Abstract
Context: Corchorus olitorius L. (Malvaceae) has industrial importance in world jute production and is a widely cultivated and consumed crop in Cyprus and in some Arabic countries.
Objective: The present study investigated cytotoxic and genotoxic effects of leaf extracts (LE) and seed extracts (SE) of the C. olitorius on the multiple myeloma-derived ARH-77 cells. The extracts were also evaluated for their total phenol content (TPC) and free radical scavenging activity (FRSA).
Materials and methods: C. olitorius was collected from Nicosia, Cyprus. TPC and FRSA were measured by Folin–Ciocalteu and DPPH free radical methods, respectively. Cytotoxicity was evaluated by the MTT assay (4–2048 µg/mL range), and DNA damage (at IC50 and ½IC50) was measured by the comet assay.
Results and discussion: The LE had significantly higher total phenol (78 mg GAE/g extract) than the SE (2 mg GAE/g extract) with significantly higher FRSA (IC50 LE: 23 µg/mL and IC50 SE: 10 401 µg/mL). Both LE and SE exerted cytotoxic effects on cells after 48 h. The IC50 of SE (17 µg/mL) was lower than LE (151 µg/mL), which demonstrates its higher cytotoxicity on cells. The extracts were applied at 150 and 75 µg/mL for LE and at 17 and 8.5 µg/mL for SE, and the results of the comet assay revealed that the extracts induced genotoxic damage on ARH-77 cells. In both 48 h leaf and seed extract treatments, genotoxic damage significantly increased with increasing concentrations at relevant cytotoxic concentrations.
Conclusion: To our knowledge, this is the first report demonstrating the high cytotoxic potential of C. olitorius SE and the genotoxic potential of LE and SE.
Introduction
Corchorus (Malvaceae), a genus of about 40–100 species of flowering plants, is distributed in the tropics of both hemispheres. In North Cyprus, this genus is represented with two annual species, namely Corchorus olitorius L. and C. trilocularis L. C. olitorius (Jute) is a native plant of tropical Africa and Asia and has since spread to Australia, South America, and some parts of Europe (Meikle, Citation1977). Besides having industrial importance in world jute production, it has agricultural importance as a widely cultivated and consumed crop in Cyprus, and some Arabic countries under the Arabic name “Molukhyia”. It has traditional uses for the treatment of fever, chronic cystitis, aches and pains, dysentery, enteritis, and pectoral pains (Zakaria et al., Citation2006).
Plant-derived compounds comprise diverse biological activities with different mechanisms of actions. Some studied biological activities of different parts of Corchorus olitorius L. are cardiovascular, antihistaminic, hepatobiliary, renal, anticonvulsant, antiesterogenic, antimalarial, and hematological changes (Khan et al., Citation2006). Nishiumi et al. (Citation2006) demonstrated suppressive effect of ethanol extract on aryl hydrocarbon receptor (AhR) transformation induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin. In addition, both hydrophilic and lipophilic extracts of the leaf have shown antioxidant activity (Oboh et al., Citation2009).
Medically screened plants that are used as traditional remedies increase the chance of finding new bioactive principles. Epidemiological studies have suggested that the medicinal plants can have considerable anticarcinogenic effect, and as well inhibiting the genotoxicity and carcinogenicity of anticancer drug to normal cells (Newman et al., Citation2003). It is important to screen anticarcinogenic potential of plants, either in the form of crude extracts or as components isolated from them. Screening of anticancer activities of various plants comprises the basis of studies on the pharmacological mechanisms and searching for chemical structures from herbal extract for new anticancer drugs (Cragg & Newman, Citation1999). A number of other plant-derived compounds such as vinblastine, vincristine, etoposide, and taxotere are currently used as anticancer drugs.
The present study investigated in vitro effects of LE and SE of C. olitorius on the growth of multiple myeloma-derived ARH-77 cells with their genotoxic potential. The extracts were also evaluated for their total phenol content (TPC) and free radical scavenging activity (FRSA).
Materials and methods
Plant material and extraction
Leaves and seeds of C. olitorius were collected from Nicosia, Cyprus, in August and October (2010), respectively. Voucher specimens were identified by Dr. Evren Cabi (Department of Biology, Namık Kemal University, Tekirdağ, Turkey) and stored as herbarium materials (NGBB 3935) at the Nezahat Gökyiğt Botanical Garden, İstanbul, Turkey. Plants were also cultured from seeds in the Greenhouse of Institute of Transplantation and Gene Sciences, Baskent University (Kazan-Ankara, Turkey). Dry material was powdered by using a coffee blender. Powder (10 g) was mixed with 100 mL of pure methanol (Merck, Darmstadt, Germany) and incubated for 24 h at room temperature (dark) with continuous shaking. The solution was filtered (Whatman No. 40), and the filtrate was lyophilized by using a freeze-dryer at −50 °C, 0.50 hPa (LyoPro 3000; Thermo Scientific (Heto), Waltham, MA). Dry material was recovered in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO) and stored at −20 °C. Extract yields [(g lyophilized material/10 g powder) ×100] were calculated as 15% and 21% for LE and SE, respectively.
Analysis of TPC of the extracts
The Folin–Ciocalteu method was used to assay total phenol (Folin & Ciocalteu, Citation1927; Slinkard & Singleton, Citation1977). Two microliters of sample (0.05 g/mL), 50 μL Folin’s reagent (Sigma-Aldrich), and 300 μL 10% (w/v) sodium carbonate (Sigma-Aldrich) were sequentially added to 1 mL assay mixture, and the mixture was incubated at 40 °C in a water bath for 30 min. The absorbance was measured at 765 nm, and the TPC was represented as mg gallic acid (Sigma-Aldrich) equivalents (GAE) per g extract using gallic acid calibration curve. The assays were performed as triplicate experiments.
DPPH radical scavenging assay
FRSA of the plant extracts have been performed using DPPH radical. The method is based on the reduction of DPPH in methanol solution in the presence of a hydrogen donating antioxidant due to the formation of the nonradical form DPPH-H that is measured spectrophotometrically according to decoloration of purple-colored solution of DPPH (Sharma & Bhat, Citation2009). Three milliliters of serial extract dilutions were mixed with 1 mL 200 µM methanol solution of DPPH• (Sigma-Aldrich), vortexed, and incubated at room temperature (dark) for 30 min. The absorbance was measured at 517 nm. Inhibition of DPPH• was calculated as:
Inhibitory concentration 50 (IC50) of extracts were calculated from trend lines of I versus extract concentration plots. Butylated hydroxytoluene (BHT; Sigma-Aldrich), l-ascorbic acid (AscA; Sigma-Aldrich), and α-tocopherol (α-Toc; Sigma-Aldrich) were used as positive controls in the assays. The assays were performed as triplicate experiments.
Cell culture
Multiple myeloma-derived ARH-77 cells were maintained in RPMI 1640 medium (Biochrom AG, Berlin, Germany) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Biochrom AG), 2 mM l-glutamine (Biochrom AG), and streptomycin (100 μg/mL)–penicillin (100 U/mL) mix (Biological Industries, Kibbutz Beit-Haemek, Israel) at 37 °C in a humidified atmosphere of 5% CO2 (Heraeus, Hanau, Germany).
Assay for cytotoxicity
The effects of LE and SE on the proliferation of ARH-77 cells were colorimetrically tested by biochemical reduction of MTT [3-(4,5-dimethyl-2-thiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide; Sigma-Aldrich]. The extracts were diluted from 2048 µg/mL to 4 µg/mL horizontally in 96-well microtiter plates. DMSO cytotoxicity was tested at the solvent dilution range. Ten thousand cells were seeded to each well with the exception of medium control wells. The plates were incubated for 48 h, and then 20 µL of MTT solution (5 mg/mL) were added to each well. After incubation for 4 h, 100 µL sodium dodecyl sulfate (Sigma-Aldrich) solution (10% w/v) were added to each well. The plates were further incubated overnight to allow the dissolution of formazan crystals that were produced by the mitochondrial activity of viable cells. The inhibition of cell proliferation was determined by measuring the optical density of the chromogenic product at 540 nm with an ELISA reader (Biotek Instrument ELx800; Winooski, VT). Inhibition of cell proliferation and inhibitory concentration 50 (IC50) values, which are concentrations at which 50% of cells are viable, were calculated from the logarithmic trend lines of the viability graphs.
Alkaline comet assay (alkaline single cell gel electrophoresis)
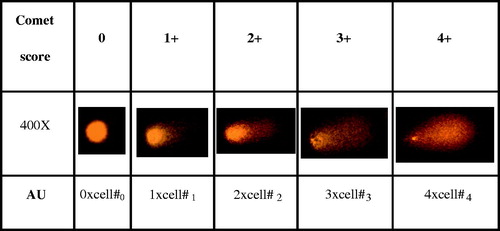
One of the quantitative parameters to measure DNA damage induced by various agents in cells is the comet assay (Yurtcu et al., Citation2011, Citation2012). Extracts were applied to ARH-77 cells at IC50 and half concentrations (½IC50) as 150 and 75 µg/mL for LE, and 17 and 8.5 µg/mL for SE, respectively, for 48 h. Control groups were assayed without any treatment in each experiment. The highest DMSO concentration was also applied in separate flasks (DMSO controls), in order to determine solvent effect on experimental setups. For the determination of genotoxic effects of treatments on ARH-77 cells, alkaline single cell gel electrophoresis (SCGE) was performed as previously described (O’Brien et al., Citation2000). In brief, cells were resuspended in 0.5 mL phosphate buffered saline (PBS; Sigma-Aldrich), and 5 µL of cell suspension was mixed with 35 µL of 1% (w/v) low-melting point agarose (LMPA; Sigma-Aldrich) and added on to the slides coated with 0.5% (w/v) normal melting point agarose (NMPA; Sigma-Aldrich). Coverslips were placed and slides were incubated on ice packs until the solidification of the agarose. Coverslips were removed, and 40 µL 1% (w/v) LMPA were added on to the slides. Slides were incubated in lysis solution (2.5 M NaCl, 100 mM ethylenediaminetetraacetic acid (EDTA) disodium salt, 10 mM Tris; pH 10) at 4 °C (dark) for 2 h. Slides were incubated in electrophoresis buffer (300 mM NaOH, 1 mM (EDTA) disodium salt; pH > 13) for 20 min at dark, and electrophoresis was performed at 24 V (300 mA) for 30 min. After neutralization (0.4 M Tris; pH 7.5), slides were stained with 2 µg/mL ethidium bromide and observed under a fluorescence microscope (Eclipse 600, Nikon, Tokyo, Japan) with 400× magnification. A minimum of three SCGE slides were prepared for each treatment, and 100 nuclei were blindly scored per slide. Tail moment of DNA obtained by comet assay were expressed as arbitrary units (AU) in the present study, which were evaluated by visual scoring of nuclei representing DNA damage () (Zhao et al., Citation2006). Nuclei were scored as 0, 1+, 2+, 3+, and 4+ by a blinded observer according to apparent relative proportion of DNA in the tail and head (). Each counted nucleus was multiplied by its score, and total scores were expressed as AU.
Statistical analysis
All data are expressed as mean ± standard error of the means (SEM). All statistical analyses were performed using SPSS Software (SPSS Inc., Chicago, IL). Treatments were statistically evaluated by one-way analysis of variance at 0.05 levels, and post-hoc Tukey analysis was carried out to find groups whose mean differences were significant. The results were subjected to two-tailed t-test where necessary (α = 0.05).
Results
TPC of the extracts
The TPC of the extracts is given in in terms of mg GAE per g extract. The LE of C. olitorius had significantly higher level of TPC than the SE (p < 0.05).
Table 1. TPC, IC50 of DPPH• scavenging activity, and IC50 of cytotoxicity.
Free radical scavenging activity
The LE (IC50: 22.7 µg/mL) had significantly higher FRSA than SE (p < 0.05) (). IC50 (FRSA) of standards AscA, BHT, and α-Toc were 5.9 ± 0.3, 12.6 ± 0.5, and 5.3 ± 0.5 µg/mL, respectively.
Cytotoxic effects of extracts on ARH-77 Cells
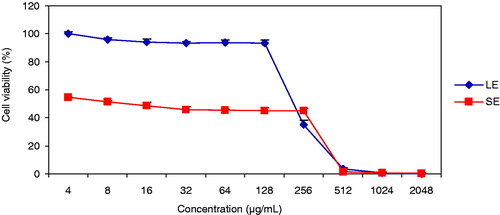
Both LE and SE exerted cytotoxic effects on ARH-77 cells in a range of 4–2048 µg/mL (). The solvent DMSO did not have any cytotoxic effect in the dilution range. The IC50 of SE (17 µg/mL) was ∼9-fold lower than LE (), which demonstrates its higher cytotoxicity on ARH-77 cells.
LE and SE caused dose-dependent genotoxic damage on ARH-77 cells
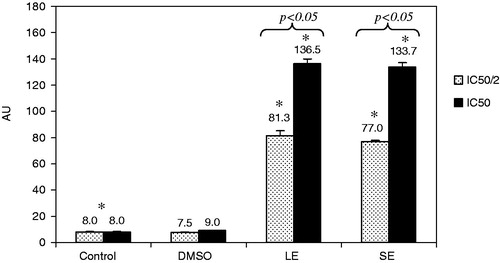
The visual analysis of comet assay measures the amount of DNA in the head and in the tail based on the principle that migration of nuclei with intact and damaged DNA on agarose differs (). The results obtained with the comet assay are summarized in . Both the extracts were applied at their respected IC50 and half of IC50 and induced genotoxic damage on ARH-77 cells. The control groups had 8 ± 0.4, whereas it increased to 81.3 ± 3.7 and 136.5 ± 3.5 after 48 h of 1/2 IC50 (75 µg/mL) and IC50 (150 µg/mL) leaf extract treatments, respectively. In concordance, genotoxic damage increased to 77.0 ± 1.0 and 133.7 ± 3.5 after 48 h of 1/2 IC50 (8.5 µg/mL) and IC50 (17 µg/mL) seed extract treatments, respectively. In both leaf and seed extract treatments, genotoxic damage significantly (p < 0.05) increased with increasing concentrations at relevant cytotoxic concentrations. DMSO used as solvent control did not cause significant increase in DNA damage.
Discussion
There is a good positive correlation between antioxidant capacity and TPC of spices, medicinal herbs, and other dietary plants due to their electron transfer/hydrogen donating ability (Lu et al., Citation2011). In our study, phenol content of the extracts was well correlated to DPPH• scavenging activity. Phenol contents and FRSA of LE were considerably higher than the SE. Moreover, FRSA of the LE (i.e., IC50 of 22.7 ± 1.8 µg/mL) was comparable to the activity of natural and synthetic antioxidants tested. Azuma et al. (Citation1999) identified 5-caffeoylquinic acid to be the most predominant antioxidant of C. olitorius leaves. Furthermore, flovonoidal glycosides (astragalin, isoquercitrin, tolifolin and jugulanin), and coumarin glycosides (4,7-dihydroxycoumarin, cichoiriine and scopolin) besides quercetin derivatives have been identified in C. olitorius leaves and seeds (Mukherjee et al., Citation1998; Khan et al., Citation2006).
Concentration and time-dependent pro-oxidant effects of many antioxidants on cancer cells have been demonstrated (Bjelakovic et al., Citation2004; Woods et al., Citation1999; Zhang & Omaye, Citation2001). According to , LE with considerably higher TPC and FRSA had lower cytotoxic effects on cells, at concentration which was above the IC50 of FRSA. Higher concentrations of LE may be required to shift to pro-oxidant effects and exert cytotoxicity. On the other hand, SE had higher cytotoxic potential with an IC50 of far below its IC50 of FRSA. In the case of SE with considerably low FRSA, antioxidant/pro-oxidant effect seems to be less important. So, high cytotoxic potential may be attributed to corchorusins which are abundant in seeds of Corchorus sp. (Khan et al., Citation2006), having structural similarity with saikosaponins (Hsu et al., Citation2000; Mahato & Pal, Citation1987). Saikosaponins and saikosaponin-like compounds have been reported to possess potent antitumor activity (Bachran et al., Citation2008). In a study of Mallick et al. (Citation2010), methanol extract of Corchorus acutangulus, its n-butanol fraction, and corchorusin-D inhibited cell growth and produced significant cytotoxicity in leukemic cell lines U937 and HL-60 via mitochondrial apoptotic pathway.
Depending on the extracted tissue, cytotoxic and genotoxic effects may vary. The IC50, the concentration of a toxic substance at which the half viability is attained relative to control, is a useful parameter when selecting the test concentrations. In both half of IC50 LE and SE applied groups, DNA damage values increased ∼10 folds. DNA damage increased ∼17-fold with respect to control when extract concentrations were doubled. Doubling the extract concentration resulted in significant increase in DNA damage in nuclei of ARH-77 cells demonstrating genotoxicity of the extracts. As stated above, compounds with high antioxidant activity may exhibit pro-oxidant behavior, and pro-oxidant activity can accelerate damage to molecules such as DNA, carbohydrates, or proteins (Aruoma et al., Citation1997). We have previously demonstrated that both ascorbic acid and β-carotene induce concentration and time-dependent genotoxic and cytotoxic damage on HepG2 human hepatocellular carcinoma cells together with increased oxidative damage at their relevant achievable plasma level concentrations (Yurtcu et al., Citation2011) in contrast to their protective effect on lymphocytes (Yurtcu et al., Citation2012). In addition, many compounds of plant origin may directly and irreversibly bind/damage to proteins involved in DNA replication, repair, and transcription, leading to damage at nucleic acid level. DNA damage observed in nuclei of ARH-77 cells might have been induced by direct or indirect acting of extract components on nucleic acids, and these deleterious effects may show variation depending on the extract component of different plant parts.
Conclusions
In the present study, we demonstrated the in vitro cytotoxic effect of C. olitorius LE and SE on ARH-77 cells, with relation to TPC and FRSA of the extracts. The LE had high TPC and FRSA, and cytotoxic IC50 on ARH-77 cells above its IC50 for FRSA. On the other hand, SE had lower TPC and FRSA in comparison to LE but highly cytotoxic to ARH-77 cells. Both LE and SE induce genotoxic damage at relevant cytotoxic concentrations. In vitro cytotoxic effect and genotoxicty of LE may be attributed to pro-oxidant effect of phenol compounds, whereas cytotoxic potential of SE may be related to direct effect of bioactive constituents. Although more studies with other cancer cell lines are required, this study gives a preliminary insight to further research at molecular level and in vivo research on C. olitorius. To our knowledge, this is the first report demonstrating the high cytotoxic potential of C. olitorius SE and the genotoxic potential of LE and SE.
Declaration of interest
The authors report no declarations of interest. This study was supported by Baskent University Research Fund.
Acknowledgements
The Department of Medical Biochemistry, Faculty of Medicine, Baskent University is greatly acknowledged for lyophilization process. Dr. Evren Cabi and Dr. Meltem Demirel Kars are greatly acknowledged for their contributions. This study was approved by Baskent University Institutional Review Board (Project no: DA11/29).
References
- Aruoma OI, Whiteman M, England TG, Halliwell B. (1997). Antioxidant action of ergothioneine: Assessment of its ability to scavenge peroxynitrite. Biochem Biophys Res Commun 231:389–91
- Azuma K, Nakayama M, Koshioka M, et al. (1999). Phenolic antioxidants from the leaves of Corchorus olitorius L. J Agric Food Chem 47:3963–6
- Bachran C, Bachran S, Sutherland M, et al. (2008). Saponins in tumor therapy. Mini Rev Med Chem 8:575–84
- Bjelakovic G, Nikolova D, Simonetti RG, Gluud C. (2004). Antioxidant supplements for prevention of gastrointestinal cancers: A systematic review and meta-analysis. Lancet 2:1219–28
- Cragg GM, Newman DJ. (1999). Screening of natural and synthetic compounds for their antimicrobial and anticancer activity. Cancer Lett 17:153–62
- Folin O, Ciocalteu V. (1927). Tyrosine and tryptophane determination in proteins. J Biol Chem 27:627–50
- Hsu MJ, Cheng JS, Huang HC. (2000). Effect of saikosaponin, a triterpene saponin, on apoptosis in lymphocytes: Association with c-myc, p53, and bcl-2 mRNA. Brit J Pharmacol 131:1285–93
- Khan MSY, Bano S, Javed K, Mueed MA. (2006). A comprehensive review on the chemistry and pharmacology of Corchorus species – A source of cardiac glycosides, triterpenoids, ionones, flavonoids, coumarins, steroids and some other compounds. J Sci Indust Res 65:283–98
- Lu M, Yuan B, Zeng M, Chen J. (2011). Antioxidant capacity and major phenolic compounds of spices commonly consumed in China. Food Res Int 44:530–6
- Mahato SB, Pal BC. (1987). Triterpenoid glycosides of Corchorus acutangulus Lam. J Chem Soc Perkin Trans 1:629–34
- Mallick S, Ghosh P, Samanta SK, et al. (2010). Corchorusin-D, a saikosaponin-like compound isolated from Corchorus acutangulus Lam., targets mitochondrial apoptotic pathways in leukemic cell lines (HL-60 and U937). Cancer Chemother Pharmacol 66:709–19
- Meikle RD. (1977). Corchorus L. In: Meikle RD, ed. Flora of Cyprus. Kew, London: Royal Botanic Gardens, 317–24
- Mukherjee KK, Mitra SK, Ganguly SN. (1998). A new coumarine from the seeds of Jute (Corchorus olitorius L.). Nat Prod Sci 4:51–2
- Newman DJ, Cragg GM, Snader KM. (2003). Natural products as sources of new drugs over the period 1981–2002. J Nat Prod 66:1022–37
- Nishiumi S, Yabushita Y, Fukuda I, et al. (2006). Molokhia (Corchorus olitorius L.) extract suppresses transformation of the aryl hydrocarbon receptor induced by dioxins. Food Chem Toxicol 44:250–60
- Oboh G, Raddatz H, Henle T. (2009). Characterization of the antioxidant properties of hydrophilic and lipophilic extracts of Jute (Corchorus olitorius) leaf. Int J Food Sci Nutr 60:124–34
- O’Brien NM, Woods JA, Aherne SA, O’Callaghan YC. (2000). Cytotoxicity, genotoxicity and oxidative reactions in cell-culture models: Modulatory effects of phytochemicals. Biochem Soc Trans 28:22–6
- Sharma OP, Bhat TK. (2009). DPPH antioxidant assay revisited. Food Chem 113:1202–5
- Slinkard K, Singleton VL. (1977). Total phenol analyses: Automation and comparison with manual methods. Am J Enol Viticul 28:49–55
- Woods JA, Bilton RF, Young AJ. (1999). beta-Carotene enhances hydrogen peroxide induced DNA damage in human hepatocellular HepG2 cells. FEBS Lett 23:255–8
- Yurtcu E, Iseri OD, Sahin FI. (2011). Effects of ascorbic acid and β-carotene on HepG2 human hepatocellular carcinoma cell line. Mol Biol Rep 38:4265–72
- Yurtcu E, Kasapoğlu E, Sahin FI. (2012). Protective effects of β-carotene and silymarin on human lymphocytes. Turk J Biol 36:47–52
- Zakaria ZA, Sulaiman MR, Arifah AK, et al. (2006). The anti-inflammatory and antipyretic activities of Corchorus olitorius in rats. J Pharmacol Toxicol 1:139–46
- Zhang P, Omaye ST. (2001). DNA strand breakage and oxygen tension: Effects of β carotene, alpha-tocopherol and AsA. Food Chem Toxicol 39:239–46
- Zhao X, Aldini G, Johnson EJ, et al. (2006). Modification of lymphocyte DNA damage by carotenoid supplementation in postmenopausal women. Am J Clin Nutr 83:163–9