Abstract
Context: Primary dysmenorrhea is one of the most frequent gynecological disorders in young women. Chinese herbal medicine has the advantage in terms of multi-targeting efficacy, lower toxicity, as well as lower cost. Core licorice is the hard and atropurpureus heart part in root and rootstock of Glycyrrhiza uralensis Fisch (Leguminosae), having a therapeutic effect on dysmenorrhea.
Objective: This experiment indicated the spasmolytic effect of core licorice aqueous extract (CLE) on spontaneous rhythmic contractions and spasmogen-provoked contractions of stilbestrol primed, estrogen-dominated, non-pregnant mouse isolated uterine horns and its spasmolytic mechanism.
Materials and methods: We investigated the spasmolytic effect of CLE (0.025–0.1 mg/mL) on spontaneous contractions and potassium chloride (KCl, 40 mM), acetylcholine (ACh, 5 μg/mL), carbachol (CCh, 5 μg/mL), oxytocin (OT, 2 U/L) or bradykinin (5 ng/mL)-provoked contractions of mouse isolated uterine horns. Contractions were recorded by tension force transducers using Biolap 420F software on a PC.
Results: Our present study showed that graded, escalated concentrations of CLE (0.025–0.1 mg/mL) significantly inhibited the amplitude of spontaneous phasic contractions (15.03–55.10%), as well as the contractions produced by KCl (40 mM; 20.16–53.99%), ACh (5 μg/mL; 14.65–48.32%), CCh (5 μg/mL; 38.40–76.70%), OT (2 U/L; 21.53–58.49%) or bradykinin (5 ng/mL; 58.01–79.44%) of the estrogen-dominated isolated mice uterine horn preparations in a concentration-related manner.
Discussion and conclusion: The spasmolytic effect of CLE observed in the present study lends pharmacological support to the traditional use of core licorice in the management, control and treatment of primary dysmenorrhea.
Introduction
Primary dysmenorrhea is one of the most common gynecologic disorders in young women. Women with primary dysmenorrhea have been found to produce a higher level of prostaglandins (PGF2α) in their endometria compared with asymptomatic controls (Rasmussen et al., Citation1985). PGs exert a direct effect on the myometrium, causing severe uterine smooth muscle contraction (Maxson & Rosenwaks, Citation1993; Speroff et al., Citation1999). Dysmenorrhea is usually treated with nonsteroid anti-inflammatory drugs (NSAIDs) in clinical medicine, but most of NSAIDs show severe side effects in long-term therapy that limits their clinical uses (Evans et al., Citation1997; Gutthann et al., Citation1996; Rodríguez et al., Citation1994).
The project was proposed to investigate the mechanism of Chinese herbal medicine for treatment of the dysmenorrhea. Core licorice is the hard and atropurpureus heart part in root and rootstock of Glycyrrhiza uralensis Fisch (Leguminosae) from various northwest provinces of China. Our results demonstrated that the core licorice aqueous extract (CLE) exerted protective effects on the mouse uterine horns. Licorice is a leguminous perennial herb, which is commonly used as a Chinese herbal medicine, enjoying the reputation of “king of Chinese herbal medicine”. Licorice has always been the research focus because of the significant efficacy and clear pharmacological effects. The chemical composition of licorice are very complex, including flavonoids, triterpenoids coumarin, polysaccharides, amino acids, bio-estrogen and organic acids, but the active ingredients are flavonoids and triterpenoids, and the main components of flavonoids are licorice glycosides and isoliquiritigenin (Li et al., Citation2002). Licorice flavonoid compounds have a variety of biological activity, in addition to anti-inflammatory, antibacterial, antioxidant and anticancer activities they also have an obvious role as an antispasmodic (Kwon et al., Citation2008; Sato et al., Citation2007; Siracusa et al., Citation2011).
According to folk medicine experience, we used core licorice in the clinical treatment of dysmenorrhea and received good effects. The present study was, therefore, undertaken to investigate the effect of CLE on mice isolated uterine muscle contractility.
Materials and methods
Plant material
The core licorice was collected from a piece of open grassland in Yanchi county, Wuzhong City, Ningxia Hui Autonomous Region, China, in September 2011. A voucher specimen was deposited at the herbarium of Pharmaceutical Information Institute, Department of Pharmacology, Ningxia Medical University, Yinchuan, China. One kilogram of dried core licorice was washed with distilled water, milled into fine powder in a blender, and decocted twice, on each occasion with 5 L of distilled water. The blended aqueous extract solutions were filtered and concentrated into 50% liquid medicine (0.5 g/mL) under reduced pressure in a rotary evaporator (CFA-02). Without any further purification, the plant’s crude aqueous extract was diluted with distilled water into the concentration we used for our experiments.
Animals
Healthy, young adult, female ICR mice (25–30 g) were selected from Experimental Animal Center, Ningxia Medical University, Yinchuan, China. The animals were kept and maintained at a constant laboratory condition of temperature, humidity and light, and allowed free access to food and drinking water ad libitum. The animals were pretreated with stilbestrol (0.025 mg/10 g ip.) 24 h before the experiments. All the animals were fasted for 12 h, but still allowed free access to drinking water, before the commencement of our experiments. The animal experiments were performed according to the rules and regulations of the Institutional Animal Ethics Committee. The Animal Ethic Review Committees of Ningxia Medical University approved all procedures.
Tissue preparation
Animals were killed by cervical dislocation. The abdomens were opened by midline incisions, following which the two uterine horns were quickly removed from the animals. Uterine strips (15 mm long) were cleaned free from fat and connective tissues.
Drugs
The following reference drugs were used: stilbestrol (Shanghai General Pharmaceutical Co., Shanghai, China), acetylcholine chloride (Ach, Shanghai General Pharmaceutical Co.), carbachol chloride (CCh, Shan Dong Bausch and Lomb Freda Pharmaceutical Co., Shan Dong, China), potassium chloride (KCl, Shanxi Jingxi Pharmaceutical Co., Shanxi, China), oxytocin (OT, Shanghai Harvest Pharmaceutical Co., Shanghai, China), bradykinin (Sigma-Aldrich, St. Louis, MO), propranolol (Shanxi YunPeng Pharmaceutical Co., Shanxi, China). All drugs were dissolved and diluted in distilled water just before usage. Drug concentrations quoted in the text refer to final organ-bath concentrations. The volume that added to the muscle bath never exceeded 5% of its total volume.
Effect of CLE on mice isolated uterine horns
Each isolated uterine horn preparation was suspended in a 20 mL isolated organ bath containing dejalon solution (in mM: NaCl, 154; KCl, 5.63; NaHCO3, 5.95; CaCl2, 0.65; and glucose, 2.77; pH adjusted to 7.4). The bathing solution was maintained at 37 ± 1 °C and continuously aerated with carbogen (95% O2 + 5% CO2 gas mixture, 60–70 bubbles/min). In order to make allowance for changes in tissue sensitivity, two isolated uterine horn preparations from the same animal were always set up at a time; one used as “control” and the other one used as “test” (i.e., CLE- or reference drug-treated) preparation. One end of the tissue was tied to glass holder and the other end of the tissue was tied to an tension transducer (Biolap 420F, Chengdu TaiMeng Technology Co., Sichuan, China) and preloaded with 0.5 g. Tissue was allowed to equilibrate for 30 min until a stable baseline was attained, during which time the bathing solution was changed every 15 min.
After the equilibration period, concentration-response curves were measured for 10 min. Subsequently, CLE or reference drugs were added to the bath-fluid sequentially.
Mice were assigned randomLy to three groups: (1) the control group; uterine horn strips were only treated with distilled water equivalent to the volume of CLE or reference drugs used. (2) CLE group; cumulative concentrations of CLE were administered and concentration related relaxations were observed. A series concentration of CLE was added to the bathing solution and allowed to react for 15 min. (3) Reference drugs and CLE group; uterine horn strips were elicited by sequential, exogenous additions of KCl (40 mM), ACh (5 μg/mL), CCh (5 μg/mL), OT (2 U/L), bradykinin (5 ng/mL) or propranolol (10−6 M) to the bath fluid. A series concentration of CLE (0.025, 0.05 and 0.1 mg/mL final concentration in the organ bath) was added to the bathing solution and allowed to react for 15 min. Contractions were recorded by tension force transducers using Biolap 420F software on a PC.
Data analysis
The results were presented as the standard errors of the means. The differences in responses among the different groups were analyzed by using one-way analysis of variance (95% confidence interval), followed by Dunnett’s post hoc test. Differences were considered to be significant when p < 0.05.
Results
Effect of CLE on mouse isolated uterine horns
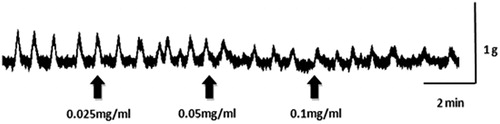
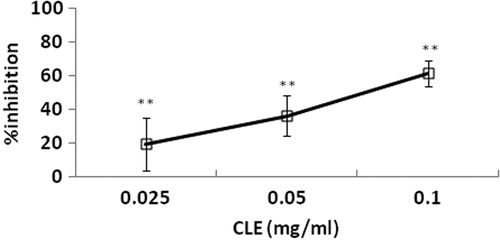
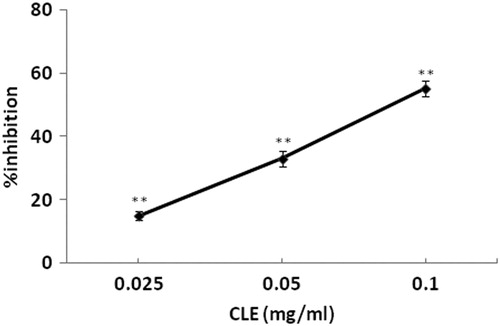
Isolated uterine horns taken from normal female mice exhibited spontaneous phasic contractions periodically, with a mean amplitude of 0.512 ± 0.05 g (n = 11). Relatively low to high concentrations of CLE (0.025, 0.05, 0.1 mg/mL) cumulative-adding to the bath-fluid, caused concentration-related and significant (p < 0.05) reductions of phasic contractions (15.03, 33.05 and 55.10%). and show an inhibition effect of CLE (0.025, 0.05 and 0.1 mg/mL) on uterine horn preparations. However, preincubation of the uterine strips with propranolol (10−6 M) did not alter the relaxant effects of CLE (0.025, 0.05 and 0.1 mg/mL) on the uterine horn preparations (19.62, 36.35 and 61.62%; and ).
Figure 1. Effect of CLE on a mouse isolated uterine horn. CLE (0.025, 0.05 and 0.1 mg/mL) was added to the bath-fluid at the upward-pointing solid arrow.
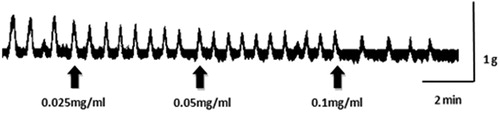
Figure 2. Concentration-effect curve of CLE (0.025, 0.05 and 0.1 mg/mL) on contractions of spontaneously contracting mice isolated uterine horns. Vertical bars represent standard deviation of mean (SD), n = 10. **p < 0.01; versus distilled water-treated controls.
Effects of CLE on spasmogen-induced contractions of uterine horns isolated from non-pregnant mice
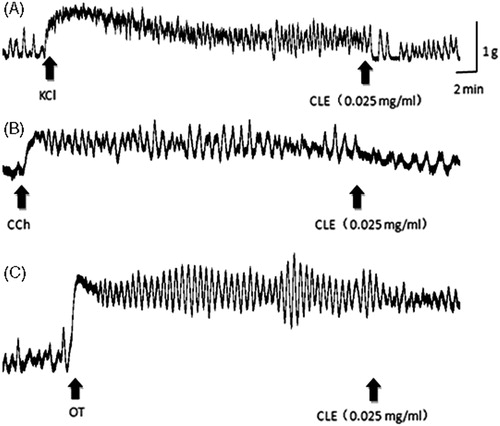
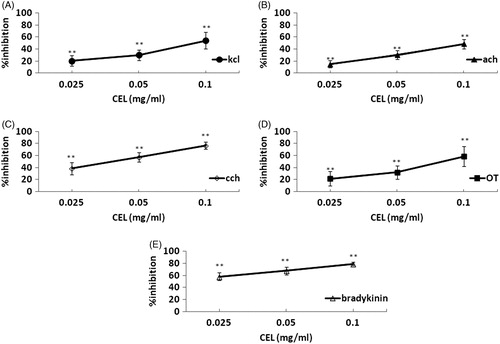
The same low to high concentrations of CLE (0.025, 0.05 and 0.1 mg/mL) inhibited contractions produced by KCl (40 mM; 20.16, 29.77, 53.99%), ACh (5 μg/mL; 14.65, 29.95, 48.32%), CCh (5 μg/mL; 38.40, 56.99, 76.70%), OT (2 U/L; 21.53, 32.27, 53.99%), bradykinin (5 ng/mL; 58.01, 67.85, 79.44%), in a concentration-related manner, in the isolated, estrogen-dominated and uterine horn muscles (). summarizes the inhibitory effects of CLE on KCl-, CCh- and OT-induced uterine horn contractions (n = 10).
Figure 5. Concentration-effect curves of CLE (0.025, 0.05, 0.1 mg/mL) on contractions of mice isolated uterine horns induced by (A) KCl (40 mM), (B) ACh (5 μg/mL), (C) CCh (5 μg/mL), (D) OT (2U/L) and (E) bradykinin (5 ng/mL)-contractile. Vertical bars represent the SD of mean, n = 10. **p < 0.01; versus distilled water-treated controls.
Discussion
The experimental results indicated that CLE (0.025, 0.05 and 0.1 mg/mL) significantly inhibited the spontaneous phasic contractions, as well as the contractions elicited by ACh, CCh, OT, bradykinin or high concentration of KCl in estrogen-dominated, isolated uterine horn preparations of mice.
Dysmenorrhea is caused by irregular convulsive contraction of uterine smooth muscle which is mediated mainly via increased intracellular Ca2+ and accomplished by excitation-contraction coupling mechanisms (Zhang et al., Citation2005). When the intracellular Ca2+ concentration in uterine smooth muscle exceeds 10−6 M, uterine contractions will be induced (Brody & Larner, Citation1993). The intracellular Ca2+ concentration is regulated by two different Ca2+ channels: receptor- and voltage-operated channels (D’Ocon et al., Citation1991; Hurwitz, Citation1986).
A high concentration of K+ in the medium depolarizes the muscle cell membrane, thus inducing the movement of extracellular Ca2+ into the cell through voltage-operated Ca2+ channels, causing its contraction (Horowitz et al., Citation1996; Rodger, Citation1992). We used a high concentration of KCl (40 mM) to induce uterine muscle contraction. Then we found that CLE inhibited the contractions effectively (), showing its effect on Ca2+ entry into the cell through the voltage-operated channels.
It is accepted that Ach- and CCh-induced contractile response following receptor activation requires an increase in intracellular Ca2+ which is provided by both calcium influx through l-type Ca2+ channels and Ca2+ release from intracellular calcium stores (Tavonic et al., Citation2000). We used Ach (5 μg/mL) and CCh (5 μg/mL) for evoking the uterine muscle contraction, which are thought to cause the smooth muscle contraction by increasing the intracellular Ca2+ concentration through the muscarinic cholinoceptor operated channels. In addition, we used OT, which leads to the uterine muscle contraction via an increase in the influx of extracellular Ca2+, thereby causing an increase in intracellular Ca2+ concentration. According to our study, we found that CLE inhibited contractions induced by ACh, CCh and OT in a concentration-related manner, in the isolated uterine horn muscles (). Therefore, the inhibitory effects of CLE on uterine muscle contraction are likely to be due to the inhibition of the increase in the influx of extracellular Ca2+ through the muscarinic cholinoceptor and OT-receptor operated channels.
To find more about the mechanisms of CLE, we used bradykinin to induce the uterine muscle contraction. Bradykinin could stimulate the uterus directly and arouse the release of PGs in the endometrium. Our study shows that CLE inhibits bradykinin-induced uterine contractions in vitro (). This indicates that CLE is likely to relax the uterine contractions by inhibiting the composition and release of PGs.
However, propranolol, as a β2-adrenergic antagonist, failed to inhibit uterine relaxant effect of CLE, which strongly suggests that CLE-induced uterine relaxation is probably not mediated via β2-adrenoceptor activation.
Licorice is often used clinically to treat various abdominal spasmodic symptoms as a component of Shaoyao-Gancao-Tang, whose efficacy has been demonstrated in a placebo-controlled double-blind parallel study (Katsura, Citation1995; Kumada et al., Citation1999). In addition, the smooth muscle relaxing activity of licorice has been shown in ACh-induced contracted guinea pig ileum and carbachol-induced contracted mice jejunum (Maeda et al., Citation1983; Sato et al., Citation2006).
However, the mechanisms involved in the inhibitory effects of CLE in mouse uterus are complex. CLE produces inhibition of calcium influx by blocking the voltage-operated channels, muscarinic cholinoceptor and OT-receptor operated channels, inhibition of the composition and release of PGs.
In conclusion, the spasmolytic effects of CLE on spasmogen-induced contractions of uterine horn muscle strips taken from stilboesterol-primed, estrogen-dominated, non-pregnant mice would appear to be mediated through a non-specific spasmolytic mechanism.
Conclusions
There are several clinical studies proved that CLE has a reliable treatment effect on dysmenorrhea and the gastrointestinal tract reaction caused by it. Our experiments on mouse isolated uterine horn indicate that CLE has multiple actions on the uterine smooth muscles to suppress its contraction aroused by various spasmogen. Thus, CLE could be recommended as a natural therapeutic method in the management, control and treatment of primary dysmenorrhea.
Declaration of interest
The study was supported by the National Natural Science Foundation of China (Grant No. 309605060) and the Natural Science Foundation of NingXia (Grant No. NZ11212). There is no conflict of interest.
References
- Brody TM, Larner JS. (1993). Estrogens, progestins and oral contraceptives. In: Wingard LB, ed. Human Pharmacology. Missouri: Mosby-Year Book Inc., 493–513
- D’Ocon P, Blasco R, Candenas L, et al. (1991). Inhibition of calcium entry induced by cularines and isocrasfoline in uterine smooth muscle. Eur J Pharmacol 196:183–7
- Evans JM, McMahon AD, Murray F.E., et al. (1997). Non-steroidal anti-inflammatory drugs are associated with emergency admission to hospital for colitis due to inflammatory bowel disease. Gut 40:619–22
- Gutthann SP, Rodríguez LAG, Raiford DS, et al. (1996). Nonsteroidal anti-inflammatory drugs and the risk of hospitalization for acute renal failure. Arch Int Med 156:2433–9
- Horowitz A, Menice CB, Laporte KG. (1996). Mechanisms of smooth muscle contraction. Phys Rev 76:967–1003
- Hurwitz L. (1986). Pharmacology of calcium channels and smooth muscle. Pharmacol Toxicol 26:225–58
- Katsura T. (1995). The remarkable effect of Kanzo-to and Shakuyakukanzo-to in the treatment of acute abdominal pain. Jpn J Orient Med 46:293–9
- Kumada T, Kumada H, Yoshiba M, et al. (1999). Effects of Shakuyaku-kanzo-to on muscle cramps accompanying cirrhosis in a placebo-controlled double-blind parallel study. J Clin Ther Med 15:499–523
- Kwon HS, Park JH, Kim DH, et al. (2008). Licochalcone A isolated from licorice suppresses lipopolysaccharide-stimulated inflammatory reactions in RAW264.7 cells and endotoxin shock in mice. J Mol Med 86:1287–95
- Li W, Koike K, Asada Y, et al. (2002). Flavonoids from Glycyrrhiza pallidiflora hairy root cultures. Phytochemistry 60:351–5
- Maeda T, Shinozuka K, Baba K, et al. (1983). Effect of shakuyaku-kanzoh-toh, a prescription composed of shakuyaku (Paeoniae radix) and kanzoh (Glycyrrhizae radix) on guinea pig ileum. J Pharmacobio-Dyn 6:153–60
- Maxson WS, Rosenwaks Z. (1993). Dysmenorrhea and premenstrual syndrome. In: Larry JC, Jarrell JF, McGregor JA, eds. Textbook of Gynecology. Philadelphia (PA): W.B. Saunders Company, 398–413
- Rasmussen AB, Johannesen P, Allen J, et al. (1985). Plasma prostaglandin F2a and plasma 13,14-dihydro-15-keto- prostaglandin F2a levels in women during induction of labor with i.v. infusion of prostaglandin F2a in relation to uterine contractions. J Perinat Med 13:15–21
- Rodger IW. (1992). Airway smooth muscle. Br Med Bull 48:97–107
- Rodríguez LAG, Williams R, Derby LE, et al. (1994). Acute liver injury associated with nonsteroidal anti-inflammatory drugs and the role of risk factors. Arch Int Med 154:311–16
- Sato Y, Akao T, He JX, et al. (2006). Glycycoumarin from Glycyrrhizae radix acts as a potent antispasmodic through inhibition of phosphodiesterase 3. J Ethnopharmacol 105:409–14
- Sato Y, He JX, Nagai H, et al. (2007). Isoliquiritigenin, one of the antispasmodic principles of Glycyrrhiza ularensis roots, acts in the lower part of intestine. Biol Pharm Bull 30:145–9
- Siracusa L, Saija A, Cristani M, et al. (2011). Phytocomplexes from liquorice (Glycyrrhiza glabra L.) leaves – Chemical characterization and evaluation of their antioxidant, anti-genotoxic and anti-inflammatory activity. Fitoterapia 82:546–56
- Speroff L, Glass RH, Kase NG. (1999). Clinical Gynaecologic Endocrinology and Infertility. Baltimore: Lippincott Williams and Wilkins
- Tavonic A, Jimenez M, Fernandez E. (2000). Lack of effect of nitric oxide on KCl-, acetylcholine-and substance P-induced contractions in ileal longitudinal muscle of the rat. Life Sci 67:531–41
- Zhang WW, Li Y, Wang XQ, et al. (2005). Effects of magnolol and honokiol derived from traditional Chinese herbal remedies on gastrointestinal movement. World J Gastroenterol 11:4414–8