer a number of technological advantages, including better storage stability in comparison to liposomes, the possibility of steam sterilization and lyophilization, large scale production with qualified production lines and the use of substances that are generally accepted as safe (Doktorovova et al., Citation2011; Lobovkina et al., Citation2011; Olbrich et al., Citation2001; Vighi et al., 2007).
However, the lack of efficient site specific delivery systems impeded the prevalent practical realization of non-viral gene therapy (Kim et al., Citation2010). Therefore, surface modification has been applied to potentiate these gene delivery vectors (Choi et al., Citation2006). Surface modification of nanocarriers with specific ligands can assist in targeting and internalization of the nanocarriers to specific cell populations, such as cancers and disease organs (Yu et al., Citation2010).
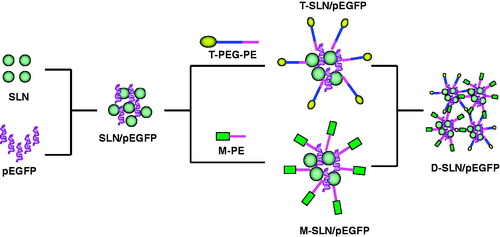
Polyethylene glycol-phosphatidylethanolamine (PEG-PE) conjugates with various PEG lengths and terminal-targeted moieties can provide extremely stable, long-circulating and actively targeted nanocarriers, which spontaneously accumulate at specific sites (Lukyanov et al., Citation2002; Torchilin Citation2005, Citation2006). In this study, we would like to provide a dual ligand modified system which could both target HepG2 cells and Kupffer cells (KCs) in liver. T-PEG-PE and M-PE were applied as the target ligands. Cationic SLN were used as the vectors and plasmid enhanced green fluorescent protein (pEGFP) was used as the model gene. This kind of gene delivery nano-system for liver targeted transfection was evaluated in vivo for the determination of the target ability. Single ligand modified and unmodified systems were used as controls.
Materials and methods
Materials
pEGFP-N1 was kindly provided by the Shandong University (Shandong, China). Injectable soya lecithin was obtained from Shanghai Taiwei Pharmaceutical Co. Ltd (Shanghai, China). Maleimide-PEG-COOH was purchased from Shanghai Yarebio Co., Ltd. (Shanghai, China). Stearic Acid, Human Tf (iron-free), mannan, L-α-PE, dimethyldioctadecylammonium bromide (DDAB) and Cell Counting Kit-8 (CCK-8) were purchased from Sigma-Aldrich Co. Ltd (St Louis, MO). Quant-iT™ PicoGreen® dsDNA quantitation reagent was obtained from Invitrogen by Life Technologies (Carlsbad, CA). HepG2 cells and KCs were obtained from the American type culture collection (Manassas, VA). All other chemicals were of analytical grade or higher.
Animals
BALB/c mice (4–6 weeks old, 20 ± 2 g weight) were purchased from the Medical Animal Test Center of Shandong University and housed under standard laboratory conditions. All animal experiments complied with the requirements of the National Act on the Use of Experimental Animals (People’s Republic of China).
Synthesis of T-PEG-PE and M-PE
T-PEG-PE ligands were synthesized using the method reported previously (Wang et al., Citation2012). Maleimide-PEG-COOH (1 g) was dissolved with dimethyl sulfoxide (DMSO) and stirred with PE (0.5 g) as a mixture. 1-[3-(Dimethylamino)propyl]-3-ethylcarbodiimide (EDC·HCl) (1 g) and triethylamine (TEA, 1 equivalent of EDC·HCl) were dissolved in DMSO and added dropwise into the mixture in an ice bath, stirred for 24 h to produce Maleimide-PEG-CO-NH-PE (PEG-PE). Tf was firstly modified with 1 equivalent of Traut’s reagent to complete thiolation of Tf (Jiang et al., Citation2007). The thiolated Tf was then added to the PEG-PE solution and the whole was incubated for 4 h at room temperature with gentle stirring. The product was dialyzed against Milli-Q water for 24 h to form T-PEG-PE solution. The mixture was centrifuged at 10 000g for 30 min at 4 °C, and then resuspended in PBS (pH 7.4).
M-PE ligands were synthesized as the previous method (Yu et al., Citation2010). Mannan (1 g) was dissolved with sodium hydroxide (1 M, 10 mL) and stirred for 30 min for alkalinization, then chloroacetic acid (20%, 1 mL) was added into the solution and stirred in an oil bath 60 °C) for 6 h. After that, hydrochloric acid (1 M) was added until pH 2–3 to complete the carboxymethylation of mannan. Carboxymethylated mannan was then stirred with PE in DMSO solution and EDC·HCl mixed with TEA (1 equivalent of EDC·HCl, in DMSO) were added dropwise into the solution in an ice bath, stirred for 24 h. DMSO was moved by rotary evaporation, and the product was dialyzed against Milli-Q water for 24 h to finally form M-PEG-PE.
Preparation of cationic SLN/pEGFP
SLN were prepared following the solvent displacement method (Endres et al., Citation2012). Stearic acid (50 mg) and injectable soya lecithin (30 mg) was accurately weighted and dissolved in 10 ml acetone. The organic phase was added dropwise into the 0.2% DDAB solution being stirred at 600 rpm at room temperature. When complete evaporation of the organic solvent had occurred, the redundant stabilizers were separated by ultracentrifugation at 1000 g, 4 °C for 20 min. The pellet was vortexed and resuspended in Milli-Q water, washed three times, filtered through a 0.45 μm membrane, and adjusted to pH 7.0 ± 0.1 with sodium hydroxide. The obtained SLN suspensions were stored at 2–8 °C.
SLN/pEGFP complexes were prepared by incubated the SLN with pEGFP. Briefly, pEGFP was mixed with SLN by vortexing the particles with a 1 mg/mL solution of pEGFP for 20 s. Incubation of the mixture for 20 min at RT facilitated the formation of SLN/pEGFP.
Modification of SLN/pEGFP with T-PEG-PE and M-PE
T-PEG-PE ligands were dissolved in 5 mL of PBS (pH 7.4). Then the solution was added dropwise into 20 mL of SLN/pEGFP complexes that was stirred at 600 rpm at RT leading to the immediate modification. The obtained complexes was resuspended in Milli-Q water, washed three times, and filtered through a membrane with 0.80 μm pore size to obtain T-PEG-PE-SLN/pEGFP (T-SLN/pEGFP).
M-PE ligands were dissolved in 5 mL of PBS (pH 7.4). The solution was then added dropwise to 20 mL of SLN/pEGFP complexes and stirred at 600 rpm in room temperature, leading to immediate modification. The complexes obtained were resuspended in Milli-Q water, washed three times, and filtered through a membrane with a pore size of 0.80 μm to obtain M-PE-SLN/pEGFP (M-SLN/pEGFP).
Dual ligands modified SLN/pEGFP complexes (D-SLN/pEGFP) were obtained by mixing the two kinds of modified vectors together and stirred at 400 rpm for 20 min to get the double targeted system ().
Characterization of D-SLN/pEGFP
Size, size distribution and zeta potential
The mean particle size, polydispersity index (PDI) and zeta potential of SLN, SLN/pEGFP, T-SLN/pEGFP, M-SLN/pEGFP and D-SLN/pEGFP were analyzed by photon correlation spectroscopy with a Zetasizer 3000 (Malvern Instruments, Malvern, England). The average particle size was expressed as volume mean diameter and the reported value was represented as mean ± SD (n = 3).
Gene loading capacity: PicoGreen-fluorometry assay
The pEGFP was isolated from D-SLN/pEGFP by centrifugation at 1000 g, 4 °C for 30 min. The concentration of pEGFP was determined by fluorescence, comparing with the supernatant from blank SLN. The amount of pEGFP loaded in the SLN was calculated according to the linear calibration curve of pEGFP. Gene loading quantity (%) = (Total amount of pEGFP − The amount of free pEGFP)/Total amount of DNA × 100.
In vitro cytotoxicity evaluation
To examine the cytotoxicity, HepG2 cells and KCs were seeded in 96-well plates at 8 × 103 cells/well and incubated for 24 h to allow cell attachment (Suzuki et al., Citation2008). The cells were incubated with SLN/pEGFP and D-SLN/pEGFP complexes at various concentrations (10, 20, 50, 100 and 200 μg/ml) for 48 h at 37 °C and 5% CO2 atmosphere, respectively. Lipofectamine™ 2000 (Lip) was used as positive control according to the manufacturer’s procedures. Cells without incubation were used as negative control. Cellular viability was assessed using CCK-8 according to the manufacturer’s procedures and the absorbance at 450 nm was measured using a microplate reader (Model 680, BIO-RAD, Hercules, CA). Cells without the addition of CCK-8 were used as a blank to calibrate the spectrophotometer to zero absorbance. The relative cell viability (%) was calculated as (Abssample − Absblank)/(Abscontrol − Absblank) × 100.
Animal model preparation
Tumor-bearing mice were prepared by inoculating (s.c.) a suspension of HepG2 cells (2 × 106 cells) into the right armpit of BALB/c mice (Li et al., Citation2012; Liu et al., Citation2011). Briefly, the mise were acclimatized at a temperature of 25 ± 2 °C and a relative humidity of 70% ± 5% under natural light/dark conditions for 1 week before dosing. Then the mice were injected subcutaneously in the right armpit with HepG2 cells suspended in PBS. Tumors were permitted to reach 8–10 mm in diameter before initiation of the studies.
In vivo transfection studies
In vivo transfection activity of D-SLN/pEGFP was evaluated against HepG2 solid tumors in mice. Five groups of tumor-bearing mice (right per group) were used. The mice were injected intravenously with naked DNA, SLN/pEGFP, T-SLN/pEGFP, M-SLN/pEGFP and D-SLN/pEGFP. The mice were sacrificed at 48 or 72 h after injection and the tumor tissue samples and liver were taken out. The tumor tissues were homogenized by pressing the samples through a 30 μm cell mesh with the plunger of a 10 ml syringe; erythrocyte lysis buffer was added during homogenization to lyse the red blood cells (Li et al., Citation2012). The homogenates were washed three times with PBS containing 0.5% bovine serum albumin and then filtered. The cells were finally obtained after centrifugation (4 °C, 100g, 5 min) and were seeded into 24-well plates in 1 ml of Dulbecco’s Modified Eagle’s Medium with 10% fetal bovine serum (FBS). KCs were isolated from mice under pentobarbitone anesthesia (Liu et al., Citation2007; Olynyk et al., Citation1998). In brief, the rat portal vein was cannulated and perfused with Hank’s buffered salt solution for 10 min, the liver was excised, and the perfusate was discarded. The liver was perfused with 0.2% pronase, then with a recirculating solution of 0.05% pronase and 0.05% collagenase until the liver was digested. Next the liver was cut into small pieces, suspended in a solution containing 0.02% pronase, 0.05% collagenase and 0.005% DNase, and then agitated. Following digestion, the liver homogenate was filtered through sterile gauze and centrifuged at 1000g and 4 °C for 10 min. The supernatant was removed and the pellet then resuspended in Percoll™ gradient. Aliquots of this cell suspension were added to aliquots of Percoll gradient. These were carefully overlaid with Hank’s balanced salt solution and centrifuged. The nonparenchymal cell-enriched layer observed at the interface between the two layers was carefully harvested and diluted with Hank’s balanced salt solution. The suspension was then centrifuged to precipitate the KCs, which were then seeded into a 96-well microtiter plate at a density of 2 × 104 cells/well in RPMI 1640 supplemented with 10% FBS and antibiotics. After incubation at 37 °C for 2 h under 5% CO2 atmosphere, the culture medium was replaced by 200 μL of fresh RPMI 1640 to yield the purified KCs.
Flow cytometry was applied to quantitate the amount of cells that have been successfully transfected. The cells were washed with 1 ml of PBS (100g, 4 °C for 5 min) and were detached with trypsin/EDTA. The supernatant was discarded and resuspended with 300 μl of PBS and added into the flow cytometry to quantitate the amount of HepG2 cells and KCs which have been successfully transfected.
Statistical analysis
All studies were repeated three times and all measurements were carried out in triplicate. Results were reported as means ± SD (SD = standard deviation). Statistical significance was analyzed using the Student’s t-test. Differences between experimental groups were considered significant when the p value was less than 0.05.
Results
Structure confirmation of T-PEG-PE and M-PE
The structure of T-PEG-PE and M-PE was confirmed by 1H NMR spectroscopy. The structure of M-PE was confirmed by infrared spectroscopy (IR) and nuclear magnetic resonance (1H NMR) spectroscopy. IR ν/cm−1: 3437.1 (–NH–, –OH); 1819.5 (–C=O); 1548.6 (–HN–CO–, evidence of the mannan linked with PE); 1089.2 (–C–O–C–, secondary alcohol structure in mannan). 1H NMR (DMSO-d6, 300 MHz) δ: 2.51 (CH2CO), 3.24 (CH2N) 6.01 (NH). δ (3.0–6.5), the similar peaks were observed as the spectra of mannan; δ (0–3.0), the peaks were in accordance with PE. The production rate was around 65%. The 1H NMR data of T-PEG-PE (DMSO-d6, 300 mHz) δ 2.48 (CH2CO, OCH2CH2NHCO), 3.29 (CH2N), 3.21–3.50 (OCH2CH2), 3.68 (NHCO) and 6.10 (NH). The production rate was around 70%.
Characterization of D-SLN/pEGFP
Mean particle size, PDI and zeta potential of SLN, SLN/pEGFP, T-SLN/pEGFP, M-SLN/pEGFP and D-SLN/pEGFP as well as gene loading capacity were characterized and summarized in .
Table 1. Particle size, zeta potential and gene loading quantity of different vectors.
In vitro cytotoxicity evaluation
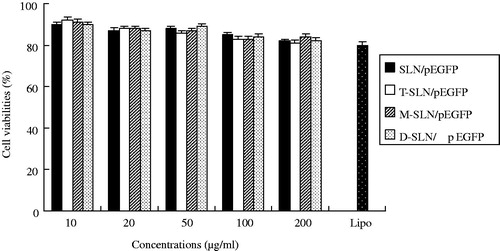
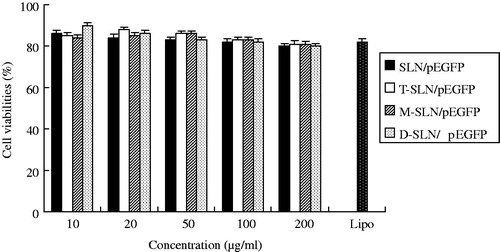
In vitro cytotoxicity of SLN/pEGFP, T-SLN/pEGFP, M-SLN/pEGFP and D-SLN/pEGFP were evaluated by CCK-8 in HepG2 cells () and KCs () at different concentrations. The cell viabilities of the vectors over the studied concentration range (10–200 μg/ml) were between 80% and 100% compared with controls.
In vivo gene delivery
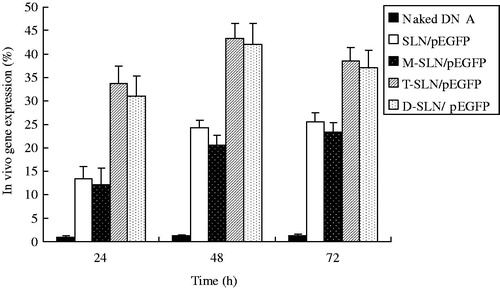
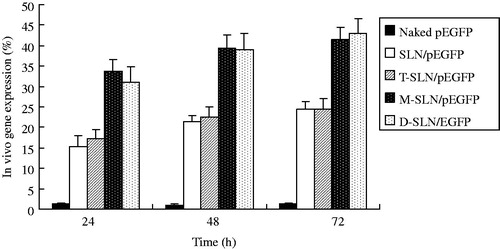
The in vivo transfection efficiency of SLN/pEGFP, T-SLN/pEGFP, M-SLN/pEGFP and D-SLN/pEGFP were evaluated against HepG2 solid tumors in mice. In HepG2 cells (), D-SLN/pEGFP achieved almost the same transfection efficiency when compared with T-SLN/pEGFP (p > 0.05), and had the higher transfection efficiency at different time intervals compared to other vectors (p < 0.05). In KCs (), D-SLN/pEGFP gain the same transfection efficiency when compared with M-SLN/pEGFP (p > 0.05), and displayed remarkably higher transfection efficiency than others (p < 0.05).
Discussion
With a deep understanding of gene delivery process, it is acknowledged that efficient gene delivery asks for multifunctional gene vectors that possess a long circulation time, cellular or tissue targeting (Kamiya et al., Citation2003). The aim of this study is to develop a kind of dual ligand modified system, which could both target HepG2 cells and KCs in liver. This kind of dual ligand system could target two different kinds of cells in liver at the same time, deliver the therapeutic materials at different sites, and enhance the therapeutic effect of the drugs/genes and decrease the side effects.
HepG2 is a human liver carcinoma cell line that is widely used for the study of polarized human hepatocytes (Pinti et al., Citation2003). Tf is an iron-binding glycoprotein. When Tf loaded with iron encounters Tf receptor (TfR) on the surface of cell, they bind and are consequently transported into the cell (Bellocq et al., Citation2003; Singh, Citation1999). Tf is especially useful in targeting to cancer cells, since many cancer cells over-express TfR on their surface (Li et al., Citation2002; Maruyama et al., Citation2011; Wagner et al., Citation1994). So, Tf containing ligand (T-PEG-PE) was used as the first target moiety that could bind to the TfR on the HepG2 cells.
Macrophages are important targets for gene therapy and which are also key components of cancer-promoting inflammatory reactions (Kawakami et al., Citation2008; Mantovani et al., Citation2006). Tumor-associated macrophages represent the major inflammatory component of the stroma of many tumors and can affect different aspects of the neoplastic tissue (Sica et al., Citation2006). Thus, therapeutic targeting of macrophages-derived mediators may provide innovative therapeutic strategies against tumor invasion and metastasis. KCs are liver-specific resident macrophages that play an integral part in physiological homeostasis of the liver. They have significant roles in acute and chronic responses of the liver to bacterial and viral infections, toxic or carcinogenic attack, as well as mediating hepatotoxicity (Kitani et al., Citation2010). Several studies have confirmed KCs express mannose receptor (MR) on their surfaces and the feasibility of using mannose- or mannan-modified nanocarriers to target them (Cui et al., Citation2003, Citation2004; Ezekowitz et al., 1991). So, mannan containing ligand (M-PE) was used as the second target moiety that could bind to the MR on the KCs.
In recent years, much attention has been paid to the use of cationic SLN as gene carriers (Jiang et al., Citation2012; Wang et al., Citation2012). The in vivo experiments concluded that both of the vectors achieved better transfection efficiency compared to the unmodified ones on the target cells. In the present study, we would like to make a combination of them and develop a dual ligands system that has the ability to target different kinds of cells at one injection. During the modification procedure, T-PEG-PE ligands and M-PE were coated onto the SLN/DNA surface separately to form two kinds of single ligand systems first (T-SLN/pEGFP and M-SLN/pEGFP), and then they were mixed carefully to form a uniform dual ligands system (D-SLN/pEGFP).
The mean particle size of D-SLN/pEGFP is around 198 nm (), which is in accordance with T-SLN/pEGFP (203 nm) and M-SLN/pEGFP (192 nm). D-SLN/pEGFP has a zeta potential of +20 mV, it is also the same with the single ligand system (T-SLN/pEGFP +19 mV, M-SLN/pEGFP +21 mV). The gene loading efficiency of D-SLN/pEGFP was 89%, which marked no significant difference from T-SLN/pEGFP (90%) and M-SLN/pEGFP (88%). These results could demonstrate the uniformity and stability of the dual ligands system.
In vitro cytotoxicity of D-SLN/pEGFP and other vectors were analyzed in both HepG2 cells and KCs. The cell viabilities of HepG2 and KCs treated with D-SLN/pEGFP, T-SLN/pEGFP, M-SLN/pEGFP and SLN/pEGFP over the studied concentration range were between 80 and 100% compared with controls ( and ). D-SLN/pEGFP showed no higher cytotoxicity than other vectors at all concentrations.
The gene delivery ability of D-SLN/pEGFP and others were tested further in animal models. In vivo gene delivery and expression studies were evaluated against HepG2 tumors bearing mice. After intravenous injection, the rats were euthanized at 24, 48 and 72 h, at each time point HepG2 cells and KCs were isolated and analyzed. In HepG2 cells (), D-SLN/pEGFP achieved almost the same transfection efficiency when compared with T-SLN/pEGFP (p > 0.05), and had the higher transfection efficiency at different time intervals compared to other vectors (p < 0.05). In KCs (), D-SLN/pEGFP gain the same transfection efficiency when compared with M-SLN/pEGFP (p > 0.05), and displayed remarkably higher transfection efficiency than others (p < 0.05). D-SLN/pEGFP gained high efficiency in both HepG2 cells and KCs in one dose of injection, which could be the evidence of the double targeted ability of the dual ligands system. These observations above strongly support the active targeting ability of Tf and mannan modified SLN/pEGFP in both HepG2 cells and KCs and the resulting vectors would be very useful for in vivo gene delivery.
Conclusions
This study showed that dual ligands (Tf and mannan) mediated targeting can successfully enhance the gene expression in liver cancer cells and liver macrophages. It could also demonstrate that having the modification ligands, such as T-PEG-PE and M-PE, in SLN formulations can significantly improve the transfection efficiency of the carriers in target cells. D-SLN/pEGFP had remarkably higher transfection efficiency both in HepG2 cells and KCs in vivo which demonstrate the double targeted capability of the vectors. It could be concluded that Tf and mannan could function as an excellent active targeting ligand to improve the cell targeting ability of the carriers and the resulting dual ligands nanomedicine could be used as a promising double targeted gene delivery system.
Declaration of interest
The work was supported by the Natural Science Foundation of Shandong Province (ZR2011HQ032, ZR2011HM030). The authors do not have any conflict of interest with the content of the manuscript.
Reference
- Bellocq NC, Pun SH, Jensen GS, Davis ME. (2003). Transferrin-containing, cyclodextrin polymer-based particles for tumor-targeted gene delivery. Bioconjug Chem 14:1122–32
- Choi JS, Ko KS, Park JS, et al. (2006). Dexamethasone conjugated poly(amidoamine) dendrimer as a gene carrier for efficient nuclear translocation. Int J Pharm 320:171–8
- Cui ZG, Han SJ, Huang L. (2004). Coating of mannan on LPD particles containing HPV E7 peptide significantly enhances immunity against HPV-positive tumor. Pharm Res 21:1018–25
- Cui ZR, Hsu CH, Mumper RJ. (2003). Physical characterization and macrophage cell uptake of mannan-coated nanoparticles. Drug Dev Ind Pharm 29:689–700
- Doktorovova S, Shegokar R, Rakovsky E, et al. (2011) Cationic solid lipid nanoparticles (cSLN): Structure, stability and DNA binding capacity correlation studies. Int J Pharm 420:341–9
- Endres T, Zheng M, Beck-Broichsitter M, et al. (2012). Optimising the self-assembly of siRNA loaded PEG-PCL-lPEI nano-carriers employing different preparation techniques. J Control Release 160:583–91
- Ezekowitz RA, Williams DJ, Koziel H, et al. (1991). Uptake of Pneumocystis carinii mediated by the macrophage mannose receptor. Nature 351:155–8
- Fields RJ, Cheng CJ, Quijano E, et al. (2012). Surface modified poly(β amino ester)-containing nanoparticles for plasmid DNA delivery. J Control Release 164:41–8
- Jiang Y, Liu C, Hong M, et al. (2007). Tumor cell targeting of transferrin-PEG-TNF-alpha conjugate via a receptor-mediated delivery system: Design, synthesis, and biological evaluation. Bioconjug Chem 18:41–9
- Jiang Z, Sun C, Yin Z, et al. (2012). Comparison of two kinds of nanomedicine for targeted gene therapy: Premodified or postmodified gene delivery systems. Int J Nanomed 7:2019–31
- Kamiya H, Akita H, Harashima H. (2003). Pharmacokinetic and pharmacodynamic considerations in gene therapy. Drug Discov Today 8:990–6
- Kawakami S, Higuchi Y, Hashida M. (2008). Nonviral approaches for targeted delivery of plasmid DNA and oligonucleotide. J Pharm Sci 97:726–45
- Kim SK, Park KM, Singha K, et al. (2010). Galactosylated cucurbituril-inclusion polyplex for hepatocyte-targeted gene delivery. Chem Commun (Camb) 46:692–4
- Kitani H, Takenouchi T, Sato M, et al. (2010). A novel isolation method for macrophage-like cells from mixed primary cultures of adult rat liver cells. J Immunol Methods 360:47–55
- Kong F, Zhou F, Ge L, et al. (2012). Mannosylated liposomes for targeted gene delivery. Int J Nanomed 7:1079–89
- Li P, Liu D, Miao L, et al. (2012). A pH-sensitive multifunctional gene carrier assembled via layer-by-layer technique for efficient gene delivery. Int J Nanomed 7:925–39
- Li H, Qian ZM. (2002). Transferrin/transferrin receptor-mediated drug delivery. Med Res Rev 22:225–50
- Liu C, Yu W, Chen Z, et al. (2011). Enhanced gene transfection efficiency in CD13-positive vascular endothelial cells with targeted poly(lactic acid)-poly(ethylene glycol) nanoparticles through caveolaemediated endocytosis. J Control Release 151:162–75
- Liu H, Cao H, Wu ZY. (2007). Isolation of Kupffer cells and their suppressive effects on T lymphocyte growth in rat orthotopic liver transplantation. World J Gastroenterol 13:3133–6
- Lobovkina T, Jacobson GB, Gonzalez-Gonzalez E, et al. (2011). In vivo sustained release of siRNA from solid lipid nanoparticles. ACS Nano 5:9977–83
- Lukyanov AN, Gao Z, Mazzola L, Torchilin VP. (2002). Polyethylene glycoldiacyllipid micelles demonstrate increased accumulation in subcutaneous tumors in mice. Pharm Res 19:1424–9
- Mantovani A, Schioppa T, Porta C, et al. (2006). Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev 25:315–22
- Maruyama K. (2011). Intracellular targeting delivery of liposomal drugs to solid tumors based on EPR effects. Adv Drug Deliv Rev 63:161–9
- Olbrich C, Bakowsky U, Lehr CM, et al. (2001). Cationic solid-lipid nanoparticles can efficiently bind and transfect plasmid DNA. J Control Release 77:345–55
- Olynyk JK, Clarke SL. (1998). Isolation and primary culture of rat Kupffer cells. J Gastroenterol Hepatol 13:842–5
- Pinti M, Troiano L, Nasi M, et al. (2003). Hepatoma HepG2 cells as a model for in vitro studies on mitochondrial toxicity of antiviral drugs: Which correlation with the patient? J Biol Regul Homeost Agents 17:166–71.
- Sica A, Schioppa T, Mantovani A, Allavena P. (2006). Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: Potential targets of anti-cancer therapy. Eur J Cancer 42:717–27
- Singh M. (1999). Transferrin as a targeting ligand for liposomes and anticancer drugs. Curr Pharm Des 5:443–51
- Suzuki R, Takizawa T, Kuwata Y, et al. (2008). Effective anti-tumor activity of oxaliplatin encapsulated in transferrin-PEG-liposome. Int J Pharm 346:143–50
- Torchilin VP. (2005). Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov 4:145–60
- Torchilin VP. (2006). Multifunctional nanocarriers. Adv Drug Deliv Rev 58:1532–55
- Vighi E, Ruozi B, Montanari M, et al. (2007). Re-dispersible cationic solid lipid nanoparticles (SLNs) freeze-dried without cryoprotectors: Characterization and ability to bind the pEGFP-plasmid. Eur J Pharm Biopharm 67:320–8
- Wagner E, Curiel D, Cotton M. (1994). Delivery of drugs, proteins and genes into cells using transferrin as a ligand for receptor-mediated endocytosis. Adv Drug Deliv Rev 14:113–35
- Wang W, Zhou F, Ge L, Kong F. (2012). Transferrin-PEG-PE modified dexamethasone conjugated cationic lipid carrier mediated gene delivery system for tumor targeted transfection. Int J Nanomed 7:2513–22
- Yu W, Liu C, Liu Y, et al. (2010). Mannan-modified solid lipid nanoparticles for targeted gene delivery to alveolar macrophages. Pharm Res 27:1584–96