Abstract
Context: Scientific validation of an ethnomedicinal combination consisting of Semecarpus kurzii Engler (Anacardeaceae) leaves (SKL) and Hernandia peltata Meisn (Hernandeaceae) stem-bark (HPB), traditionally used in ailments related to inflammation, pain and fever.
Objective: To validate in vivo and in vitro analgesic and antiinflammatory activities of methanol extract of SKL, HPB and their combination.
Materials and methods: Analgesic activity was tested by acetic acid induced writhing reflex and tail flick in Swiss albino mice, while the anti-inflammatory activity was studied in acute, subacute and chronic model on Wistar rats. The vascular permeability, membrane stabilization and protein denaturation were examined to know the possible mode of action.
Results: Significant (p < 0.01) analgesic (78.04% inhibition of writhing) and antiinflammatory (72.54% inhibition of paw edema) activity was observed in combination of SKL and HPB extracts at 250 mg/kg each. The SKL extract alone inhibits acetic acid-induced vascular permeability (64.4%) at 500 mg/kg, while in combination at 250 mg/kg each, the inhibition was 69.49% (p < 0.01). Furthermore, SKL in combination with HPB (0.25 mg/mL each) prevent RBC hemolysis (61.91%) and inhibition of protein denaturation (76.52%)-like indomethacin.
Discussion and conclusion: The SKL and HPB extract, alone (500 mg/kg) and in combination, (250 mg/kg each) had significant analgesic and antiinflammatory activity, probably by inhibiting the release of certain inflammatory mediators and membrane stabilization, due to the presence of triterpenes, tannins and related phytochemicals in the extracts. Thus, our results demonstrated that this combination provide the scientific rationale of its folk use.
Introduction
The traditional medicines and medicinal plants has been widely used for primary health care in most developing countries (UNESCO, Citation1996), including India. Out of four biodiversity zones Andaman and Nicobar Island, situated about 1200 km away from the mainland India, is one of the richest tropical biodiversity zone. The tropical rain forest of Andaman and Nicobar Islands harbor about 2500 angiospermic plants, of which 52 are used as medicaments by its aboriginal populations namely, Great Andamanese, Onge, Jarawas and Sentinels of Andaman group and Nicobarese and Shompen of the Nicobar group of Islands. These tribes used diverse herbal combinations containing several endemic or extra-Indian plant species as time tested remedies (Dagar & Dagar, Citation1991) for cancer to common cold. Furthermore, due to congenial environment several plant species of these islands, they may possess unique bioactive compounds useful for new drug development.
Ethnomedicinal combination of Semecarpus kurzii Engler (Anacardeaceae) leaf (SKL) and Hernandia peltata Meisn (Hernandeaceae) stem bark (HPB) at 1:1 ratio was used by Onge, Nicobarese and Shompen tribes of Andaman and Nicobar Islands for fever, pain and skin ailments (Mandal et al., Citation2000). S. kurzii, locally known as Bara Bhilawa, is an endemic species of South Andaman Islands (Mandal et al., Citation2000), and its leaves are used for treating skin ailments and pain (Bhargava, Citation1983; Chakraborty & Vasudeva Rao, Citation1988; Dagar & Dagar, Citation1991); while resin for painful wound, fever, allergy, and helminthic infections (Das et al., Citation2006). On the other hand, H. peltata, locally known as jack box, is a littoral tree of the seashores, used to treat wounds, tumor, headache, epilepsy and fits (Bhargava, Citation1983; Chakraborty & Vasudeva Rao, Citation1988; Dagar & Dagar, Citation1991). Until now, there were no pharmacological studies of these ethnomedicinal remedy, either alone or in combination. Thus, the present study was undertaken to evaluate the in vivo analgesic and antiinflammatory efficacies and possible mode of action of these two plants alone and in combination (1:1), as used by the ethnic community.
Materials and methods
Plant materials, preparation of extract(s) and traditional formulation
The SKL and HPB were collected from the rain forest of South Andaman and Great Nicobar Islands, in August, December and April of 2007–2008. The plants were identified and authenticated by Dr. Sreekumar, Botanical Survey of India (BSI), Port Blair, and the voucher specimens (No. ABM 071BSI/PB: 2008 and ABM 072BSI/PB: 2008) were deposited in the BSI, Port Blair. The plant parts were shade-dried, separately powdered, passed through a 40-mesh sieve and stored for future use. The powdered materials (200 g) of respective plant parts were extracted with 95% methanol for 72 h at room temperature (Arunachalam et al., Citation2002). The whole extract was separately collected, repeatedly filtered (Whatman No. 1 filter paper), and solvent evaporated to dryness under reduced pressure in an Eyela Rotary Evaporator (Tokyo, Japan) at 40–45 °C. The concentrated extracts of SKL (yield 8.9% ± 0.13%) and HPB (9.15% ± 0.11%) were stored in a desiccator for further study. A weighted amount of the dried extract(s) were dissolved in 2% (w/v) Tween-80 and diluted in sterile distilled water; while the combination or formulation was made by mixing the dried extract of SKL and HPB (1:1). The combination was dissolved in 2% (w/v) Tween-80 diluted in sterile distilled water for pharmacological studies, whenever required.
Standardization of the extract(s) was done by physicochemical constant, fluorescence analysis and phytochemical tests (Arunachalam et al., Citation2009; WHO, Citation1998). Physicochemical parameters like total ash, acid insoluble ash and water content, and the property of powdered drug in different solutions under UV312 nm and visible light was determined following WHO (Citation1998) Guideline. Preliminary phytochemical studies to know the chemical groups present within the extracts were performed by standard methods (Arunachalam et al., Citation2009; Chhabra et al., Citation1984).
HPLC instrumentation and experimental conditions
HPLC analysis was done by the method of Kontogianni et al. (Citation2009), using a Shimadzu liquid chromatograph consisting of binary LC-20AD pumps, coupled with a SPD-M20A photo-diode array detector. Injection was done through a Rheodyne 7725 injector equipped with a 20 μL loop, and a reverse phase C18, 250 × 4.6 mm, 5 µm, Zorbax column (Agilent Technology, Inc., Santa Clara, CA). Both the column and HPLC system were kept at ambient (25 °C) conditions. The mobile phase was 1% acetic acid in HPLC grade water:methanol (10:90) for SKL methanol extract and 1% acetic acid in HPLC grade water:methanol (35:65) for HPB methanol extract with the flow rate of 1 ml/min. The absorbance was monitored at 210 nm with the injection volume of 20 μL.
The stock solutions of pure ursolic acid (UA) and chlorogenic acid (CA) (Sigma, St. Louis, MO) prepared by dissolving 10 mg of pure compounds separately in 10 mL of methanol. All the samples were filtered (0.45 μm membrane), and 20 μl of the sample solution was injected into the HPLC system for analysis. Identification of UA and CA was based on their retention time and comparison of their UV spectra. Quantitative analysis was achieved by five point calibration curves for UA and CA, at concentrations of 5–1000 μg/mL and from the equation Y = 6987.2X + 60941 for UA and Y = 6977.3X + 60943 for CA, respectively, where Y represents the area of the extract and X represents the concentration of either UA or CA. The regression coefficient values were 0.9985 for UA and 0.9945 for CA. Real samples were diluted accordingly to fit the dynamic linear range of the regression line, when necessary. All measurements were performed in triplicates.
Animals
Healthy inbreed Swiss albino male mice (20 ± 2 g) and adult Wistar male rats (150–180 g) were housed at (23 ± 4 °C, humidity 60–70%) in the animal house facility (s) of the Pharmaceutical Technology Department, Jadavpur University, Kolkata, India and were fed with standard pellet diet and water ad libitum. All the animals were acclimatized for 1 week before the experiments, and all experiments were carried out according to the Institutional Animal Ethics committee guidelines (Permission No. JU/Pharm/007/2008; dated 07.07.2008).
Drugs and chemicals
Paracetamol, morphine sulfate, diclofenac disodium, carrageenan, dextran powder and all other analytical grade chemicals were purchased from the respective manufacturers.
Acute toxicity study
The acute toxicity of SKL and HPB extracts alone and in combination was conducted on 13 groups of mice (n = 6) at oral (p.o.) doses of 200–2000 mg/kg body weight, including a control group. While the same in Wistar rats at 200, 500, 1000 and 2000 mg/kg body weight, using nine groups of animals (n = 6). Simultaneously, the acute toxicity of SKL and HPB extracts was tested in Wistar rats at 200, 500, 1000 and 2000 mg/kg body weight orally along with a control group. The animals were observed for toxic symptoms and death at 6, 12, 18 and 24 h and then daily for next 14 d (OECD, Citation2000). The study showed neither any observable toxic effects (agility, muscular tonus, tremors, convulsions, problem in breathing and water or food intake) nor any death following treatment, and hence, the procedure was repeated up to 5000 mg/kg. On the basis of toxicity data the dose regimen for further tests were selected as 400 and 500 mg/kg of extracts alone and 250 mg/kg (each) in combination.
Analgesic activity
Acetic acid-induced writhing tests
The writhing test was performed according to the method of Koster et al. (Citation1959). Briefly, Swiss albino mice of either sex were divided into seven groups of six animals each. The first group served as control; the second group received paracetamol (50 mg/kg), while the 3rd to 6th groups received SKL and HPB extract at 400 and 500 mg/kg, respectively, and the last group was treated with a combination of SKL and HPB (250 mg/kg each) as i.p. After 30 min of drug administration, the animals were orally fed with 1% v/v fresh acetic acid solution (10 ml/kg), and immediately counted the numbers of writhing or stretches (abdominal contraction, trunk twist response and extension of hind limbs) for 10 min. A reduction in the writhing number compared to the control was considered as evidence of analgesia (Pradeepa et al., Citation2012). The percentage inhibition of writhing was calculated as: % Inhibition = C − T/C × 100, where C is the mean number of writhes produced by the control group and T represents the mean number of writhes produced by the test groups.
Tail flick test
Swiss albino mice of either sex weighing 20 ± 2 g were divided into seven groups of five animals each. The tail of each mouse was placed on the nichrome wire of an analgesiometer (Techno Lab, Lucknow, India) and the time taken by the animal to withdraw (flick) its tail from the hot wire was taken as a reaction time. The extract of SKL, and HPB at 400 and 500 mg/kg and the combination of SKL and HPB at 250 mg/kg each, were injected i.p., using morphine sulfate (5 mg/kg) as standard drug. Analgesic activity was measured after 30 min of administration of test and standard drug (Das et al., Citation2011) and the percentage inhibition was calculated by the same formula as above.
Anti-inflammatory activity
Carrageenan-induced rat paw edema (acute model)
Wister male rats were divided into seven groups of six animals (n = 6) each. The extracts of SKL and HPB at 400 and 500 mg/kg body weight were administered orally to the animals of groups I–IV, while the Vth group received a combination of SKL and HPB (250 mg/kg each), 60 min prior to carrageenan injection. The VIth group serves as vehicle control, and the VIIth group received diclofenac disodium (20 mg/kg) as drug control, for assessing comparative pharmacological significance. Edema was induced by sub-plantar injection of 0.05 mL of fresh carrageenan suspension (1% w/v) in normal saline into the right hind paw of each rat (Winter et al., Citation1962). The paw volume (linear circumference) was measured at 0 h and at 1–5 h after carrageenan injection by a plethysmometer (Oyemitan et al., Citation2008). The anti-inflammatory activity was evaluated using the ratio of the changes in paw diameter in treated and untreated group by the formula: anti-inflammatory activity (%) = (1 − D/C) × 100; where D is the change in paw diameter in treated group and C is the change in paw diameter in untreated group.
Dextran-induced rat paw edema (sub-acute model)
The hind paw edema in each rat was induced by sub-plantar injection of 0.1 mL of 1% dextran solution (Das et al., Citation2011) in the right foot. Paw volumes were measured 30 min before and after dextran injection. The treatment of extracts (test), control (vehicle), and standard drug was the same as described for carrageenan model. The percentage inhibition of edema was calculated by the method of Arunachalam et al. (Citation2002).
Cotton pellet-induced granuloma (chronic model)
The rats were divided into seven groups of six animals each, and the animals were anaesthetized after shaving of the fur. Sterile preweighed cotton pellets (10 mg) were implanted in the axilla region of each rat through a single needle incision (D’Arcy et al., Citation1960). The extracts of SKL and HPB were treated orally at 400 and 500 mg/kg to groups I–IV, and a combination of SKL and HPB (250 mg/kg each) to group V. The group VI serves as vehicle control, while group VII received diclofenac disodium (20 mg/kg), for consecutive seven days from the first day of implantation. On 8th day, the animals were anesthetized; cotton pellets were removed surgically and made free from extraneous tissues. To obtain constant weight, the pellets were incubated at 37 °C for 24 h and dried at 60 °C. The granuloma formation was measured by the increase in dry weight of the pellets (Winter & Porter, Citation1957).
Acetic acid-induced vascular permeability in mice
The vascular permeability was tested by the method of Whittle (Citation1964). Briefly, mice were divided into seven groups, six per group (n = 6). Group I served as vehicle control, groups II–V were treated orally with 400 and 500 mg/kg of SKL and HPB extract; group VI with a combination of SKL and HPB (250 mg/kg each), and group VII received indomethacin (20 mg/kg). One hour after the treatment, 0.2 mL of 0.2% Evans’ blue in normal saline was injected intravenously to each mouse through tail vein and 30 min later, each mouse was injected i.p. with 0.2 mL of 0.6% acetic acid in normal saline. After 1 h, the animals were sacrificed, and the abdominal cavity was exposed and washed with 5 mL normal saline to collect the content in a sterile test tube. The content was centrifuged to eliminate contaminants, and the absorbance (A) of the solution was measured by a spectrophotometer at 590 nm. The vascular permeability effects, expressed as the absorbance (A590), represented the total amount of dye leaked into the intraperitoneal cavity.
Membrane stabilizing activity
This was assessed by hypotonic solution-induced human erythrocyte hemolysis (Shinde et al., Citation1999). Whole blood collected from a healthy volunteer (DC) in a heparinized tube was washed three times with isotonic buffer (154 mM NaCl in 10 mM sodium phosphate buffer, pH 7.4) for 10 min at 3000 g. The test sample consisted of RBC (0.50 ml) in 5 mL hypotonic solution (50 mM NaCl in 10 mM sodium phosphate buffer saline, pH 7.4) containing the extracts (0.2–1.0 mg/ml) alone or in combination, or indomethacin (0.1 mg/ml). The control tube contain RBC (0.5 ml) mixed with hypotonic-buffered saline. The mixtures were incubated for 10 min at room temperature, centrifuged (3000 g for 10 min) and the absorbance of the supernatant was measured at A540 nm. The percentage inhibition of hemolysis or membrane stabilization, tested in triplicate, was calculated as % inhibition of hemolysis = 100 × {OD1 − OD2/OD1}, where OD1 is the optical density of hypotonic buffer saline and OD2 is the optical density of test sample in hypotonic solution.
Effect on in vitro protein denaturation
Test solution (1 ml) containing different concentrations of extracts (100–1000 μg), alone or in combination or indomethacin (100 μg) was mixed with 1 mL of egg albumin (1 mM) and incubated at 27 ± 1 °C for 15 min. The mixture was then placed at 70 °C in a water bath for 10 min to induce denaturation of albumin. After cooling, the turbidity was measured at 590 nm (Mizushima, Citation1966) and the average of three sets was taken. The percentage inhibition of denaturation was calculated relative to the control as % inhibition of denaturation = (Abs of control − Abs of treated)/Abs of control × 100) (Ramalingam et al., Citation2010).
Statistical analysis
The results were expressed as mean, mean ± SD and SEM (standard error mean). The significance between the two groups was evaluated by Student’s t-test, and the significance of difference among the various treated and control group were analyzed by one-way ANOVA followed by Dunnett’s t-test (Woodson, Citation1987).
Results
In this study, analgesic and anti-inflammatory activity of methanol extract of SKL and HPB and their combination (1:1), as used by the ethnic community, was tested by in vivo and in vitro screening methods.
Standardization of sample and physicochemical study of extract
The methanol extract of plant parts collected in different session showed that the post rainy session samples (August) had the minimum amount of water, total ash and acid insoluble ash content, with maximum yield (), and thus selected for further study. Under visible and UV312 nm light the color of powdered SKL extracts was yellowish to light brown; while it was gray to light brown in HPB extract. Preliminary phytochemical screening of the SKL extract revealed the presence of flavonoids, triterpene, tannins, saponins and glycosides; while HPB extract contains alkaloids, flavonoids, steroids and tannins.
Table 1. The physicochemical parameters of SKL and HPB.
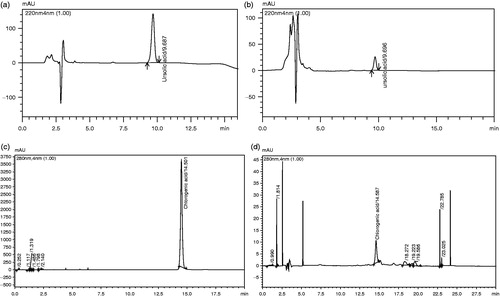
The quantification by HPLC analysis revealed that UA (triterpene) in the methanol extract of SKL was 0.39% (w/w) while the amount of CA in HPB extract was 0.46% (w/w). We have used UA and CA as marker compounds for the quantification of SKL and HPB extracts, as in our phytochemical estimation, we have found the presence of triterpene and tannin as major groups, and these compounds are common in majority of medicinal plants. Moreover, no report of their presence from these two plants has appeared. HPLC chromatograms of the standard UA and methanol extract of SKL are presented in , while the same for standard CA and methanol extract of HPB are presented in .
Acute toxicity
A toxicity study conducted over 14 d showed no observable toxic effects (convulsion, ataxia, agility, dyspnea, water or food intake, diarrhea or diuresis) or any death, signifying that both SKL and HPB extracts possess good safety profiles. However, slightly reduced motor activity, ataxia and hyperventilation was observed in mice with an oral dose of 5 g/kg. The median lethal dose (LD50) of SKL and HPB extract(s) was found to be 3.6 and 3.2 g/kg, respectively, in mice; while the same in rats was 4.0 and 3.6 g/kg. Thus, the dose regimen for further study was selected as 400 and 500 mg/kg alone, and 250 mg/kg (each extract) in combination.
Analgesic activity
Effect of acetic acid-induced writhing test
The results of acetic acid-induced writhing test with SKL and HPB extracts (400 and 500 mg/kg), and their combination (250 mg/mL each) presented in , showed that the inhibition of writhing reflexes was 59.87% at 400 mg/kg and 64.70% at 500 mg/kg of SKL. The response was 61.66% at 400 mg/kg, and 73.08% at 500 mg/kg of HPB extract (p < 0.01). Interestingly, the combination of SKL and HPB extracts (250 mg/kg each) produced maximum inhibition (78.04%), which was similar to the paracetemol-treated group (81.95%).
Table 2. Analgesic activity of ME of SKL and HPB and its combination, by acetic acid-induction and tail flick methods.
Effect of tail flick induced test
The results of tail flick test, presented in , showed that the SKL extract had reaction time of 9.25 s (57.24%) at 400 mg/kg and 7.67 s (64.53%) at 500 mg/kg i.p doses. While the reaction times in vehicle control and morphine sulfate (5 mg/kg) group was 21.63 and 5.20 s (75.95%), respectively. On the other hand, HPB extracts at 400 mg/kg showed reaction time as 9.11 s with 57.88% inhibition; while at 500 mg/kg, it was 6.51 s (69.90% inhibition). Thus, significant (p < 0.01) activity was noted with both the extracts at 500 mg/kg alone and 250 mg/kg (each) in combination. The maximum inhibition of 71.70% was observed at 6.12 s, which is close to the morphine (75.95%) treated group.
Antiinflammatory activity
Effect of carrageenan-induced paw edema
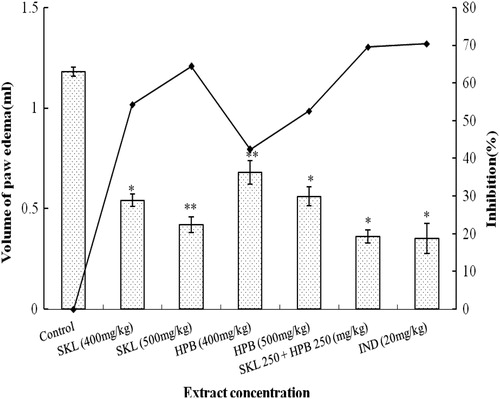
The results of antiinflammatory activity of SKL and HPB extracts and their combination, presented in , showed that SKL at 500 mg/kg exhibited 65.08% inhibition of paw edema; while at 400 mg/kg the inhibition was 56.94%. Here the standard drug diclofenac disodium (20 mg/kg) produced 74.23% inhibition of edema volume after 3 h of treatment. The HPB extract exhibited 43.05% inhibition at 400 mg/kg and 52.20% at 500 mg/kg, respectively. However, the combination of SKL and HPB extracts (at 250 mg/kg each) produced the highest inhibition (72.54%) which is highly significant (p < 0.01), compared to the drug control group.
Table 3. Anti-inflammatory activity of ME of SKL and HPB and its combination, by carrageenan- and dextran-induced paw edema methods.
Effect of dextran-induced paw edema
In dextran-induced paw edema test the maximum inhibition (74.36%) of edema swelling was noted with 20 mg/kg of diclofenac disodium, followed by SKL extract at 400 mg/kg (55.37%) and 500 mg/kg (64.24%), respectively. However, HPB extract showed significant (p < 0.05) inhibition (50.63%) of paw edema at the 500 mg/kg dose only. Here again, the combination of SKL and HPB extracts (250 mg/kg each) revealed highest inhibition (72.15%). These data are significant (p < 0.01) with respect to the control groups, indicating the possible antiinflammatory activity of these extracts ().
Effect of cotton pellet-induced granuloma
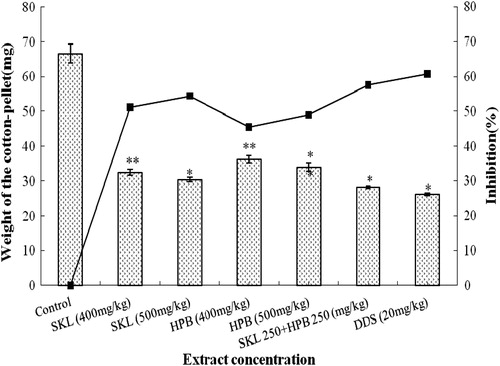
The results presented in showed that SKL and HPB extracts significantly inhibited the granuloma weight. The SKL extract produced 54.40% inhibition at 500 mg/kg (p < 0.01) compared to diclofenac disodium (60.77%) at 20 mg/kg (p < 0.05); while, 49% inhibition of granuloma weight was recorded with 500 mg/kg HPB extract. Interestingly, the combination of SKL and HPB extracts produced 57.55% inhibition, nearly equal to diclofenac disodium (60.77%).
Figure 2. Antiinflammatory activity of ME of S. kurchi leaf and H. peltata bark and the combination of the both by Cotton pellet granuloma in rats.
Inhibition of acetic acid-induced vascular permeability in mice
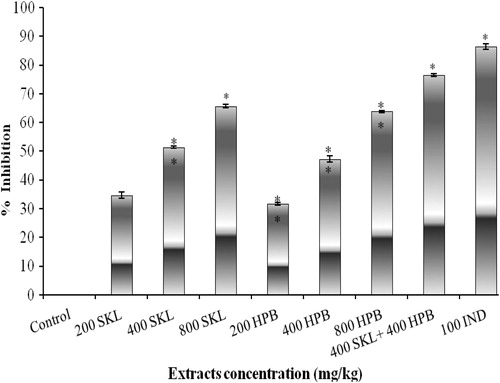
Effect of the oral administration of SKL and HPB extracts (400 and 500 mg/kg) on acetic acid-induced vascular permeability in mice is presented in . Results showed that SKL at 400 and 500 mg/kg inhibited 54.23 and 64.4% vascular permeability, compared to indomethacin (70.33%). The HPB extract at 400 and 500 mg/kg produced 42.37% and 52.54% inhibition of vascular permeability. Here again the highest inhibiton (69.49%) was recorded when both the extracts were combined. All these data are significant at the 5% level (p < 0.05).
Membrane stabilizing activity
The non-specific membrane stabilization study was carried out with both the extracts alone and in combination for further validation of in vivo data. The results, presented in , showed that the SKL and HPB extracts can protect the erythrocyte membrane against lysis induced by the hypotonic solution, as both extracts at 0.5 mg/mL significantly (p < 0.01) protect the erythrocyte membrane (59.45 and 54.79% inhibition) against lysis, compared to indomethacin (63.69% inhibition) at 0.1 mg/ml. Interestingly, the combination of SKL and HPB achieved 61.91% protection in half the concentration (0.25 mg/ml) of each extract, which is highly significant (p < 0.01) and nearly equal to the standard drug.
Table 4. Effect of ME of SKL and HPB and its combination extract on human RBC-induced sensitivity and on membrane stabilization.
Inhibition of protein denaturation
The inhibitory effect of SKL and HPB extracts on protein denaturation, presented in , showed that both the extracts (200 and 400 μg/1000 µL) moderately inhibited (34.64–51.37%) the denaturation of egg albumin. However, at 800 μg/1000 µL, extracts produced 65.64 and 63.80% inhibition, compared to indomethacin (86.36%) at 100 μg/1000 µL. Here again, the combination of SKL and HPB (400 µg/1000 µL each), produced 76.52% inhibition (p < 0.01), similar to indomethacin (86.36%).
Discussion
We have studied the in vivo analgesic and anti-inflammatory activity along with the vascular permeability, in vitro membrane stabilization of RBC and inhibition of protein denaturation of the standardized extracts of two ethnomedicinal plants S. kurzii and H. peltata, alone and in combination, used by some ethnic community of Andaman and Nicobar Islands, India. The importance of this study lies on their ethnomedicinal use and endangered nature of one of its component S. kurzii, which have not been validated yet. The HPLC analysis of the methanol extract of SKL showed the presence of UA while HPB showed CA as one of the components, and both these compounds are known to have analgesic and antiinflammatory activity (Azza et al., Citation2011; Liu, Citation1995). Nevertheless, our findings for the first time scientifically validated this combination for analgesic and antiinflammatory activity, which is probably due to several group of compounds like triterpene, tannin, and flavonoid including CA and UA.
The analgesic activity, tested by acetic acid-induced writhing model, indicated that the numbers of writhing movements were significantly less in mice treated with SKL and HPB extracts alone and in combination, comparable to the untreated group. The effect of the extracts when compared to paracetemol, suggests that both the extracts might have peripheral analgesic effect. Furthermore, the analgesic effect tested by the tail flick test was comparable to that of morphine treated control, suggesting a central analgesic effect.
Formation of edema by a complex array of enzyme activation, mediator release, cell migration, extravasations of fluid, tissue breakdown and repair is one of the cardinal signs of inflammation (Vane & Bolting, Citation1995). We used the most acceptable carrageenan-induced paw edema model, as carrageenan helps to release the chemical mediators in an orderly and biphasic manner (Di Rosa, Citation1972; Sur et al., Citation2002), where the vasoactive histamine and 5HT (bradykinin and serotonin) released in the early phase and prostaglandins (kinin) in the late phase (Heller et al., Citation1998), resulting in increased vascular permeability, leading to the accumulation of fluid in the tissues to form edema (White, Citation1999). On the other hand, dextran-mediated inflammation was reduced, probably due to antihistaminic effects of the extracts, as dextran cause inflammation through the release of histamine and serotonin (Ghosh et al., Citation1963). Carrageenan- and dextran-induced paw edema tests revealed that SKL and HPB extracts possesses significant anti-inflammatory activity at 400 and 500 mg/kg oral dose, while the combination of both extracts at 250 mg/kg showed much better activity (p < 0.01) at 3 h, compared to diclofenac disodium. Thus, the ability of SKL and HPB extracts alone or in combination to reduce the edema volume, suggests that the phytochemicals of these extracts, including UA and CA, may block the release of some mediators like histamine, bradykinin, serotonin and prostaglandins. Furthermore, the decrease in the cotton-pellet-induced granuloma weight by the extracts is due to the inhibition of proliferative phage of inflammation (Rajavel et al., Citation2009), as the inflammatory response induced by the cotton pellet can modulate the release of mediators leading to the tissue proliferation and granuloma formation (Stoeck et al., Citation2004).
Acetic acid-induced vascular permeability, a capillary permeability assay, was used to confirm the antiinflammatory potential of the tested extracts. The acetic acid cause dilation of blood vessels and increased permeability through the release of histamine, prostaglandins and leukotrienes by stimulating mast cells (Brown & Roberts, Citation2006). During inflammation these mediators increase vascular permeability, while acetic acid cause an immediate sustained reaction over 24 h and its inhibition by the test extracts alone or in combination, suggests that SKL and HPB may effectively suppress the exudative phase of acute inflammation.
Furthermore, the non-specific RBC membrane stabilization test showed the protective effect on hypotonic saline induced RBC lysis, an index of antiinflammatory activity (Oyedapo & Famurewa, Citation1995). When RBC is exposed to hypotonic medium, it undergoes hemolysis and oxidation of hemoglobin (Augusto et al., Citation1982; Ferrali et al., Citation1992) due to excessive accumulation of fluid within the cell. Such injury renders the RBC more susceptible to secondary damage through free radical-induced lipid peroxidation (Ferrali et al., Citation1992). This is consistent with the observation that the breakdown of bio-membranes induces free radical generation, which in turn enhances cellular damage (Maxwell, Citation1995). Thus, compounds with membrane-stabilizing properties can protect cell membranes against injurious substances (Liu et al., Citation1992; Perez et al., Citation1995; Shinde et al., Citation1999) by interfering the release of phospholipases to trigger the formation of inflammatory mediators (Aitadafoun et al., Citation1996). Here the significant (p < 0.01) membrane stabilizing activity of SKL and HPB extracts, alone and in combination, suggests that the extracts might inhibit the release of chemical mediators in inflammatory events.
Moreover, protein denaturation is a well-known cause of inflammation (Mizushima, Citation1966), and several anti-inflammatory drugs dose-dependently inhibit thermally induced protein denaturation (Grant et al., Citation1970). Here, the ability of the tested extracts to bring down thermal denaturation of protein is possibly a contributing factor for its anti-inflammatory activity, due to the presence of bioactive groups like flavonoids, triterpene, tannin and related CA and UA, as reported with other plants (Havsteen, Citation2002; Middleton et al., Citation2000).
The contemporary literature revealed that the Malaysian species of H. peltata Meisn, used by the Samoan healers, has anti-hypertensive (Dittmar, Citation1991; Singh, Citation1986), antitumor (Pernet, Citation1971), piscicide (Nishino & Mitsui, Citation1973) and anti-arteriosclerosis (Ebel & Roth, Citation1987) activity. H. peltata from Malaysia is reported to contain benzylisoquinoline alkaloid hebridamine (Chalandre et al., Citation1986) that inhibit chloroquine-resistant Plasmodium falciparum (Angerhofer et al., Citation1999), and artherosclerosis (Lakshmi et al., Citation2009). Moreover, the lignans such as podophyllotoxins (deoxypodophyllotoxin and deoxypicropodophyllin) isolated from H. peltata have anticancer activity (Pettit et al., Citation2004) while hernanol, benzopyran, epiaschantin and deoxypodorhizone inhibit Neisseria gonorrhoeae (Pettit et al., Citation2004). Interestingly, another plant of the formulation, S. kurzii, is reported to contain 9-hydroxy-cis-12-enoic (isoricinoleic) acid and fatty acids (Farooqi et al., Citation1985). However, the present study with the combination of SKL and HPB showed the presence of flavonoids, tannins, saponins, glycosides, alkaloid and steroid, and the HPLC revealed the presence of UA and CA as one of their compounds.
Conclusion
In conclusion, this study suggests that the traditional formulations of SKL and HPB in combination (250 mg/kg each) may offer beneficial effects in the management of inflammatory conditions, as both the extracts significantly inhibited writhing reflexes, paw edema, vascular permeability, membrane-stabilization and protein denaturation, probably due to presence of CA, UA and related phytophores in both the extracts. Further studies involving the purification of the chemical constituents and the investigations on biochemical pathways are required to establish the exact mechanism of its action. However, these findings endorsed the vernacular medicinal use of both of these unique plants to treat ailments related to inflammatory conditions.
Declaration of interest
There is no conflict of interest in any form between the authors.
Acknowledgements
The authors deeply acknowledged the ICMR Virus Unit and the Department of Pharmaceutical Technology, Jadavpur University for laboratory facilities, and the School of Natural Product Studies, Jadavpur University for HPLC analysis.
References
- Aitadafoun M, Mounieri C, Heyman SF, et al. (1996). 4-Alkoxybenzamides as new potent phosholipase A2 inhibitors. Biochem Pharm 51:737–42
- Angerhofer CK, Guinaudeau H, Wongpanich V, et al. (1999). Antiplasmodial and cytotoxic activity of natural bisbenzylisoquinoline alkaloids. J Nat Prod 62:59–66
- Arunachalam G, Chattopadhyay D, Mandal AB, et al. (2002). Evaluation of antiinflammatory activity of Alstonia macrophylla wall ex A.DC. leaf extract. Phytomedicine 9:632–5
- Arunachalam G, Bag P, Chattopadhyay D. (2009). Phytochemical and phytotherapeutic evaluation of Mallotus peltatus (Geist.) Muell. Arg. var acuminatus and Alstonia macrophylla Wall ex A. DC: Two ethnomedicine of Andaman Islands, Ind J Pharma Phytother 1:1–13
- Augusto O, Kunze KL, Montellano PR. (1982). N-phenylprotoporphyrin formation in the haemogolobin-phenylhydrazine reaction. J Biol Chem 257:6231–41
- Azza EM, Yieldez B, Mahmoud K, Abdullatif M. (2011). Chlorogenic acid as potential anti-inflammatory analgesic agent: An investigation of the possible role of nitrogen-based radicals in rats. Int J Pharmacol Toxicol Sci 1:24–33
- Bhargava N. (1983). Ethnobotanical studies of the tribes of Andaman and Nicobar Islands, India. I. Onge. Econ Bot 37:110–19
- Brown JN, Roberts J. (2006). Histamine, bradykinin, and their antagonists, In: Gilman AG, Hardman JG, Limbird LE, Goodman and Gilman’s the Pharmacological Basis of Therapeutics, 11th ed. New York: McGraw Hill Co, 645–67
- Chakraborty T, Vasudeva Rao MK. (1988). Ethnobotanical studies of the Shompen of Great Nicobar Islands. J Econ Taxon Bot 12:43–54
- Chalandre MC, Cabalion P, Guinaudeau H. (1986). Étude des Hernandiacées. XII. Dimères aporphine–benzylisoquinoléine originaux isolés de Hernandia peltata. Can J Chem 64:123–6
- Chhabra SC, Viso FC, Mshin EN. (1984). Phytochemical screening of Tanzanian medicinal plants. J Ethnopharmacol 11:157–79
- D’Arcy PD, Howard EM, Muggleton PW, Townsend SB. (1960). The antiinflammatory action of griseofulvin in experimental animals. J Pharm Pharmacol 12:659–65
- Dagar HS, Dagar JC. (1991). Plant folk medicines among Nicobarese of Katchal Island, India. Econ Bot 45:114–19
- Das S, Haldar PK, Pramanik G, et al. (2011). Evaluation of analgesic and anti-inflammatory activity of Diospyros cordifolia extract. Afr J Trad Comp and Alt Med 8:11–14
- Das S, Sheeja TE, Mandal AB. (2006). Ethnomedicinal uses of certain plants from Bay Islands. Ind J Trad Knowledge 5:207–11
- Di Rosa M. (1972). Biological properties of carrageenan. J Pharm Pharmacol 24:89–102
- Dittmar A. (1991). The effectiveness of Hernandia species (Hernandiaceae) in traditional Samoan medicine and accordining to scientific analysis. J Ethnopharmacol 33:243–51
- Ebel S, Roth H. (1987). Lexikon der Pharmazie. Stuttgart: Thieme
- Farooqi JA, Jamal S, Ahmad I. (1985). Isoricinoleic acid in Semecarpus kurzii seed oil. J Am Oil Chem Soc 62:1702–3
- Ferrali M, Signorni C, Ciccoli L, Comporti M. (1992). Iron release and membrane damage in erythrocytes exposed to oxidizing agents, phenylhydrazine, divicine and isouramil. Biochem J 285:295–301
- Ghosh MN, Banerjee RH, Mukherjee SK. (1963). Capillary permeability increasing property of hyaluronidase in rat. Indian J Physiol Pharmacol 7:17–21
- Grant NH, Alburn HE, Kryzanauskas C. (1970). Stabilization of serum albumin by anti-inflammatory drugs. Biochem Pharmacol 19:715–22
- Havsteen BH. (2002). The biochemistry and medical significance of the flavonoids. Pharmacol Therapeut 96:67–202
- Heller A, Koch T, Schmeck J, Acker VK. (1998). Lipid mediators in inflammatory disorders. Drugs 55:487–96
- Kontogianni VG, Exarchou V, Troganis A, Gerothanassis LP. (2009). Rapid and novel discrimination and quantification of oleanolic and ursolic acids in complex plant extracts using two-dimensional nuclear magnetic resonance spectroscopy comparison with HPLC methods. Anal Chim Acta 635:188–95
- Koster R, Anderson M, De Beer EJ. (1959). Acetic acid for analgesics screening. Fed Proceed 18:412–17
- Lakshmi V, Pandey K, Mishra SK, et al. (2009). An overview of family Hernandiaceae. Record Nat Prod 3:1–22
- Liu J. (1995). Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol 49:57–68
- Liu GT, Zhang TM, Wang BE, Wang YW. (1992). Protective action of seven natural phenolic compounds against peroxidative damage to biomembranes. Biochem Pharmacol 43:147–52
- Mandal AB, Chattopadhyay D, Coomar T. (2000). Rare and endangered flowering plants of Bay Islands with special reference to endemics and extra Indian Taxa. Ind Forest 126:389–96
- Maxwell SRJ. (1995). Prospects for the use of anti-oxidant therapies. Drugs 49:345–61
- Middleton E, Kandaswami C, Theoharides TC. (2000). The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease and cancer. Pharmacol Rev 52:673–751
- Mizushima Y. (1966). Screening test for anti-rheumatic drugs. Lancet 288:442--3
- Nishino C, Mitsui T. (1973). Lignans from Hernandia ovigera. Tethedron Lett 4:335–8
- OECD Guideline 425 (2000). Acute oral toxicity. Environmental Health and Safety Monograph series on Testing and Assessment No. 24
- Oyedapo OO, Famurewa AJ. (1995). Anti-protease and membrane stabilizing activities of extracts of Fagra zanthoxiloides, Olax subscropioides and Tetrapleura tetraptera. Int J Pharmacog 33:65–9
- Oyemitan IA, Iwalewa EO, Akanmu MA, Olugbad TA. (2008). Antinociceptive and anti-inflammatory effects of essential oil of Dennettia tripentala G. Baker (Annonaceae) in rodents, AJTCAM 5:355–62
- Perez RM, Perez S, Zavala MA, Salazar M. (1995). Anti-inflammatory activity of the bark of Hippocratea excelsa. J Ethnopharmacol 47:85–90
- Pernet R. (1971). Revue des Hernandiacees. Planta Med 20:314–19
- Pettit GR, Meng Y, Gearing RP, et al. (2004). Antineoplastic agents. 522. Hernandia peltata (Malaysia) and Hernandia nymphaeifolia (Republic of Maldives). J Nat Prod 67:214–20
- Pradeepa K, Krishna V, Venkatesh, et al. (2012). Antinociceptive activity of Delonix elata leaf extract. Asian Pac J Trop Biomed 2:S229–31
- Rajavel R, Sivakumar T, Jagadeeswaran M, Malliga P. (2009). Evaluation of analgesic and anti-inflammatory activities of Oscillatoria willei in experimental animal models. J Med Plants Res 3:533–7
- Ramalingam R, Bindhu Madhabi B, Ravinder Nath A, et al. (2010). In vitro antidenaturation and antibacterial activity of Zizyphus oenoplia. Der Pharma Lett 2:87–93
- Shinde UA, Phadke AS, Nair AM, et al. (1999). Membrane stabilizing activity – a possible mechanism of action for the anti-inflammatory activity of Cedrus deodara wood oil. Fitoterapia 70:251–7
- Singh YN. (1986). Traditional medicine in Fiji: some herbal folk cures used by Fiji Indians. J Ethnopharmacol 15:57–88
- Stoeck M, Riedel R, Hochhaus G, et al. (2004). In vitro and in vivo anti-inflammatory activity of the new glucocorticoid ciclesonide. J Pharmacol Exp Therap 309:249–58
- Sur TK, Pandit S, Bhattacharya DK, et al. (2002). Studies on anti-inflammatory activity of Betula alnoides bark. Phytother Res 16:669–71
- UNESCO. (1996). Culture and health, orientation texts-world decade for cultural development 1998–1997. Document CLT/DEC/PRO-1996; Paris, France; pp. 129
- Vane JR, Bolting RM. (1995). New insights into the mode of action of anti-inflammatory drugs. Inflammation Res 44:1–10
- White M. (1999). Mediators of inflammation and inflammatory process. J Allergy Clin Immunol 103:5378–81
- Whittle BA. (1964). The use of changes in capillary permeability in mice to distinguish between narcotic and nonnarcotic analgesics. Brit J Pharmacol 22:246–53
- WHO. (1998). Macroscopic and Microscopic Examination: Quality Control Methods for Medicinal Plant Materials. Geneva: WHO, ISBN-13
- Winter CA, Porter CC. (1957). Effect of alteration in side chain upon anti-inflammatory and liver glycogen activities in hydrocortisone esters. J Am Pharmacol Soc 46:515--19
- Winter CA, Risley EA, Nuss GW. (1962). Carrageenan induced oedema in hind paw of rat as assay for antiinflamatory drugs. Proc Soc Exp Biol Med 111:544–7
- Woodson RF. (1987). Statistical Methods for the Analysis of Biomedical Data, Probalility and Mathematical Statistics. Chichester, UK: Wiley, 315–16