Abstract
Context: Fagopyrum cymosum (Trey.) Meisn (Polygonaceae) (EFC) has long been used as a folk medicine to treat various ailments of the lung, dysentery and rheumatism in China.
Objective: The present study evaluated the anti-arthritic effect of 95% ethanol extract of EFC (extract of Fagopyrum cymosum).
Materials and methods: The anti-arthritic activity was investigated by adjuvant arthritic (AA) rat model induced by Freund’s complete adjuvant (FCA). The AA rats were randomly separated into different groups and then treated with EFC (40, 80 and 160 mg/kg) from day 7 to day 28 after immunization. Arthritis was evaluated by hind paw swelling, polyarthritis index, body weight and index of immune organs. In addition, the severity of arthritis in the knee joints was evaluated by histopathological and hemorheological examination. The levels of interleukin 1 (IL-1) and tumor necrosis factor alpha (TNF-α) in the serum were assessed by ELISA.
Results: The high dose level of EFC (160 mg/kg) significantly suppressed the swelling of hind paw of AA rats (p < 0.01) and inhibited their body weight loss (p < 0.01). Based on histopathological examination, all EFC groups showed great amelioration compared with the model group. EFC (80 and 160 mg/kg) also decreased the plasma viscosity in different shear rates (p < 0.01). Moreover, EFC significantly reduced the production of IL-1 and TNF-α in the serum of AA (p < 0.01).
Discussion and conclusion: This study provides a scientific basis for the claims that F. cymosum is effective in preventing and suppressing the development and progression of experimental arthritis, with reductions in inflammatory response.
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease, which leads to swelling of the joints, inflammation of the synovium and destruction of the adjacent cartilage and bone (Sweeney & Firestein, Citation2004). Steroidal agents, nonsteroidal anti-inflammatory drugs (NSAIDs) and immunosuppressants are used to treat RA. However, these drugs could produce various side effects such as immunodeficiency, gastrointestinal disorders and humoral disturbances (Simon, Citation2003). In recent years, attention to natural therapies has aroused a new wave of research interest in traditional practices because natural products have been used efficiently for several centuries with no obvious toxicities or side effects. Efforts need to be made to seek more effective and new natural drugs that can be used for long-term RA administration (Gao et al., Citation2008; Li et al., Citation2010; Wang et al., Citation2006).
Adjuvant arthritis (AA) is a kind of experimental arthritis model that is induced by the injection of Freund’s complete adjuvant (FCA). AA, which shares some features with human RA in aspects of histology and immunology, such as swelling of the extremities, cartilage degradation, loss of joint function and lymphocyte infiltration, is often used to evaluate new drugs for the treatment of RA (Cicala et al., Citation2000).
Although the causes of RA remain unknown, some studies have revealed that cytokines play an important role in the inflammation cascade and some of the important pro-inflammatory cytokines involved in RA such as interleukin 1 (IL-1) and tumor necrosis factor (TNF-α) are thought to play key roles in the destruction of cartilaginous and bony tissues in joints affected by RA (Dayer, Citation2002; Feldmann et al., Citation1996).
Fagopyrum cymosum (Trev.) Meisn (Polygonaceae) grows mainly in Vietnam, Thailand, Nepal, India and China (Anonymous, Citation1999). Its rhizome is regarded as a folk medicine in China to treat various ailments of the lung, dysentery and rheumatism (Gao & Meng, Citation1993; Liu et al., Citation1981). Previous chemical studies have shown that there are some phenolics and other compounds in the rhizome, such as hecogenin, β-sitosterol, ferulic acid, p-coumaric acid and shakuchirin (Liu et al., Citation1983; Wang et al., Citation2005). However, no in vivo study on the therapeutic effects of F. cymosum for RA has been reported and the detailed mechanisms of F. cymosum on arthritis have not been understood.
The current study evaluated the 95% ethanol extract of the rhizomes of Fagopyrum cymosum on AA rats. An evaluation system with arthritis damage, index of immune organs and histopathological and hematological parameters was employed in the study (Chen et al., Citation2009), and the possible underlying mechanisms in which some pro-inflammatory cytokines IL-1 and TNF-α are involved were further investigated.
Materials and methods
Plant material
Fagopyrum cymosum was collected from the Huangpi district, Wuhan, Hubei province, in November 2010 and identified by Professor Yonghui Zhang, Department of Chinese Medicine, Tongji Medical College. The voucher specimen (No.PS10-2010 Hubei) has been deposited in the laboratory of Pharmacy Department, Tongji Hospital.
Preparation of extract of F. cymosum
The rhizomes of F. cymosum were cleaned and dried in an airy room and then powdered. This powder (1.0 kg) was extracted by maceration in 95% ethanol (3 × 10 L) for 48 h at room temperature. Then the extracts were combined and filtered. The combined extract was concentrated under reduced pressure to give a residue (100 g). The extract was dried and solubilized in 0.5% CMC-Na (sodium carboxyl methyl cellulose) before being fed to the rats.
Experimental animal
Male Sprague-Dawley (SD) rats weighing 160–180 g were obtained from the experimental animal center of Tongji Medical College and maintained in a temperature (23 ± 2 °C) and humidity (55 ± 5%) with 12 h light–dark cycle for at least one week before the experiment. The animals were kept in plastic cages and allowed free access to food and water. All animals welfare and experimental procedures were carried out in compliance with the Guide for the Care and Use of Laboratory Animals (the project was approved by the Laboratory Animal Center of Tongji Medical College). Ethical approval for this study was obtained from the Ethics Committee of Tongji Medical University (licence number: SCXK 2010-0007). Six animals of each group were adopted in all tests.
Reagents and chemicals
Tripterygium glycosides (TPT), extracted from the Chinese herb Tripterygium wilfordii Hook. f. (Tripterygium) known for hundreds of years as a therapeutic agent for RA (Wang et al., Citation2004), was used as the positive control in this study and was also solubilized in 0.5% CMC-Na. TPT was obtained from Fudan Fuhua Pharmaceutical Factory (Shanghai, China). FCA was purchased from Sigma, Santa Clara, CA. ELISA kits for assessing the levels of IL-1 and TNF-α were all obtained from Shanghai Senxiong Technology Industry Co., Ltd. (Shanghai, China).
Adjuvant-induced arthritis in rats
AA was induced by subcutaneous injection with 0.1 ml of FCA into the right footpad of each rat. On the 7th day after immunized with FCA, the SD rats were randomly divided into five groups and treated as follows: group A treated with the extract of F. cymosum (EFC) (40 mg/kg); group B treated with EFC (80 mg/kg); group C treated with EFC (160 mg/kg); group D (positive control) treated with TPT (40 mg/kg); group E (model control) fed with 0.5% CMC-Na. An extra group (F) was the unimmunized male rats as a normal control. The tested doses of EFC were calculated according to the human doses of crude drug and the ratio of extract. The whole period of treatment was 21 days.
Assessment of AA in rats
The hind paws of rats were measured with a water replacement plethysmometer before immunization with FCA (baseline, day 0) and on days 8, 12, 16, 20, 24 and 28 after immunization throughout the total experiment. Paw swelling at each time point was defined as the increase in paw volume since immunization, given in milliliters. The arthritic severity in each paw was evaluated by a macroscopic scoring system ranging from 0 to 4: 0 – paws with no swelling and focal redness; 1 – paws with swelling of finger joints; 2 – paws with mild swelling of ankle or wrist joints; 3 – paws with severe inflammation of the entire paws; 4 – paws with deformity or ankylosis. The cumulative score for all four paws of each rat was used as the polyarthritis index with a maximum value of 16 (Hughes et al., Citation1994).
Index of immune organs
At day 28 after immunization, the rats were killed by cervical dislocation. The thymus and spleen were promptly removed and weighed. The index of thymus and spleen was expressed as the percentage (%) of thymus and spleen wet weight versus body weight, respectively (Li et al., Citation2010).
Histopathological assessment of ankle joint
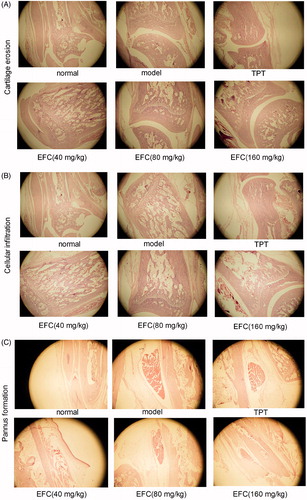
For the histologic analysis of the knee joints, the hind paws above the ankle joints were amputated and were fixed in 10% neutral-buffered formalin, decalcified in 5% formic acid and then embedded in paraffin. Tissue sections were stained with hematoxylin and eosin (H&E) and were evaluated under a light microscope (OlympusLX70, Olympus, Tokyo, Japan). Bone destruction, vascular proliferation, synovial hyperplasia and inflammatory cell infiltration were assessed. The severity of lesions was evaluated using the following scoring system (Schramm et al., Citation2004; Wang et al., Citation2011): synovial proliferation: Grade 0, proliferation was absent; Grade 1, proliferation was mild with two to four layers of reactive synoviocytes; Grade 2, proliferation was moderate with four plus layers of reactive synoviocytes, increased mitotic activity and mild or absent synovial cell invasion of adjacent bone and connective tissue; and Grade 3, proliferation was severe and characterized by invasion and effacement of joint space and adjacent cartilage, bone and connective tissue. Cellular infiltration: Grade 0, no changes; Grade 1, few focal infiltrates; Grade 2, extensive focal infiltrates; and Grade 3, extensive infiltrates invading the capsule with aggregate formation. Cartilage erosion: Grade 0, no changes; Grade 1, superficial, localized cartilage degradation in more than one region; Grade 2, localized deep cartilage degradation; and Grade 3, extensive deep cartilage degradation at several locations. Pannus formation: Grade 0, no changes; Grade 1, pannus formation at up to two sites; Grade 2, pannus formation at up to four sites, with infiltration or flat overgrowth of joint surface; and Grade 3, pannus formation at more than four sites or extensive pannus formation at two sites. Pathological evaluation was performed randomly by a pathologist trained in rat joint pathology who was blind to the treatment of the samples.
Hemorheological effects of rats
On the last day of treatment, all rats were anesthetized with 10% chloral hydrate and then the blood was drawn from abdominal vein to determine hemorheological variables. The blood or plasma was used to determine the viscosity with a cone-plate viscometer (Model LG-R-80B, Steellex Co., Beijing, China) at different shear rates maintained at 37 °C. One part of the blood was anticoagulated with heparin (20 U/mL) for plasma viscosity and hematocrit measurements. The whole blood viscosity was measured at high shear rate (200 s−1) and low shear rate (50 s−1) (Li et al., Citation2009).
IL-1 and TNF-α determination
The cytokines, including IL-1 and TNF-α, produced from the serum of the animals, were measured by ELISA kits according to the manufacturers’ instructions (ADL Systems, Shanghai Senxiong Technology Industry Co., Ltd, Shanghai, China). Each serum sample was assayed in duplicate.
Statistical analysis
Results are expressed as mean standard deviation (S.D.). Data were analyzed by the one-way analysis of variance to determine significant differences between means of groups. p Value below 0.05 was considered statistically significant.
Results
EFC inhibits AA
AA developed rapidly with clinical signs such as redness, edema and swelling in the joints of the feet. The results showed that the redness and swelling of joints appeared around day 12 and peaked on day 20 after immunization () (p < 0.01). EFC (80, 160 mg/kg) administration suppressed the severity of clinical arthritis compared with the model group, as demonstrated by both the paw swelling () (p < 0.01) and the polyarthritis index () (p < 0.01).
Evaluation of body weight and index of organs
The body weight and index of organs were considered as apparent indicators of arthritis to evaluate the arthritic progression of adjuvant-induced arthritis. As shown in , the model group had significantly lower body weight from day 12 after adjuvant injection than the normal group. From the 16th day after immunization, the body weight of different EFC groups and TPT group was significantly higher than that of the model group. At the same time, the body weight of EFC (80 and 160 mg/kg) was higher than that of the TPT group, but the EFC (40 mg/kg) group was lower. Compared with the model group, EFC groups showed a gradient increase in a dose-dependent manner after the drug administration. Moreover, administration of EFC (40, 80 and 160 mg/kg) obviously increased the index of thymus and spleen of AA rats compared with the model group. The same results were also observed in the TPT group ().
EFC prevents ankle joint histopathology
The disease severity and the effect of EFC on joint histology was illustrated by photomicrographs of sections stained with H&E (). No inflammation, cartilage erosion and pannus formation were shown in section from normal rats. In contrast, the model group was marked extensive inflammation, marked proliferation of synoviocytes, cellular infiltration and pannus formation with resultant erosion of articular cartilage. EFC-treated (80 mg/kg) rats exhibited moderate cartilage degradation and mild pannus formation; EFC (160 mg/kg) and TPT (40 mg/kg) ameliorated joint destruction. Histopathological evaluation demonstrated that EFC (80, 160 mg/kg) treatment resulted in statistically significant reductions in synovial proliferation, cellular infiltration, cartilage erosion and pannus formation scores in AA rats (). The same results were also shown in the TPT group.
Effect of EFC on hemorheological disorders in AA rats
Hemorheological disorders may play an important role in the development and pathogenesis of many diseases. The whole blood viscosity of all groups in different shear rates (50, 100 and 200 s−1) is shown in . The plasma viscosity significantly increased at all shear rates in the model group compared to the control group, and decreased in the TPT as well as 40, 80 and 160 mg/kg EFC groups compared to the model group (p < 0.01); and the EFC groups were in dose-dependent manner.
Table 1. Effect of EFC on hemorheological disorders in rats.
EFC regulates the concentrations of IL-1 and TNF-α in serum
The levels of IL-1 and TNF-α are shown in . The levels in the control group were 59.36 and 360.73 ng/L while the serum IL-1 level increased to 132.47 ng/L and the serum TNF-α level rose to 517.24 ng/L in the model group. The serum levels of the EFC groups were significantly decreased, in comparison with the model group.
Table 2. Effect of EFC on the IL-1 and TNF-α levels in rats serum.
Discussion
In China, F. cymosum has long been used for medical purposes. For example, WeiMaiNing (WMN), which is the commercial product of F. cymosum, is nontoxic and partially inhibits tumor growth to treat patients with lung cancer in clinical trial (reported in International Publication Number WO99/163 19). However, there are scarcely reports about the anti-inflammatory and anti-arthritic activities of Fagopyrum cymosum. For this reason, we analyzed the effects of EFC in established AA.
AA is a well-established in vivo model that has been used in numerous studies to investigate the pathogenesis of RA and for identifying potential therapeutic targets (Wooley, Citation1991). AA is very similar to human RA both in pathological and serological changes (Jacobson et al., Citation1999), including the involvement of paw edema, arthrodynia, decreased body weight and cartilage degradation. Decrease in body weight occurs in many chronic diseases, such as diabetes, cancer, as well as in inflammatory diseases such as Crohn’s disease, sepsis and RA.
In this study, we used paw swelling, polyarthritis index, the changes of body weight and immune organs index to assess the disease progression. As a result, treatment with EFC markedly suppressed the development of arthritis ( and ), with these effects observed throughout the course in the article.
In addition, suppression of disease progression by EFC was further supported by the histopathological analysis from these animals. EFC (80 and 160 mg/kg) improved arthritic histopathological in AA rats, such as in cellular infiltration, cartilage erosion and pannus formation (). AA scores were reduced across all disease parameters assessed in the study (). The current findings support that EFC can prevent disease progression and attenuate joint inflammation and destruction.
In light of the impressive therapeutic effect of EFC in AA, we investigated the effect of EFC on hemorheological disorders in AA rats. EFC in different doses can reduce the whole blood viscosity (50, 100 and 200 s−1) and packed cell volume () to improve the local lesion capillary permeability and prevent the inflammatory exudates. At the same time, it suggests that EFC may inhibit platelet aggregation and increase the local blood supply.
AA is a T-cell-mediated chronic inflammatory stress (Skapenko et al., Citation2005). The effect of EFC on inflammatory mediators was determined by the analysis of cytokines. The production of pro-inflammatory cytokines IL-1 (Joosten et al., Citation1999) and TNF-α (Han et al., Citation2013) were released from activated T cells and macrophage in RA patients, which are considered to be the important participants in the pathophysiology of RA (Bonecchi et al., Citation1998; Robinson et al., Citation1975; van den Berg, Citation2001), decreased after treatment with EFC in serum (). These findings suggest that EFC was the downregulation of pro-inflammatory cytokines and their associated inflammatory pathways.
Conclusion
Taken together, the findings of this study demonstrate that EFC has profound therapeutic effects on AA. It directs toward the control of arthritis progression and prevents synovial proliferation, cartilage and bone destruction of the arthritic joints of AA rats. In addition, EFC changes the hemorheological disorders and reduces the level of pro-inflammatory cytokines (IL-1and TNF-α). The data obtained in this study also warrant the further investigation of EFC, including intensive mechanism studies and confirmation of anti-arthritic effects in other animal models.
Declaration of interest
The authors declare no potential conflict of interest and are responsible for the writing and content of the paper.
Acknowledgements
The authors deeply thank Biyu Zhang for assistance in hemorheology.
References
- Anonymous. (1999). Zhong Hua Ben Cao (China Herbal). Shanghai: Shanghai Science and Technology Publisher and Distributor
- Bonecchi R, Bianchi G, Bordignon PP, et al. (1998). Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med 187:129–34
- Chen H, Shoumura S, Emura S, Isono H. (2009). Tibetan medicated-bath therapy may improve adjuvant arthritis in rat. Evid Based Complement Alternat Med 6:211–7
- Cicala C, Ianaro A, Fiorucci S, et al. (2000). NO-naproxen modulates inflammation, nociception and downregulates T cell response in rat Freund’s adjuvant arthritis. Br J Pharmacol 130:1399–405
- Dayer JM. (2002). Interleukin 1 or tumor necrosis factor-alpha: Which is the real target in rheumatoid arthritis? J Rheumatol Suppl 65:10–15
- Feldmann M, Brennan FM, Maini RN. (1996). Role of cytokines in rheumatoid arthritis. Annu Rev Immunol 14:397–440
- Gao Q, Shan J, Di L, et al. (2008). Therapeutic effects of daphnetin on adjuvant-induced arthritic rats. J Ethnopharmacol 120:259–63
- Gao Z, Meng FH. (1993). Effect of Fagopyrum cymosum root in on clonal formation of four human tumor cells. Zhongguo Zhong Yao Za Zhi 18:498–500, 511
- Han W, Xiong Y, Li Y, et al. (2013). Anti-arthritic effects of clematichinenoside (AR-6) on PI3K/Akt signaling pathway and TNF-α associated with collagen-induced arthritis. Pharm Biol 1:13–22
- Hughes C, Wolos JA, Giannini EH, Hirsch R. (1994). Induction of T helper cell hyporesponsiveness in an experimental model of autoimmunity by using nonmitogenic anti-CD3 monoclonal antibody. J Immunol 153:3319–25
- Jacobson PB, Morgan SJ, Wilcox DM, et al. (1999). A new spin on an old model: In vivo evaluation of disease progression by magnetic resonance imaging with respect to standard inflammatory parameters and histopathology in the adjuvant arthritic rat. Arthritis Rheum 42:2060–73
- Joosten LA, Helsen MM, Saxne T, et al. (1999). IL-1 alpha beta blockade prevents cartilage and bone destruction in murine type II collagen-induced arthritis, whereas TNF-alpha blockade only ameliorates joint inflammation. J Immunol 163:5049–55
- Li HX, Han SY, Wang XW, et al. (2009). Effect of the carthamins yellow from Carthamus tinctorius L. on hemorheological disorders of blood stasis in rats. Food Chem Toxicol 47:1797–802
- Li R, Cai L, Xie XF, et al. (2010). Hesperidin suppresses adjuvant arthritis in rats by inhibiting synoviocyte activity. Phytother Res 24:S71–6
- Liu WF, Song YM, Wang LZ, et al. (1981). Some pharmacological properties of Jin Qiao Mai Fagopyrum cymosum (Trev.) Meisn. Yao Xue Xue Bao 16:247–52
- Liu YL, Frang QN, Zhang XC, et al. (1983). Chemical constituents of Fagopyrum cymosum (Trev) Meisn. Yao Xue Xue Bao 18:545–7
- Robinson DR, Tashjian AJ, Levine L. (1975). Prostaglandin-stimulated bone resorption by rheumatoid synovia. A possible mechanism for bone destruction in rheumatoid arthritis. J Clin Invest 56:1181–8
- Schramm C, Kriegsmann J, Protschka M, et al. (2004). Susceptibility to collagen-induced arthritis is modulated by TGFbeta responsiveness of T- cells. Arthritis Res Ther 6:114–19
- Simon RA. (2003). Prevention and treatment of reactions to NSAIDs. Clin Rev Allergy Immunol 24:189–98
- Skapenko A, Leipe J, Lipsky PE, Schulze-Koops H. (2005). The role of the T-cell in autoimmune inflammation. Arthritis Res Ther 7 Suppl 2:4–14
- Sweeney SE, Firestein GS. (2004). Rheumatoid arthritis: Regulation of synovial inflammation. Int J Biochem Cell Biol 36:372–8
- Van den Berg WB. (2001). Anti-cytokine therapy in chronic destructive arthritis. Arthritis Res 3:18–26
- Wang B, Ma L, Tao X, Lipsky PE. (2004). Triptolide, an active component of the Chinese herbal remedy Tripterygium wilfordii Hook F, inhibits production of nitric oxide by decreasing inducible nitric oxide synthase gene transcription. Arthritis Rheum 50:2303–995
- Wang D, Chang Y, Wu Y, et al. (2011). Therapeutic effects of TACI-Ig on rat with adjuvant arthritis. Clin Exp Immunol 163:225–34
- Wang KJ, Zhang YJ, Yang CR. (2005). Antioxidant phenolic constituents from Fagopyrum dibotrys. J Ethnopharmacol 99:259–64
- Wang Y, Wei D, Lai Z, Le.Y. (2006). Triptolide inhibits CC chemokines expressed in rat adjuvant-induced arthritis. Int Immunopharmacol 6:1825–32
- Wooley PH. (1991). Animal models of rheumatoid arthritis. Curr Opin Rheumatol 3:407–20

![Figure 1. Effects of EFC on paw swelling and polyarthritis index in an AA rat model [n = 6, mean (S.D.)]. Noninjected hind paw swelling (A) and polyarthritis index (B) were assessed at each point at the respective time.](/cms/asset/6c2b8409-275e-42e7-8925-aad09fca63c3/iphb_a_766892_f0001_b.jpg)
![Figure 2. Effects of EFC on body weights and index of immune organs of AA rats [n = 6, mean (S.D.)]. Changes in body weights (A) and index of organs (B) were assessed at each point at the respective time. *p < 0.05, **p < 0.01 versus the model group.](/cms/asset/795e3d13-c1b8-48a0-b536-ceae00ff9a02/iphb_a_766892_f0002_b.jpg)
![Figure 4. Histopathological evaluation of ankle joint in AA rat models. Histological appearance was scored for the presence of synovial proliferation, cellular infiltration, cartilage erosion and pannus formation. *p < 0.05, **p < 0.01 compared with the model group [n = 6, mean (S.D.)].](/cms/asset/31ee3cb0-eb43-4a4c-94d1-1cd6b076c041/iphb_a_766892_f0004_b.jpg)