Abstract
Context: Currently, famous traditional Chinese medicine formulas have undergone re-evaluation and development in China. Wei–Chang–An–Wan (WCAW) as one of them has been used for treating various gastrointestinal diseases for several decades. The secondary development of WCAW is in progress so as to interpret the effective material basis or find new pharmacological activity.
Objective: To evaluate the antinociceptive effect of methanol extract of WCAW (ME) as well as four fractions (P.E., EtOAc, n-BuOH, H2O) and obtain information on the correlation between the contents of the fractions and antinociceptive effect.
Materials and methods: ME was divided into four parts extracted by petroleum ether, ethyl acetate and n-butanol. Antinociceptive activity was evaluated by three models of acetic acid–induced writhing, formalin and hot-plate test in mice after repetitive administration of ME at 200, 400 or 800 mg/kg, P.E. 132 mg/kg, EtOAc 106 mg/kg, n-BuOH 176 mg/kg and H2O 176 mg/kg for six days. The chemical compounds were analyzed by HPLC-ESI-MS.
Results: ME at 800 mg/kg inhibited acid-induced writhing by 84.69%, and reduced the licking time of second phase in formalin test by 53.23%. The inhibition rates in acid-induced writhing of P.E., EtOAc, n-BuOH and H2O were 27.79, 33.85, 38.97 and 37.69%, respectively, and in formalin test about 50%. They had no effect on the hot-plate test. HPLC-ESI-MS analysis showed that 68 chemical compounds were detected and 41 compounds were identified from ME.
Discussion and conclusion: The results obtained herein indicate that WCAW possesses the antinociceptive activity that provides a new aspect in clinical application.
Introduction
Traditional Chinese medicine (TCM) formulas have been widely used in China since ancient times to treat different diseases. TCM formulas are believed to evolve from synergistic combinations of multiple TCM herbs. To promote TCM formulas toward the world, many encouraged innovations and redevelopments were carried out in China (Gao et al., Citation2012; Xie et al., Citation2012). Wei–Chang–An–Wan (WCAW) is a patent TCM and has been marketed in China for more than 20 years for the management of gastrointestinal diseases such as diarrhea, abdominal pain, enteritis, dysentery and vomiting (Ling et al., Citation2005). Five herbs of Aucklandia lappa Decne (Compositae), Aquilaria sinensis (Lour.), Gilg (Thymelaeaceae), Santalum album L. (Santalaceae), Citrus aurantium L. (Rutaceae) (also called Fructus Aurantii) and Magnolia officinalis Rehd. et Wils. (Magnoliaceae) in WCAW accounted for 75% (Committee of National Pharmacopoeia, Citation2010) have been widely used in treatments of abdominal pain, disorders of gastrointestinal tracts or inflammation (Fang et al., Citation2009; Guo et al., Citation2012; Kakino et al., Citation2010; Kim et al., Citation2012; Watanabe et al., Citation1983). Fructus Aurantii, Magnolia officinalis and Rheum officinale (Polygonaceae) showed anti-inflammatory activity by inhibiting lipopolysaccharide-induced nitric oxide (NO) and prostaglandin E2 (PGE2) production (Tseng et al., Citation2006). Aquilaria sinensis possessed the properties of antinociceptive and anti-inflammatory activities (Zhou et al., Citation2008), and Ligusticum chuanxiong Hort. (Umbelliferae) showed analgesic effect in different animal models (Gao & Xu, Citation2010).
Based on the above information, WCAW might have an analgesic effect. Currently, WCAW is found to relieve functional gastrointestinal disorders such as irritable bowel syndrome (Liu et al., Citation2010) and inflammatory bowel disease (Dai et al., Citation2010). In our previous work, WCAW was certified for antidiarrheal (Hu et al., Citation2009) and anti-inflammatory activities (Wang et al., Citation2012) in animal models. Therefore, the present study was to investigate the antinociceptive activity of WCAW in three different animal models. Moreover, the possible active constituents of WCAW were determined by HPLC-MS.
Materials and methods
Plant material and preparation of different extracts of WCAW
As previously described (Hu et al., Citation2009), WCAW was prepared with 10 herbs: 25% the dried root of Aucklandia lappa, 10% the resinous wood of Aquilaria sinensis, 15% the immature fruit of Citrus aurantium, 15% the dried bark of Magnolia officinalis, 10% the duramen of Santalum album, 7% the dried rhizoma of Rheum officinale, 5% the mature fruit of Cronton tiglium L. (Euphorbiaceae), 0.5% Moschus moschi ferus Linnaeus, 5% the dried rhizoma of Ligusticum chuanxiong and 7.5% the fruit of Ziziphus jujuba Mill (Rhamnaceae) mill in Tianjin Lerentang Pharmaceutical Factory. These herbs were purchased from Medicinal Material Company (Hebei Province, China) and identified by Professor Wen-Yuan Gao, and all the voucher specimens (No. WCAW-111001) were deposited at the School of Pharmaceutical Science and Technology at Tianjin University (China).
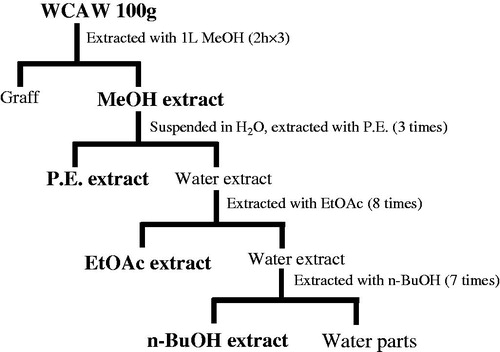
WCAW (100 g) was powdered and extracted with 1 L of methanol for 2 h in a Soxhlet apparatus. The filtrate was collected and the residue was re-extracted with 1 L of methanol twice, 2 h for each time. Then the solvent was removed under reduced pressure in a rotary evaporator (Buchi B-480) and the MeOH extract of WCAW (ME) was obtained, with a yield of 27.03% (w/w). Then ME was suspended in water and extracted with petroleum, ethyl acetate and n-butyl alcohol. After this process, we received P.E. extract (4.93 g), EtOAc extract (3.96 g), n-BuOH extract (6.59 g) and water parts (6.57 g) ().
Drugs and reagents
ME, P.E., EtOAc, n-BuOH and H2O extracts were dissolved in 0.5% carboxymethyl cellulose (0.5% CMC). Pethidine injection (1 mL:50 mg) was donated by Affiliated Hospital of Medical College of Chinese People’s Armed Police Forces. Aspirin effervescent tablets (0.5 g/10 tablets) obtained from the drugstore were produced by AstraZeneca Pharmaceutical Co., Ltd (Jiangsu, China).
Acetonitrile was of HPLC grade from Merck (Germany); acetic acid was purchased from Tianjin Guangfu Fine Chemical Research Institute (Tianjin, China). Reference standards of naringin, hesperidin, neohesperidin, aloe-emodin, rhein, emodin, honokiol, dehydrocostus lactone, costunolide, magnolol, chrysophanol and physcion were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China).
Animals
Adult male and female KM mice (18–22 g) were purchased from Tianjin Experimental Animal Center (License No. SCXK (Jin) 2009-0002) and involved in this trial. The animals were housed in polycarbonate cages (10 animals in each cage) with white wood chips for bedding, and given free access to food and drinking water, under controlled temperature (23 ± 2 °C), humidity (50–60%) and photoperiod (12 h light, 12 h dark) at the Animal Breeding Laboratory of Institute of Radiation Medicine, Chinese Academy of Medical Sciences, Tianjin, China. This animal study was approved by the Institutional Animal Care and Use Committee of China, and institutional guidelines for animal welfare and experimental conduct were followed.
Experiment design
ME was evaluated in three different analgesic models at first to identify the antinociceptive effect. ME showed analgesic activity in acetic acid-induced abdominal pain and formalin test models at the dose of 800 mg/kg. According to the yield of the extracts, the doses of P.E., EtOAc, n-BuOH and H2O were determined at 132, 106, 176 and 176 mg/kg.
Acetic acid-induced writhing response in mice
Mice were treated with ME (200, 400, 800 mg/kg), P.E. (132 mg/kg), EtOAc (106 mg/kg), n-BuOH (176 mg/kg) and H2O (176 mg/kg) for six days (once a day). The control group was given 0.5% CMC and the positive group was orally administered aspirin (100 mg/kg) or injected with pethidine (2.5 mg/kg). Thirty minutes after the administration of drugs, each mouse was injected intraperitoneally (i.p.) with 10 mL/kg of 0.6% acetic acid solution. A writhing is characterized by a wave of contraction of the abdominal musculature followed by the extension of the hind limbs. After the acetic acid administration, the starting time, of writhing, and the number of writhes within 30 min were recorded. Antinociception was expressed as an inhibition percentage of the number of writhes observed in control animals (Miranda et al., Citation2002).
Formalin test in the hind paw
The method described by other authors (Garcia et al., Citation2011; Godin et al., Citation2011; Miranda et al., Citation2007) was used. To perform the test, 20 μL of 2.5% formalin solution was injected into the right hind paw plantar surface (hypodermic injection). Each mouse was immediately returned to the observation chamber. The degree of pain intensity was determined as the total time spent by the animal licking or biting the injected hind paw, measured by visual observation and a digital time-out stopwatch. Mice were orally administrated ME and different extracts of WCAW as the same to the above described. The control group was given 0.5% CMC and the positive group was injected with pethidine (2.5 mg/kg). Thirty minutes after administration of drugs, mice were injected with formalin solution and the licking time between 0–5 min (first phase) and 15–30 min (second phase) was recorded. After investigating the licking time, we measured the volume of the right and left hind paws using vernier caliper, and calculated the volume by the following formula: Volume (mm/kg) = (the volume of right paw − volume of left paw)/weight × 1000.
Hot-plate test
A preliminary protocol was carried out to evaluate the latency for the nociceptive behavior after exposing the mice on plate temperatures of 55 ± 1 °C (Godin et al., Citation2011). The groups were divided as shown in acetic acid-induced writhing response in mice. Control group received 0.5% CMC and the positive group was given 2.5% pethidine by intraperitoneal injection. Mice were individually placed on the heated plate at 0, 15, 30, 60 and 120 min after treatment with drugs. The time for forepaw licking or jumping was taken as the latency time. The base line latency for this behavior was recorded with a stopwatch. The cut-off time (Toff) was fixed at 60 s to avoid skin damage.
Determination of the chemical compounds of WCAW by HPLC-MS
The chemical profiles of ME, P.E., EtOAc, n-BuOH and H2O extracts were analyzed by HPLC-MS. Briefly, they were analyzed by HPLC-MS using HPLC (Agilent’s 1200 series Diode Array detector, Agilent technologies, Tianjin, China) with an ion-trap ESI-mass spectrometer. Samples (100 μg/20 μL) were injected into a Kromasil RP-C18 column (4 mm × 250 mm). The column was equilibrated in 1% acetic acid (solution A) and elution of the components was achieved by increasing the concentration of solution B (100% acetonitrile) from 5% to 80% in 100 min at a flow rate of 1 mL/min. The molecular masses of the peaks were determined from electrospray ionization mass spectra using multiple-charged ion profile.
Source settings used for the ionization of WCWA were nebulizer gas flow, 27.6 Pa; dry gas flow, 10.0 L/min; electrospray voltage of the ion source, 3500 V; capillary temperature, 325 °C. Nitrogen (>99.99%) and He (>99.99%) were used as sheath and damping gas, respectively. The full scan of ions ranging from m/z 100 to 1000 in the negative ion mode or the positive ion mode was carried out. The fragment ions were obtained using collision energy of 35% for both MS2 and MS3 experiments. Analyses were conducted at ambient temperature and the data were operated on the Xcalibur software (Finnigan, Washington, DC).
Statistical analysis
Data were expressed as means ± standard error (S.E.M.) or percentage and analyzed for statistical significance using one-way analysis of variance followed by Dunnett’s test. Tests were performed using SPSS 17.0 system (Chicago, IL); p value less than or equal to 0.05 was considered to be statistically significant.
Results
Acetic acid-induced writhing response in mice
There was a decrease in the number of writhing in all groups treated with ME (200 mg/kg: 24.5 ± 1.43; 400 mg/kg: 14.5 ± 0.78; 800 mg/kg: 6.17 ± 0.34) compared to control (40.29 ± 0.86). At the dose of 800 mg/kg, the inhibition rate of ME was 84.69%, which was higher than aspirin (positive drug, 100 mg/kg, 79.79%) ().
Figure 2. Effect of methanol extracts of WCAW (ME) and four extracts of ME on acetic acid-induced abdominal pain in mice. ME (200, 400 and 800 mg/kg, i.g.) and aspirin (100 mg/kg) reduced the number of writhings (A). P.E. (132 mg/kg i.g.), EtOAc (106 mg/kg i.g.), n-BuOH (176 mg/kg i.g.), H2O (176 mg/kg i.g.) and pethidine (2.5 mg/kg i.p.) also reduced the number of writhings (B). Pethidine increased the latency time of first writhing markedly, although WCAW had little effect. Each bar represents the mean ± S.E.M. of 10 mice per group. *p < 0.05, **p < 0.01, ***p < 0.001 indicate significant differences from the control group.
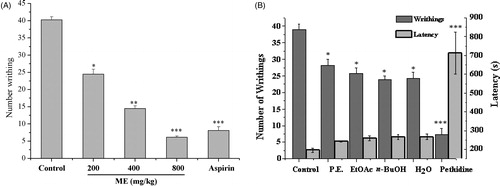
In the groups treated with different parts of ME, there was an increased latency to the first writhing appearance compared to the control group, but lower than pethidine (712.29 ± 112.13 s versus 196.00 ± 11.89 s, p < 0.001). The number of writhes also decreased after treatment with the drugs. However, all the inhibition rates were lower than the ME group (P.E.: 27.79%, EtOAc: 33.85%, n-BuOH: 38.97%, H2O: 37.69% versus Pethidine: 81.32%) ().
Formalin test in the hind paw
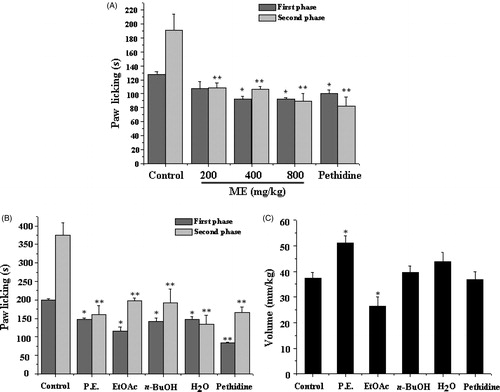
Oral administration of ME and four parts significantly reduced the time that the animal licked its stimulated paw in both phases of testing when compared with control (p < 0.05, p < 0.01) (). For the first phase, the group of EtOAc extract showed the lowest licking time compared to other extracts. In the second phase, H2O part showed the strongest effect on the licking time. However, the volumes induced by the stimulation of formalin were different when treated with extracts (). EtOAc group could reduce the volumes, but the P.E. extract increased (p < 0.05).
Figure 3. Effects of methanol extracts of WCAW (ME) and four extracts of ME on the formalin test in mice. ME (200, 400 and 800 mg/kg, i.g.) and pethidine (2.5 mg/kg i.p.) reduced the licking time (A). P.E. (132 mg/kg i.g.), EtOAc (106 mg/kg i.g.), n-BuOH (176 mg/kg i.g.), H2O (176 mg/kg i.g.) also reduced the licking time (B) and had different effect on the volume of hind paw (C). Each column represents the mean ± S.E.M. of 7 mice. *p < 0.05 and **p < 0.01 indicate significant differences from the control group.
Hot-plate test
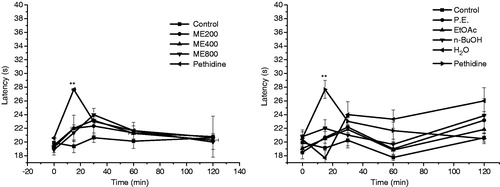
In this experiment, pethidine increased the latency for nociceptive response in the hot-plate model when a thermal stimulus of intensity (55 °C) was used at 15 min (p < 0.01) and with the extension of time, the latency was decreased. The antinociceptive effect was not observed after giving ME (200, 400 or 800 mg/kg, ) and four different parts ().
Figure 4. Effects induced by methanol extracts of WCAW (ME) (A), four different parts (B) and pethidine (2.5 mg/kg i.p.) on the nociceptive response induced by heat (hot-plate model, 55°С). Data represent the mean ± S.E.M. of 7–8 mice. *p < 0.05 and **p < 0.01 indicate significant differences from the control group.
Identification of the chemical compounds in WCAW by HPLC-MS
MS of the compounds from WCAW was acquired in the positive and negative ion modes and the base peak chromatograms of the different compounds were obtained by the HPLC-MS method (). Using the extracted ion chromatograms and the ion fragments information, 68 compounds were detected in ME and 41 compounds were identified. presents their retention times (tR), mass, ESI (±)-MSn fragmentation ions and distribution. The identified compounds were distributed in the herbals of Fructus Aurantii (17), Rheum officinale (15), Magnolia officinalis (7) and Aucklandia lappa (2). As shown in , the rule of chemical distribution of P.E., EtOAc and n-BuOH extracts seemed to exist. Twelve chemical compounds identified from P.E. extracts had the longest retention time; 20 chemical compounds identified from EtOAc extracts had a longer retention time; 28 chemical compounds identified from n-BuOH extracts had the shortest retention time.
Figure 5. Chromatograms of WCA extract and its different polar fractions by HPLC-MS. (A) TIC chromatogram of ME in the positive ESI mode. (B) TIC chromatogram of ME in the negative ESI mode. (C) TIC chromatogram of the P.E. extract in the positive ESI mode. (D) TIC chromatogram of P.E. extract in the negative ESI mode. (E) TIC chromatogram of EtOAc extract in the positive ESI mode. (F) TIC chromatogram of EtOAc extract in the negative ESI mode. (G) TIC chromatogram of the n-BuOH extract in the positive ESI mode. (H) TIC chromatogram of the n-BuOH extract in the negative ESI mode.
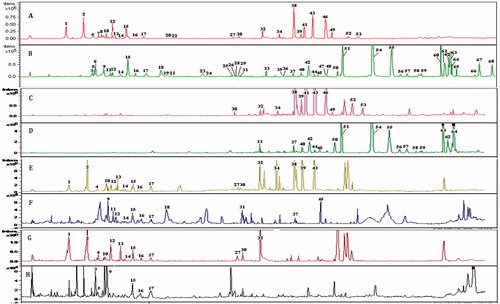
Table 1. HPLC-ESI(±)-MS data of analyses and distribution of the MeOH extract of WCAW and its fractions.
Discussion
WCAW showed lower toxicity and had antidiarrheal, antispasmodic and gut modulatory activities in vivo and in vitro (Hu et al., Citation2009). It also possessed anti-inflammatory effects in different models in rodents. As reported, the acute or chronic inflammatory process is a model of nociception and several works have shown that inflammatory mediators can generate nociceptive impulses (Ferreira et al., Citation2004). Given the relationship between anti-inflammatory and analgesic effects, the present study researched the antinociceptive effects of WCAW for the first time, and the result demonstrated that WCAW significantly inhibited acetic acid-induced abdominal writhing and reduced the licking time in the formalin test.
Acetic acid causes an increase in peritoneal fluids of prostaglandins such as prostaglandins, serotonin and histamine involved in part, which was a model commonly used for screening peripheral analgesics (Taufiq-Ur-Rahman et al., Citation2005). Our results showed that ME and its fractions produced significant analgesic activity, and these effects may be attributed to PG synthesis inhibition.
To evaluate the possible central antinociceptive effects of WCAW, the formalin and hot-plate tests were adopted. The advantage of using the formalin model of nociception is that it can discriminate pain in its central and peripheral components (Quintans-Júnior et al., Citation2010; Tjolsen et al., Citation1992). Intraplantar injection of formalin evokes a characteristic biphasic licking response. The first phase corresponding to acute neurogenic pain sensitive to drugs that interact with the opioid system, starts immediately after injection and is considered likely due to the direct stimulation of nociceptors. The second phase corresponding to inflammatory pain responses is related to the release of chemical mediators such as histamine, serotonin, bradykinin, prostaglandins and excitatory amino acids, which can be inhibited by analgesic/anti-inflammatory drugs (Pereira et al., Citation2010). ME and its fractions produced antinociception in both phases of the formalin test, but showed no effect in the hot-plate assay which involves higher brain functions and consists of responses to nociceptive stimuli organized at a supraspinal level (Srinivasan et al., Citation2003). Analgesic drugs such as aspirin and paracetamol do not have any effect in this experiment, while it is largely used to measure opioid effects (Ferreira et al., Citation2004).
In order to explain the relationship between anti-inflammatory and analgesic effects, we also detected the volume of the hind paw after injecting formalin for 1 h. Pethidine as the opium receptor drugs could not decrease the paw volumes. P.E. extract increased the paw volume, while EtOAc part decreased the paw volume in the same condition, which revealed the presence of different chemicals in different parts of WCAW.
The phytochemical analysis of ME revealed the presence of flavonoids, phenolic acids and anthraquinones compounds by HPLC (Hu et al., Citation2009). Sixty-eight compounds by HPLC-ESI-MS were detected from ME and 41 of them were identified. Saccharides or proteins contained in the H2O fraction were not detected. As shown in , the peaks were different in the extracts and certain compounds contained in the different extracts were overlapping, which may explain the different antinociceptive activities of the extracts. Most of the identified chemical compounds possessed the antinociceptive or anti-inflammatory effects. Sesquiterpene lactones such as costunolide and dehydrocostus lactone as principal components of Aucklandia lappa could inhibit the transudative, exudative and proliferative phases of inflammation (Damre et al., Citation2003). Hesperidin, as the main compound of Fructus Aurantii, stimulated the gastrointestinal movement (Fang et al., Citation2009), exhibited antinociceptive activities in the abdominal writhing and hot-plate test (Loscalzo et al., Citation2011) and showed anti-inflammatory properties (Manthey et al., Citation2001). Naringenin, magnolol and muscone showed anti-inflammatory effects (Hsu et al., Citation2004; Liang et al., Citation2010; Park et al., Citation2004; Tsai et al., Citation1999; Wang et al., Citation1995). Anthraquinone derivatives including aloe-emodin, rhein and sennosides also showed anti-inflammatory effects (Ma et al., Citation2009). Magnolol and honokiol also inhibited the second phase in the formalin test by inhibition of glutamate receptors and suppression of inflammatory mediators including PGE2 (Lee et al., Citation2011; Li et al., Citation2007; Lin et al., Citation2009). It was reported that the compounds exhibited the effects by synergy like three compounds of rhein, magnolol and naringenin exerted combined anti-inflammatory effect in a tradition Chinese formula (Tseng et al., Citation2006). From the view point of analgesic effects, ME exhibited better powerful activity than other four fractions, which may be the synergistic effect of some chemical constituents. So further work is to identify the active compounds and also to acknowledge the proportionment of the compounds.
Although we divided it into four parts by solution extraction, determination of the active component of medicines with mixtures of herbs was very complicated for the herbal interaction. The above results revealed that ME showed more influence on the acetic acid-induced writhing and formalin test than the fractions. So we calculated that mixture compounds contained in different extracts exerted the analgesic activity. Combination of the different extracts in antinociceptive models will be researched in further studies.
Conclusions
WCAW exhibited antinociceptive effect in the acetic acid-induced writhing test and the formalin test but not in the hot-plate test, indicating the possible mechanism may be related to the inflammatory mediators. Meanwhile, WCAW may exert the antinociceptive effect with different complex compounds among the parts for the reason that ME showed stronger antinociceptive effect than the four parts. This research provided a new clinical application in the redevelopment of WCAW.
Declaration of interest
We have no conflict of interest in this research. The work was supported by a grant 05ZHGCGX01000 from the Tianjin Modernization of Traditional Chinese Herbs (Tianjin, China).
References
- Committee of National Pharmacopoeia. (2010). Pharmacopoeia of People’s Republic of China (I). Beijing: Chemical Industry Press, 880–1
- Dai ZY, Wang QM, Xu XM, et al. (2010). Changes of the intestinal barrier of rats with intestinal dysfunction induced by cold restraint stress. Chin J Gastroenterol Hepatol 19:61–4
- Damre AA, Damre AS, Saraf MN. (2003). Evaluation of sesquiterpene lactone fraction of Saussurea lappa on transudative, exudative and proliferative phases of inflammation. Phytother Res 17:722–5
- Fang YS, Shan DM, Liu JW, et al. (2009). Effect of constituents from Fructus Aurantii Immaturus and Radix Paeoniae Alba on gastrointestinal movement. Planta Med 75:24–31
- Ferreira MA, Nunes OD, Fontenele JB, et al. (2004). Analgesic and anti-inflammatory activities of a fraction rich in oncocalyxone A isolated from Auxemma oncocalyx. Phytomedicine 11:315–22
- Gao D, Xu L. (2010). Comparative analgesic effect of Ligusticum chuanxiong pieces and its products in mice. Pharmacogn Mag 6:132–4
- Gao Y, Ma ZC, Zhang BL. (2012). Significance of re-evaluation and development of Chinese herbal drugs. China J Chin Mater Med 37:1–4
- Garcia GG, Miranda HF, Noriega V, et al. (2011). Antinociception induced by atorvastatin in different pain models. Pharmacol Biochem Behav 100:125–9
- Godin AM, Ferreira WC, Rocha LT, et al. (2011). Antinociceptive and anti-inflammatory activities of nicotinamide and its isomers in different experimental models. Pharmacol Biochem Behav 99:782–8
- Guo JS, Liu HY, Wang XJ, et al. (2012). Effect of the different extracts of Sandalwood on isolated the small intestine movement function and gastric emptying of mice. Chin J Exp Tradit Med Formulae 18:133–7
- Hsu MF, Lu MC, Tsao LT, et al. (2004). Mechanisms of the influence of magnolol on eicosanoid metabolism in neutrophils. Biochem Pharmacol 67:831–40
- Hu J, Gao WY, Ling NS, Liu CX. (2009). Antidiarrhoeal and intestinal modulatory activities of Wei-Chang-An-Wan extract. J Ethnopharmacol 125:450–5
- Kakino M, Tazawa S, Maruyama H, et al. (2010). Laxative effects of agarwood on low-fiber diet-induced constipation in rats. BMC Complement Altern Med 10:68--75
- Kim EJ, Hong JE, Lim SS, et al. (2012). The hexane extract of Saussurea lappa and its active principle, dehydrocostus lactone, inhibit prostate cancer cell migration. J Med Food 15:24–32
- Lee YJ, Lee YM, Lee CK, et al. (2011). Therapeutic applications of compounds in the Magnolia family. Pharmacol Ther 130:157–76
- Li N, Song Y, Zhang W, et al. (2007). Evaluation of the in vitro and in vivo genotoxicity of Magnolia bark extract. Regul Toxicol Pharmacol 49:154–9
- Liang QQ, Zhang M, Zhou Q, et al. (2010). Muscone protects vertebral end-plate degeneration by antiinflammatory property. Clin Orthop Relat Res 468:1600–10
- Lin YR, Chen HH, Lin YC, et al. (2009). Antinociceptive actions of honokiol and magnolol on glutamatergic and inflammatory pain. J Biomed Sci 16:94--103
- Ling NS, Yang J, Lv ZR. (2005). Determination of naringin in Wei–Chang–An pills by HPLC. Chin Tradit Herbal Drugs 36:1815–16
- Liu Z, Gao WY, Zhang JZ. (2010). Effect of Weichang’an Pill on the treatment of irritable bowel syndrome. Drug Eval Res 33:159–62
- Loscalzo LM, Yow TT, Wasowski C, et al. (2011). Hesperidin induces antinociceptive effect in mice and its aglicone, hesperetin, binds to mu-opioid receptor and inhibits GIRK1/2 currents. Pharmacol Biochem Behav 99:333–41
- Ma BL, Ma YM, Yan DM, et al. (2009). Effective constituents in Xiexin Decoction for anti-inflammation. J Ethnopharmacol 125:151–6
- Manthey JA, Grohmann K, Guthrie N. (2001). Biological properties of citrus flavonoids pertaining to cancer and inflammation. Curr Med Chem 8:135–53
- Miranda HF, Puig MM, Dursteler C, et al. (2007). Dexketoprofen-induced antinociception in animal models of acute pain: Synergy with morphine and paracetamol. Neuropharmacology 52:291–6
- Miranda HF, Sierralta F, Pinardi G. (2002). Neostigmine interactions with non steroidal anti-inflammatory drugs. Br J Pharmacol 135:1591–7
- Park J, Lee J, Jung E, et al. (2004). In vitro antibacterial and anti-inflammatory effects of honokiol and magnolol against Propionibacterium sp. Eur J Pharmacol 496:189–95
- Pereira SS, Lopes LS, Marques RB, et al. (2010). Antinociceptive effect of Zanthoxylum rhoifolium Lam. (Rutaceae) in models of acute pain in rodents. J Ethnopharmacol 129:227–31
- Quintans-Júnior LJ, Melo MS, De Sousa DP, et al. (2010). Antinociceptive effects of citronellal in formalin-, capsaicin- and glutamate-induced orofacial nociception in rodents and its action on nerve excitability. J Orofac Pain 24:305–12
- Srinivasan K, Muruganandan S, Lal J, et al. (2003). Antinoniceptive and antipyretic activities of Pongamia pinnata leaves. Phytother Res 17: 259–264
- Taufiq-Ur-Rahman M, Shilpi JA, Ahmed M, Faiz Hossain C. (2005). Preliminary pharmacological studies on Piper chaba stem bark. J Ethnopharmacol 99:203–9
- Tjolsen A, Berge OG, Hunskaar S, et al. (1992). The formalin test: An evaluation of the method. Pain 51:5–17
- Tsai SH, Lin-Shiau SY, Lin JK. (1999). Suppression of nitric oxide synthase and the down-regulation of the activation of NFkappaB in macrophages by resveratrol. Br J Pharmacol 126:673–80
- Tseng SH, Lee HH, Chen LG, et al. (2006). Effects of three purgative decoctions on inflammatory mediators. J Ethnopharmacol 105:118–24
- Wang L, Zhang JZ, Liu Z, Gao WY. (2012). Study on anti-inflammation effect of the extract of Wei Chang An Pill. Chin Tradit Herbal Drugs (Accept)
- Wang JP, Ho TF, Chang LC, Chen CC. (1995). Anti-inflammatory effect of magnolol, isolated from Magnolia officinalis, on A23187-induced pleurisy in mice. J Pharm Pharmacol 47:857–60
- Watanabe K, Watanabe H, Goto Y, et al. (1983). Pharmacological properties of magnolol and honokiol extracted from Magnolia officinalis: Central depressant effects. Planta Med 49:103–8
- Xie WD, Zhao YN, Du LJ. (2012). Emerging approaches of traditional Chinese medicine formulas for the treatment of hyperlipidemia. J Ethnopharmacol 140:345–67
- Zhou M, Wang H, Suolangjiba Kou J, Yu B. (2008). Antinociceptive and anti-inflammatory activities of Aquilaria sinensis (Lour.) Gilg. leaves extract. J Ethnopharmacol 117:345–50