Abstract
Context: Qindan capsule (QC), a compound used in traditional Chinese medicine, has been used as an anti-hypertensive agent in clinical settings for years. Our previous studies have shown that QC can improve the morphological index of the artery, down-regulate the collagen volume fraction in the media and inhibit the transformation of smooth muscle cells. However, the detailed mechanisms underlying its effects require further investigation, which might provide more scientific evidence for the clinical treatment of hypertensive vascular remodeling (VR).
Objective: We investigated the effects of QC-containing serum on the TGF-β1/ERK signaling pathway, cell proliferation, migration, the cell cycle, apoptosis and matrix metalloproteinase synthesis (MMPs) in rat aortic adventitial fibroblasts (AFs).
Materials and methods: AFs were cultured through tissue explants in vitro. The levels of extracellular signal-regulated kinase 1/2 (ERK1/2), phospho-ERK1/2 (p-ERK1/2), connective tissue growth factor (CTGF), MMP2 and MMP9 expression were measured by western blotting and RT-PCR. The proliferation and migration of AFs were measured by MTT and transwell migration assays. Cell cycle progression and apoptosis in AFs were analyzed by flow cytometry.
Results: The proliferation and migration rates of AFs treated with transforming growth factor β1 (TGF-β1) for 24 h were 2.4 ± 0.75 and 2.2 ± 0.06 times higher than those of untreated AFs, and increases in the expression of p-ERK1/2 (3.7 ± 0.15 times), CTGF (3.3 ± 0.24 times), MMP2 (5.7 ± 0.37 times) and MMP9 (5.4 ± 0.46 times) (p < 0.05) were observed. Treatment with QC-containing serum significantly down-regulated cell proliferation (1.9 ± 0.06 times), migration (1.6 ± 0.05 times) and the expression of p-ERK1/2 (1.3 ± 0.75 times), CTGF (1.8 ± 0.64 times), MMP2 (1.6 ± 0.65 times) and MMP9 (1.4 ± 0.46 times) (p < 0.05). We also found that QC-containing serum down-regulated the percentage of cells in the G1 phase by 1.6 ± 0.43 times and increased early-phase apoptosis by 2.3 ± 0.33 times (p < 0.05) in AFs.
Conclusions: QC effectively inhibits the proliferation and migration of AFs and changes cell bioactivity and MMPs, possibly through the TGF-β/ERK/CTGF signaling pathway. Our findings may provide new insights into the potential function of QC in preventing or treating hypertension.
Introduction
Cardiovascular disease (CVD) causes significant morbidity worldwide and contributes to more than one-third of deaths in the USA (Mason, Citation2011). Atherosclerosis is the leading cause of CVD in the developed world, and it is increasingly prevalent in developing countries (Scott, Citation2002). Hypertension is a well-established risk factor for the progression of atherosclerosis (Standridge, Citation2005). Vascular remodeling (VR) occurs during normal angiogenesis and under various pathological situations, including hypertension. The adventitia is the cell layer that is most sensitive to blood pressure (Schulze-Bauer et al., Citation2002). Fibroblasts are the most abundant cell type in the adventitia. Studies have shown that AFs play an important role in neointima formation and VR (Sartore et al., Citation2001). AFs can proliferate, differentiate into myofibroblasts and migrate to the intima, in which they are incorporated into atherosclerotic plaques under pressure from inflammation and atherosclerosis (Cai et al., Citation2009). Therefore, the study of AF bioactivity and the possible mechanisms underlying adventitial activation may provide a potential therapeutic strategy for vascular diseases.
Qindan capsule (QC), a traditional Chinese prescription medicine, has been used clinically to treat CVD for many years. QC has been shown to reverse VR in spontaneous hypertensive rats, confirming its anti-hypertensive effects. Our previous studies have shown that QC can improve the morphological index of the artery, down-regulate the collagen volume fraction in the media and inhibit the transformation of smooth muscle cells (Wang et al., Citation2006, Citation2007). However, its detailed mechanisms require further study to provide more scientific evidence for the clinical treatment of hypertensive VR.
TGF-β1 is a pleiotropic cytokine that is involved in multiple cellular processes and plays a critical role in vascular adventitial remodeling (Shi et al., Citation1996). A previous study from our group showed that QC inhibited the AF proliferation induced by TGF-β1 through the TGF-β1/Smad signaling pathway (Ren et al., Citation2010). Although TGF-β is generally associated with the Smad signaling pathway, recent findings have indicated that cell-specific TGF-β activity may be stimulated by the activation of other signaling pathways that are parallel to Smad or participate in cross-talk with the Smad pathway (Hayashida et al., Citation2003). TGF-β1 can activate the Smad, mitogen-activated protein kinase (MAPK) and integrin pathways, thus regulating AF bioactivities (Liu et al., Citation2008). However, a great deal remains to be learned about mediators of TGF-β action.
Different lines of experimental research have defined many of the details of the organization and activation of the MAPK pathway, and intracellular MAPK signaling cascades likely play an important role in the pathogenesis of cardiac and vascular disease. However, the roles of individual signaling proteins, such as ERK1/2, in the pathogenesis of hypertension, have not been clearly elucidated. Smad3 contains several ERK1/2 phosphorylation sites (Matsuura et al., Citation2005). Our previous study showed that Smad2 and Smad3 mediate the AF response to TGF-β1 (Ren et al., Citation2011).
CTGF, a downstream mediator of TGF-β1 (Chen et al., Citation2000), is important for the progression of myocardial fibrosis. In vitro studies have shown that CTGF induces fibroblast proliferation and differentiation, enhances extracellular matrix (ECM) production and regulates the activity of TGF-β in cardiac fibrosis (Ahmed et al., Citation2004; Grotendorst & Duncan, Citation2005). Another study has demonstrated that ERK1/2 is a key intracellular signal for the regulation of CTGF production (Ruperez et al., Citation2003). The ERK pathway may also be involved in fibroblast proliferation and CTGF synthesis (Leivonen et al., Citation2005). Based on our previous studies, we aimed to examine whether TGF-β1 activates other signaling transduction pathways that enhance AF functions and determine whether QC can effectively block the TGF-β/ERK/CTGF pathway and inhibit AF bioactivity.
Matrix metalloproteinase synthesis (MMPs) are extracellular matrix-degrading enzymes that play a critical role in VR (Galis & Khatri, Citation2002), and their expression can be induced by the Smad and/or MAPK pathways. MMPs are a multi-gene family of endopeptidases, and MMP2 and MMP9 play key roles in vascular pathology (Johnson & Galis, Citation2004; Kuzuya & Iguchi, Citation2003; Lessner et al., Citation2004). Lung fibroblasts can produce MMP2 and MMP9 by interfering with the Smad and MAPK pathways in vitro (Asano et al., Citation2010). Therefore, we have focused on MMP2 and MMP9 in the present study. The aim of this study was to investigate the effects of QC-containing serum on cell proliferation, migration, cell cycle progression, apoptosis and the expression of ERK1/2, p-ERK1/2, CTGF, MMP2 and MMP9 in TGF-β1-stimulated rat aortic AFs.
Materials and methods
The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication no. 85-23, revised 1996). The experiments were approved by the Committee on Ethics of Animal Experiments and conducted in accordance with the Guidelines for Animal Experiments of Shandong University Health Science Center.
Preparation of QC
QC was prepared as previously described (Ren et al., Citation2010, Citation2011). The composition of QC is presented in . The constituents of the QC compound, which were measured by thin-layer chromatography (TLC) and high-performance liquid chromatography (HPLC) for quality control, are presented in .
Table 1. Recipe for qindan-capsule formulation.
Table 2. Quality evaluation of the qindan-capsule compound.
Preparation of drug-containing serum
Losartan is a clinically effective hypotensor. Our previous experimental results showed that losartan can effectively reverse the biological activities induced by TGF-β1 in AFs (Ren et al., Citation2010, Citation2011); therefore, we chose losartan as the positive control drug. Losartan was administered at 30 mg/kg d (equivalent to six times the clinical dosage) by stomach irrigation once per day. The drug-containing serum was prepared as previously described (Ren et al., Citation2010, Citation2011). We used the same doses described previously. Briefly, 90 male Wistar-Kyoto (WKY) rats (SCXK 20050006), aged 12 weeks and weighing 250–300 g, were purchased from the Laboratory Animal Center of Shandong University (Jinan, China) and divided into three groups: a high-dose QC group (QCH), a low-dose QC group (QCL) and a losartan (MoShaDong Co., Hangzhou, China) group (Losartan) (n = 30 each). The rats in the QCH and QCL groups were treated with QC fluid at 750 and 150 mg/kg, respectively, which was delivered into the stomach twice a day for 4 d. The rats in the losartan treatment group were given 30 mg/kg losartan orally once per day for 4 d. All rats were starved for 24 h after the last full day of drug administration (i.e., the fourth day). The rats were anesthetized with diethyl ether for 2 h (QCH and QCL) or 1 h (Losartan), and blood was drawn from the abdominal aorta. The serum was separated and processed into a fine powder by vacuum freeze-drying and subsequently stored at −20 °C until use.
Cell culture
Adventitial fibroblasts (AFs) were isolated from 14-week-old WKY rats and cultured as described previously (Ren et al., Citation2010). All experiments were performed with fibroblasts at passages 2–3. The purity of the AFs was determined by immunocytochemistry through positive staining for vimentin (Wuhan Boster Biological Technology, Ltd, Wuhan, China) and negative staining for α-smooth muscle actin (SMA) (Abcam, Cambridge, UK). AFs were grown to 80% confluence and serum starved for 24 h in serum-free medium before treatment with QC.
Experimental groups
The AFs were divided into five groups as follows: positive control group (PC), AFs induced by TGF-β1 (100-21, PeproTech Inc., Rocky Hill, NJ); negative control group (NC), AFs treated with normal saline; QC high dose+ TGF-β1 group (QCH), AFs treated with the fine powder from the high-dose QC serum added to serum-free DMEM; QC low dose + TGF-β1 group (QCL), AFs treated with the fine powder from the low-dose QC serum added to serum-free DMEM; and the losartan + TGF-β1 group (Losartan), AFs treated with the fine powder from the losartan serum added to serum-free DMEM.
Measurement of cell proliferation
Proliferation assays were performed as previously described (Geng et al., Citation2011). Cells were seeded in a 96-well plate with 200 μl medium, 3-(4,5)-dimethylthiahiazo (-2-y1)-2,5-di-phenytetrazoliumromide (MTT, Sigma, St. Louis, MO) was added at a concentration of 0.5 mg/ml, and the cells were incubated for 4 h at 37 °C. The medium was removed, and dimethyl sulfoxide (DMSO, Sigma, St. Louis, MO) was added to each well. The crystals were dissolved in DMSO by shaking the plate gently for 30 min in the dark. The O.D. value of each well was determined at 570 nm using a microplate reader (Thermo Varioskan Flash, Waltham, MA).
Measurement of cell migration
The migration assay was performed using the transwell system (24 wells, 8 μm pore size with a polycarbonate membrane, BD Biosciences, San Jose, CA), as previously described (Li et al., Citation2011). Cells were stained with DAPI (Beyotime, Biotechnology, Shanghai, China), which forms fluorescent complexes with endogenous double-stranded DNA in the nuclei, producing blue fluorescence. The total number of cells invading and adhering to the lower surface was counted in six representative fields under an Olympus light microscope (Olympus, Tokyo, Japan).
Flow cytometry analysis
All materials used in this study were purchased from BD Bioscience. Flow cytometry analysis was performed as previously described (Tian et al., Citation2011). Trypsin-treated cells were washed with PBS and fixed in 70% ethanol overnight. The cells were then washed with PBS again and stained with propidium iodide (PI) (50 µg/ml) for cell cycle analysis. Cells were stained with annexin V-FITC and PI to evaluate apoptosis. The cells were analyzed using a FACSCalibur flow cytometer (BD Bioscience). Flow cytometric analysis was performed using FlowJo software (Tree Star Inc., San Carlos, CA).
RNA isolation and reverse transcription PCR
Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the suggested protocol. RT-PCR was performed as previously described (Yuan et al., Citation2008). The mRNA was reverse transcribed to cDNA using the PrimeScript RT-PCR Kit (TaKaRa, Dalian, China). The PCR primers were designed across introns to avoid genomic DNA contamination; the primer sequences are presented in . PCR products were separated on a 1.5% agarose gel and visualized by ethidium bromide (Molecular Probes, Eugene, OR) staining. The data were analyzed using Alpha Imager 2200 software (Alpha Innotech, San Leandro, CA).
Table 3. Primers for RT-PCR.
Western blot analysis
Protein expression was examined by western blot analysis, as previously described (Ren et al., Citation2010). Briefly, samples were separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred by electroblotting to a nitrocellulose membrane. The membrane was blocked in 5% bovine serum albumin and incubated with anti-CTGF (Novus Biologicals Inc., Littleton, CO), anti-ERK1/2, anti-phospho-ERK1/2, anti-mmp2 or anti-mmp9 (Cell Signaling, Danvers, MA) in 5% BSA overnight at 4 °C, washed with PBS thrice and incubated with horseradish peroxidase-labeled second antibodies for 1 h at room temperature. Bands were visualized using the super-western sensitivity chemiluminescence detection system (Pierce, Rockford, IL). Autoradiographs were quantitated by densitometry (Science Imaging System, Bio-Rad, Hercules, CA). Actin (Sigma–Aldrich, St. Louis, MO) was used as the internal control for protein normalization.
Statistical analysis
The results were presented as the mean and standard error of the mean (SEM) and analyzed with SPSS (Statistical Package for the Social Sciences) v16.0 (SPSS Inc., Chicago, IL). One-way ANOVA or t-tests were used as applicable. p < 0.05 was considered to be statistically significant. All experiments were repeated at least three times.
Results
AF identification
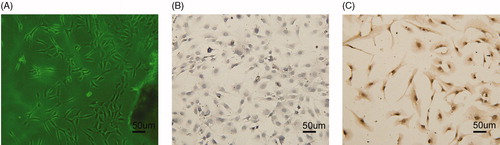
Under light microscopy (Olympus), AFs appeared to have large cell bodies with irregular shapes. A single cell layer grew from the tissue blocks 3–5 d after culture began (). The purity of the AFs was evaluated by immunocytochemistry; AFs were stained by a monoclonal antibody for vimentin () but not a polyclonal antibody for SMA ().
The effect of drug-containing serum on AF migration, as evaluated by the transwell assay
After stimulation with TGF-β1 for 24 h, the migration of AFs in the PC group was significantly increased compared with the NC group (p < 0.01). However, a noticeable down-regulation was observed in the QCH, QCL and losartan groups compared with the PC group (p < 0.05). There were no significant differences between the QCH, QCL and losartan groups (p > 0.05). These results are shown in .
Figure 2. The effects of drug-containing serum on AF migration. AFs were treated with TGF-β1 for 24 h. Cell migration was measured with the transwell assay. The values shown represent the mean ± SEM, n = 3. &, p < 0.01 compared to NC; #, p < 0.05 compared to PC; *, p > 0.05 compared to losartan; Student’s t-test.
The effects of drug-containing serum on AF proliferation, as measured by the MTT assay
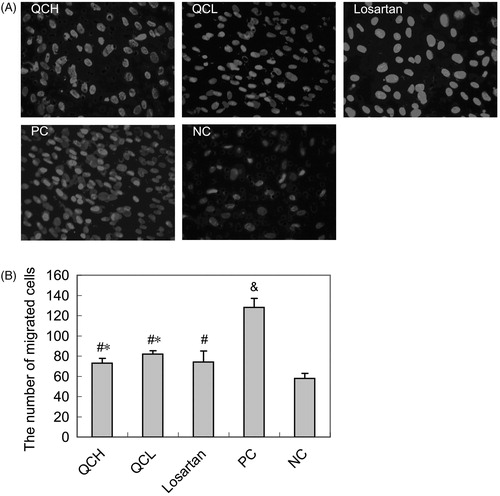
To evaluate the effects of the drug-containing serum on cell proliferation, AFs were treated with TGF-β1 for 24 h. Cell growth was measured with the MTT assay. The OD570 was significantly higher in the PC group than in the NC group (p < 0.01). However, a significant down-regulation of cell growth was observed in the QCL, QCH and losartan groups compared with the PC group (p < 0.05). Proliferation was higher in the QCL group than in the losartan group (p < 0.05), but similar in the QCH and losartan groups (p > 0.05). These results are shown in .
Figure 3. The effects of drug-containing serum on AF proliferation. AFs were treated with TGF-β1 for 24 h. Cell growth was measured with the MTT assay. The values shown represent the mean ± SEM, n = 3. &, p < 0.01 compared to NC; #, p < 0.05 compared to PC; ##, p < 0.01 compared to PC; *, p > 0.05 compared to losartan; **, p < 0.05 compared to losartan; Student’s t-test.
The effects of drug-containing serum on AF cell cycle and apoptosis, as measured by flow cytometry
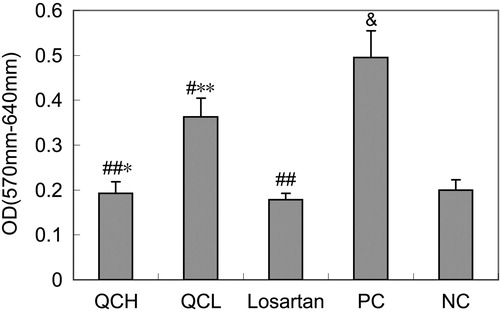
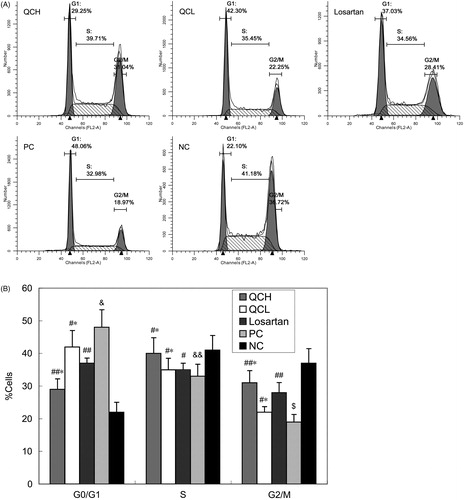
To evaluate the effects of the drug-containing serum on the cell cycle and apoptosis, AFs were treated with TGF-β1 for 24 h. Cell cycle progression and apoptosis were measured by flow cytometry. QC treatment led to G2/M cell cycle arrest. The percentage of cells in G0/G1 and G2/M was significantly different in the PC group compared with the NC group (p < 0.01 and 0.05, respectively). However, a significant reverse effect was observed in the QCH and losartan groups compared to the PC group (p < 0.05). The reverse effect was not significantly different between the QCH and losartan groups (p > 0.05). These results are shown in .
Figure 4. The effects of drug-containing serum on the AF cell cycle. AFs were treated with TGF-β1 for 24 h. Cell cycle progression was evaluated by flow cytometry. The values shown represent the mean ± SEM, n = 3. &, p < 0.01 compared to NC; $, p < 0.05 compared to NC; &&, p > 0.05 compared to NC; ##, p < 0.05 compared to PC; #, p > 0.05 compared to PC; *, p > 0.05 compared to losartan; Student’s t-test.
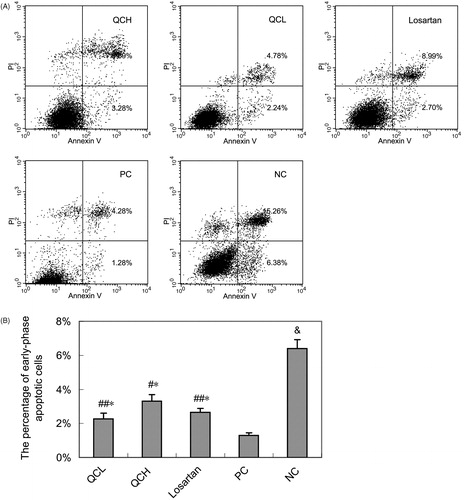
QC also increased apoptosis in AFs. The percentage of early-phase apoptotic cells was significantly lower in the PC group than in the NC group (p < 0.01). However, a significant upregulation of apoptosis was observed in the QCL, QCH and losartan groups compared to the PC group (p < 0.01 or 0.05). There was no significant difference in apoptosis between the QCH, QCL and losartan groups (p > 0.05). These results are shown in .
Figure 5. The effects of drug-containing serum on AF apoptosis. AFs were treated with TGF-β1 for 24 h. Cell apoptosis was measured by flow cytometry. (A) Representative data shows apoptosis cells. (B) Columns, mean of data obtained from three independent experiments. Bar mean SEM. &, p < 0.01 compared to NC; ##, p < 0.05 compared to PC; #, p < 0.01 compared to PC; *, p > 0.05 compared to losartan; Student’s t-test.
The effects of drug-containing serum on the TGF-β/ERK/CTGF pathway and MMP synthesis in AFs
To evaluate the effects of the drug-containing serum on the TGF-β/ERK/CTGF pathway and MMP synthesis, ERK1/2, p-ERK1/2, CTGF, MMP2 and MMP9 protein expression levels were examined by western blot analysis, and CTGF, MMP2 and MMP9 mRNA expression levels were examined by RT-PCR.
The levels of the p-ERK1/2, CTGF, MMP2 and MMP9 proteins were increased in the PC group compared to the NC group (p < 0.01). In the QCL, QCH and losartan groups, this increase was significantly inhibited (p < 0.05). There was a significant difference in the expression of these proteins between the QCL and losartan groups (p < 0.05) but no difference between the QCH and losartan groups (p > 0.05). The level of ERK1/2 protein was statistically similar in all five groups (p > 0.05). These results are shown in .
Figure 6. ERK1/2, CTGF, MMP2 and MMP9 expression. (A) and (B): protein levels of ERK1/2, p-ERK1/2, CTGF, MMP2 and MMP9. The values shown represent the mean ± SEM, n = 3. &&, p < 0.05 compared to NC; $, p < 0.01 compared to NC; &, p > 0.05 compared to NC; #, p > 0.05 compared to PC; ##, p < 0.05 compared to PC; @, p < 0.01 compared to PC; *, p > 0.05 compared to losartan; **, p < 0.05 compared to losartan; %, p < 0.01 compared to losartan; Student’s t-test. (C) and (D): CTGF, MMP2 and MMP9 mRNA expression. The values shown represent the mean ± SEM, n = 3. $, p < 0.05 compared to NC; $, p < 0.01 compared to NC; &&, p > 0.05 compared to NC; ##, p < 0.05 compared to PC; #, p > 0.05 compared to PC; *, p > 0.05 compared to losartan; **, p < 0.05 compared to losartan; Student’s t-test.
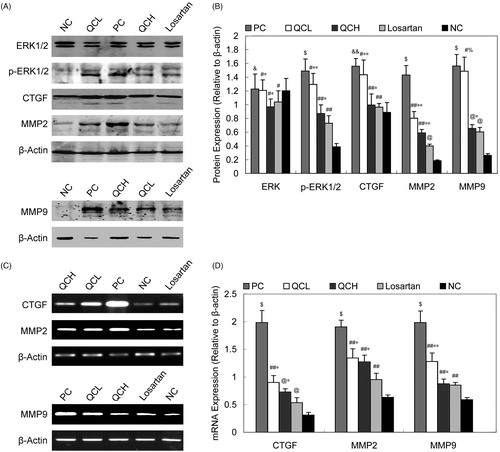
We also investigated the mRNA expression of CTGF, MMP2 and MMP9 (). Our results showed that the CTGF, MMP2 and MMP9 mRNA levels were significantly upregulated in the PC group compared to the NC group (p < 0.01). A significant downregulation of the mRNA expression of CTGF, MMP2 and MMP9 was observed in the QCH, QCL and losartan groups (p < 0.05). The difference between the QCH and losartan groups was not statistically significant (p > 0.05).
Discussion
Our study used the serum pharmacology method and previously published drug dosages (Ren et al., Citation2010). The traditional Chinese medicine serum pharmacology method was first proposed by a Japanese scholar in the 1980s and is universally used. This experimental method limits confounding factors due to the presence of other substances and accurately represents biotransformation in vivo. Rats were treated orally with QC, and their serum was used as the treatment for the reaction system in vitro. The drug concentration used in vitro was equal to that used in the in vivo system. Our experimental results have shown that this method is stable and dependable.
This study demonstrated the regulatory effects of TGF-β1 on downstream mediators expressed in cultured AFs. After stimulation with TGF-β1, ERK1/2 phosphorylation was induced, which is consistent with the observed changes in CTGF, MMP2 and MMP9 gene and protein expression. Furthermore, we showed that TGF-β1 led to changes in the proportion of cells in the G0/G1 and G2/M stages of the cell cycle, decreased apoptosis and up-regulated cell proliferation and migration in AFs. However, all these changes were reversed by treatment with QC-containing serum.
The adventitia and AFs play prominent roles in the pathogenesis of CVD (McGrath et al., Citation2005; Sartore et al., Citation2001). TGF-β1 is an important perivascular growth factor in the adventitia (Leask & Abraham, Citation2003) that is typically up-regulated at the site of vascular injury. TGF-β1 promotes proliferation, adhesion, migration and ECM synthesis in adventitial cells and myofibroblast formation in fibrosis (Kalluri & Neilson, Citation2003). The classical signal transduction pathway of the TGF-β family is dependent on Smad (Massague & Wotton, Citation2000).
QC, an effective treatment for hypertension, contains eight types of herbs; its components have been identified by both HPLC and TLC (Ren et al., Citation2009). The composition of QC is presented in , and the composition of the QC quality standard compound is presented in . Previous studies have suggested that QC and losartan could down-regulate the proliferation of AFs and the expression of collagen stimulated by TGF-β1. In addition, the mechanisms underlying collagen synthesis and AF proliferation during VR might involve the TGF-β1/Smad3 signaling pathway (Ren et al., Citation2010).
The roles of gelatinases (MMP2 and MMP9) in VR have been studied recently, but relatively little is known about the regulators and the underlying mechanisms controlling the activation and expression of these proteins in AFs. It has been shown that TGF-β1 induced an increase in the MMP2 content in fibroblasts (Overall et al., Citation1989) and that CTGF induced MMP2 expression in cultured renal interstitial fibroblasts (Yang et al., Citation2007). In this study, we found that TGF-β1 significantly induced the expression of CTGF, MMP2 and MMP9 in AFs at both the mRNA and protein level. Further evidence suggests that ERK1/2 signaling is most responsible for TGF-β1-induced CTGF, MMP2 and MMP9 expression. When AFs were incubated with TGF-β1, ERK1/2 signaling activation was observed. In this study, we demonstrated that the protein level of p-ERK1/2 was up-regulated in the PC group compared to the NC group. Our results provide preliminary evidence that another signaling pathway is activated after TGF-β1 stimulation in vitro.
The MAPK family is a family of serine-threonine protein kinases that are activated by a number of extracellular stimuli. ERK1/2 (p44/42 MAPK), p38 MAPK and JNK represent the three major subfamilies of MAPK that mediate downstream effects, such as cellular proliferation, differentiation and apoptosis, through the activation of their downstream pathways (Kyriakis & Avruch, Citation2001; Wetzker & Bohmer, Citation2003). In our study, we showed that AF proliferation and migration are up-regulated, the G2/M phase of the cell cycle is promoted and apoptosis is decreased in the PC group compared to the NC group. However, these trends were reversed by treatment with QC- or losartan-containing serum.
CTGF is a 38 kDa cysteine-rich polypeptide that was originally identified in a conditioned medium from human umbilical vein endothelial cells (Bradham et al., Citation1991). Its biological role is pleiotropic and cell-type specific. Although the specific CTGF receptor has not yet been identified, CTGF appears to exert its function through integrins, heparin sulfate-containing proteoglycans and the low-density lipoprotein receptor-related protein (LRP) (Shi-Wen et al., Citation2008). In renal myofibroblasts, CTGF induces tyrosine phosphorylation at the cytoplasmic domain of LRP and activates the ERK1/2 signaling pathway (Gao et al., Citation2008; Yang et al., Citation2004). In chondrocytes, CTGF regulates ECM via ERK1/2 signaling (Nishida et al., Citation2007). Our study shows that QC reduces TGFβ1-induced effects; the expression of MMPs and ERK is down-regulated. However, the mechanisms triggered by TGFβ1 are interdependent or sequential and need to be further studied.
Extracellular proteolysis is an essential physiological process that controls the immediate cellular environment and thus plays a key role in cell behavior and survival (Werb, Citation1997). Among the members of the MMP family, MMP2 is a key player in many physiological and pathological processes, such as cell migration, inflammation and fibrosis (Esparza et al., Citation2004). Previously, increases in MMP2 expression caused by CTGF were reported in vascular smooth muscle cells (Fan & Karnovsky, Citation2002) and renal interstitial fibroblasts (Yang et al., Citation2007). CTGF has been implicated in the regulation of ECM protein production and thus VR (Grotendorst, Citation1997). Consequently, elucidating its effects on MMP expression will be very important for a better understanding of the processes involved in VR. CTGF is up-regulated in many fibrotic disorders, including vascular diseases, which are associated with ECM accumulation (Perbal, Citation2004). In this study, we showed that CTGF is closely related to the induction of MMP2 and MMP9. However, the increased MMP2 and MMP9 production induced by CTGF was abrogated by QC and losartan.
Our previous study showed that QC inhibited the AF proliferation induced by TGF-β1 through the TGF-β1/Smad signaling pathway (Ren et al., Citation2010). In this study, we investigated the effects of QC-containing serum on the TGF-β1/ERK signaling pathway, cell proliferation, migration, the cell cycle, apoptosis and MMPs in rat aortic AFs. QC has been extensively used for the treatment of hypertension. Detailing its intracellular mechanisms shed more light on the further exploration of the compound on other related diseases such as vascular hypertrophy.
Conclusions
In conclusion, our study indicates that QC-containing serum effectively inhibits AF proliferation and migration and alters AF bioactivity and MMPs, perhaps through the TGF-β/ERK/CTGF signaling pathway. Our findings may provide new clues regarding the potential function of QC to prevent or treat CVD.
Declaration of interest
The project was supported by the Chinese National Natural Science Foundation (no. 30873324), the Shandong Province Development of Science and Technology Plan (no. 2010GWZ2O242), the Natural Science Foundation of Shandong Province (no. ZR2010HQ050), the Science and Technology Development of Traditional Chinese Medicine of Shandong Province (no. 2011-199) and the Independent Innovation Foundation of Shandong University (no. 2012TS205). The financial supporters had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare that they have no competing financial interests.
References
- Ahmed MS, Oie E, Vinge LE, et al. (2004). Connective tissue growth factor – a novel mediator of angiotensin II-stimulated cardiac fibroblast activation in heart failure in rats. J Mol Cell Cardiol 36:393–404
- Asano K, Shikama Y, Shoji N, et al. (2010). Tiotropium bromide inhibits TGF-beta-induced MMP production from lung fibroblasts by interfering with Smad and MAPK pathways in vitro. Int J Chron Obstruct Pulmon Dis 5:277–86
- Bradham DM, Igarashi A, Potter RL, Grotendorst GR. (1991). Connective tissue growth factor: A cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J Cell Biol 114:1285–94
- Cai XJ, Chen L, Li L, et al. (2009). Adiponectin inhibits lipopolysaccharide-induced adventitial fibroblast migration and transition to myofibroblasts via AdipoR1-AMPK-iNOS pathway. Mol Endocrinol 24:218–28
- Chen MM, Lam A, Abraham JA, et al. (2000). CTGF expression is induced by TGF- beta in cardiac fibroblasts and cardiac myocytes: A potential role in heart fibrosis. J Mol Cell Cardiol 32:1805–19
- Esparza J, Kruse M, Lee J, et al. (2004). MMP-2 null mice exhibit an early onset and severe experimental autoimmune encephalomyelitis due to an increase in MMP-9 expression and activity. FASEB J 18:1682–91
- Fan WH, Karnovsky MJ. (2002). Increased MMP-2 expression in connective tissue growth factor over-expression vascular smooth muscle cells. J Biol Chem 277:9800–5
- Galis ZS, Khatri JJ. (2002). Matrix metalloproteinases in vascular remodeling and atherogenesis: The good, the bad, and the ugly. Circ Res 90:251–62
- Gao X, Li J, Huang H, Li X. (2008). Connective tissue growth factor stimulates renal cortical myofibroblast-like cell proliferation and matrix protein production. Wound Repair Regen 16:408–15
- Geng F, Song K, Xing JZ, et al. (2011). Thio-glucose bound gold nanoparticles enhance radio-cytotoxic targeting of ovarian cancer. Nanotechnology 22:285101 (8 pp.)
- Grotendorst GR. (1997). Connective tissue growth factor: A mediator of TGF-beta action on fibroblasts. Cytokine Growth Factor Rev 8:171–9
- Grotendorst GR, Duncan MR. (2005). Individual domains of connective tissue growth factor regulate fibroblast proliferation and myofibroblast differentiation. FASEB J 19:729–38
- Hayashida T, Decaestecker M, Schnaper HW. (2003). Cross-talk between ERK MAP kinase and Smad signaling pathways enhances TGF-beta-dependent responses in human mesangial cells. FASEB J 17:1576–8
- Johnson C, Galis ZS. (2004). Matrix metalloproteinase-2 and -9 differentially regulate smooth muscle cell migration and cell-mediated collagen organization. Arterioscler Thromb Vasc Biol 24:54–60
- Kalluri R, Neilson EG. (2003). Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 112:1776–84
- Kuzuya M Iguchi A. (2003). Role of matrix metalloproteinases in vascular remodeling. J Atheroscler Thromb 10:275–82
- Kyriakis JM, Avruch J. (2001). Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev 81:807–69
- Leask A, Abraham DJ. (2003). The role of connective tissue growth factor, a multifunctional matricellular protein, in fibroblast biology. Biochem Cell Biol 81:355–63
- Leivonen SK, Hakkinen L, Liu D, Kahari VM. (2005). Smad3 and extracellular signal-regulated kinase 1/2 coordinately mediate transforming growth factor-beta-induced expression of connective tissue growth factor in human fibroblasts. J Invest Dermatol 124:1162–9
- Lessner SM, Martinson DE, Galis ZS. (2004). Compensatory vascular remodeling during atherosclerotic lesion growth depends on matrix metalloproteinase-9 activity. Arterioscler Thromb Vasc Biol 24:2123–9
- Li X, Kong X, Huo Q, et al. (2011). Metadherin enhances the invasiveness of breast cancer cells by inducing epithelial to mesenchymal transition. Cancer Sci 102:1151–7
- Liu P, Zhang C, Feng JB, et al. (2008). Cross talk among Smad, MAPK, and integrin signaling pathways enhances adventitial fibroblast functions activated by transforming growth factor-beta1 and inhibited by Gax. Arterioscler Thromb Vasc Biol 28:725–31
- Mason RP. (2011). Optimal therapeutic strategy for treating patients with hypertension and atherosclerosis: Focus on olmesartan medoxomil. Vasc Health Risk Manag 7:405–16
- Massague J, Wotton D. (2000). Transcriptional control by the TGF-beta/Smad signaling system. EMBO J 19:1745–54
- Matsuura I, Wang G, He D, Liu F. (2005). Identification and characterization of ERK MAP kinase phosphorylation sites in Smad3. Biochemistry 44:12546–53
- McGrath JC, Deighan C, Briones AM, et al. (2005). New aspects of vascular remodelling: The involvement of all vascular cell types. Exp Physiol 90:469–75
- Nishida T, Kawaki H, Baxter RM, et al. (2007). CCN2 (Connective Tissue Growth Factor) is essential for extracellular matrix production and integrin signaling in chondrocytes. J Cell Commun Signal 1:45–58
- Overall CM, Wrana JL, Sodek J. (1989). Independent regulation of collagenase, 72-kDa progelatinase, and metalloendoproteinase inhibitor expression in human fibroblasts by transforming growth factor-beta. J Biol Chem 264:1860–9
- Perbal B. (2004). CCN proteins: Multifunctional signalling regulators. Lancet 363:62–4
- Ren M, Wang B, Zhang J. (2009). Study on quality standard of Qindan Capsule. Chin J Integrative Med Cardio-/Cerebrovascular Dis 7:597–8
- Ren M, Wang B, Zhang J, et al. (2011). Smad2 and Smad3 as mediators of the response of adventitial fibroblasts induced by transforming growth factor beta1. Mol Med Report 4:561–7
- Ren M, Zhang J, Wang B, et al. (2010). Qindan-capsule inhibits proliferation of adventitial fibroblasts and collagen synthesis. J Ethnopharmacol 129:53–8
- Ruperez M, Lorenzo O, Blanco-Colio LM, et al. (2003). Connective tissue growth factor is a mediator of angiotensin II-induced fibrosis. Circulation 108:1499–505
- Sartore S, Chiavegato A, Faggin E, et al. (2001). Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: From innocent bystander to active participant. Circ Res 89:1111–21
- Schulze-Bauer CA, Regitnig P, Holzapfel GA. (2002). Mechanics of the human femoral adventitia including the high-pressure response. Am J Physiol Heart Circ Physiol 282:lH2427–40
- Scott J. (2002). The pathogenesis of atherosclerosis and new opportunities for treatment and prevention. J Neural Transm Suppl 63:1–17
- Shi-Wen X, Leask A, Abraham D. (2008). Regulation and function of connective tissue growth factor/CCN2 in tissue repair, scarring and fibrosis. Cytokine Growth Factor Rev 19:133–44
- Shi Y, O'Brien Jr JE, Fard A, Zalewski A. (1996). Transforming growth factor-beta 1 expression and myofibroblast formation during arterial repair. Arterioscler Thromb Vasc Biol 16:1298–305
- Standridge JB. (2005). Hypertension and atherosclerosis: Clinical implications from the ALLHAT trial. Curr Atheroscler Rep 7:132–9
- Tian Y, Yuan C, Ma D, et al. (2011). IL-21 and IL-12 inhibit differentiation of Treg and TH17 cells and enhance cytotoxicity of peripheral blood mononuclear cells in patients with cervical cancer. Int J Gynecol Cancer 21:1672–8
- Wang B, Zhang JD, Feng JB, et al. (2007). Effect of traditional Chinese medicine Qin-Dan-Jiang-Ya-Tang on remodeled vascular phenotype and osteopontin in spontaneous hypertensive rats. J Ethnopharmacol 110:176–82
- Wang B, Zhang JD, Wang SH. (2006). Effects of qindan capsule on blood pressure, endothelin, calcitonin gene-related peptide and angiotensin- II in spontaneous hypertensive rats. Chin J Integr Med 12:287–91
- Werb Z. (1997). ECM and cell surface proteolysis: Regulating cellular ecology. Cell 91:439–42
- Wetzker R, Bohmer FD. (2003). Transactivation joins multiple tracks to the ERK/MAPK cascade. Nat Rev Mol Cell Biol 4:651–7
- Yang M, Huang H, Li J, et al. (2004). Tyrosine phosphorylation of the LDL receptor-related protein (LRP) and activation of the ERK pathway are required for connective tissue growth factor to potentiate myofibroblast differentiation. FASEB J 18:1920–1
- Yang M, Huang H, Li J, et al. (2007). Connective tissue growth factor increases matrix metalloproteinase-2 and suppresses tissue inhibitor of matrix metalloproteinase-2 production by cultured renal interstitial fibroblasts. Wound Repair Regen 15:817–24
- Yuan C, Jiao L, Yang L, et al. (2008). The up-regulation of 14-3-3 proteins in Smad4 deficient epidermis and hair follicles at catagen. Proteomics 8:2230–43